Abstract
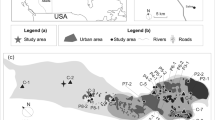
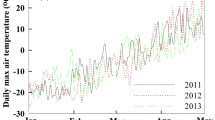
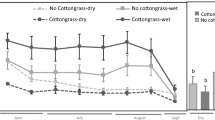
Climate change is predicted to bring about a water level (WL) draw-down in boreal peatlands. This study aimed to assess the effect of WL on the carbon dioxide (CO2) dynamics of a boreal oligotrophic fen ecosystem and its components; Sphagnum mosses, sedges, dwarf shrubs and the underlying peat. We measured CO2 exchange with closed chambers during four growing seasons in a study site that comprised different vegetation treatments. WL gradient in the site was broadened by surrounding half of the site with a shallow ditch that lowered the WL by 10–25 cm. We modeled gross photosynthesis (P G) and ecosystem respiration (R ECO) and simulated the CO2 exchange in three WL conditions: prevailing and WL draw-down scenarios of 14 and 22 cm. WL draw-down both reduced the P G and increased the R ECO, thus leading to a smaller net CO2 uptake in the ecosystem. Of the different components, Sphagnum mosses were most sensitive to WL draw-down and their physiological activities almost ceased. Vascular plant CO2 exchange, en bloc, hardly changed but whereas sedges contributed twice as much to the CO2 exchange as shrubs in the prevailing conditions, the situation was reversed in the WL draw-down scenarios. Peat respiration was the biggest component in R ECO in all WL conditions and the increase in R ECO following the WL draw-down was due to the increase in peat respiration. The results imply that functional diversity buffers the ecosystem against environmental variability and that in the long term, WL draw-down changes the vegetation composition of boreal fens.







Similar content being viewed by others
References
Aguiar MR, Paruelo JM, Sala OE, Lauenroth WK. 1996. Ecosystem responses to changes in plant functional type composition: an example from the Patagonian steppe. J Veg Sci 7:381–390
Ahti T, Hämet-Ahti L, Jalas J. 1968. Vegetation zones and their sections in northwestern Europe. Ann Bot Fenn 5:169–211
Alm J, Talanov A, Saarnio S, Silvola J, Ikkonen E, Aaltonen H, Nykänen H, Martikainen PJ. 1997. Reconstruction of the carbon balance for microsites in a boreal oligotrophic pine fen, Finland. Oecologia 110:423–431
Alm J, Schulman L, Walden J, Nykänen H, Martikainen PJ, Silvola J. 1999. Carbon balance of a boreal bog during a year with an exceptionally dry summer. Ecology 80:161–174
Aurela M, Laurila T, Tuovinen JP. 2002. Annual CO2 balance of a subarctic fen in northern Europe: importance of the wintertime efflux. J Geophys Res Atmos 107:4607. doi:10.1029/2002JD002055
Aurela M, Laurila T, Tuovinen JP. 2004. The timing of snow melt controls the annual CO2 balance in a subarctic fen. Geophys Res Lett 31:L16119. doi:10.1029/2004GL020315
Bremer DJ, Ham JM, Owensby CE, Knapp AK. 1998. Responses of soil respiration to clipping and grazing in a tallgrass prairie. J Environ Qual 27:1539–1548
Bubier JL, Bhatia G, Moore TR, Roulet NT, Lafleur PM. 2003a. Spatial and temporal variability in growing-season net ecosystem carbon dioxide exchange at a large peatland in Ontario, Canada. Ecosystems 6:353–367
Bubier J, Crill P, Mosedale A, Frolking S, Linder E. 2003b. Peatland responses to varying interannual moisture conditions as measured by automatic CO2 chambers. Global Biogeochem Cycles 17:1066. doi:10.1029/2002GB001946
Busch J, Lösch R. 1999. The gas exchange of Carex species from eutrophic wetlands and its dependence on microclimatic and soil wetness conditions. Phys Chem Earth Part B Hydrol Oceans Atmos 24:117–120
Chapin FS III, BretHarte MS, Hobbie SE, Zhong HL. 1996. Plant functional types as predictors of transient responses of arctic vegetation to global change. J Veg Sci 7:347–358
Chimner RA, Cooper DJ. 2003. Influence of water table levels on CO2 emissions in a Colorado subalpine fen: an in situ microcosm study. Soil Biol Biochem 35:345–351
Clymo RS. 1983. Peat. In: Gore AJP, Ed. Ecosystems of the of the World 4A mires: swamp, bog, fen and moor. General studies. Amsterdam: Elsevier. pp 159–224
Clymo RS, Hayward PM. 1982. The ecology of Sphagnum. In: Smith AJE, Ed. Bryophyte ecology. London: Chapman & Hall. pp 229–89
Conway V. 1937. Studies in the autoecology of Cladium mariscus R.BR. III The aeration of the subterranean parts of the plant. New Phytologist 36:64–96
Crow SE, Wieder RK. 2005. Sources of CO2 emission from a northern peatland: root respiration, exudation, and decomposition. Ecology 86:1825–1834
Drebs A, Nordlund A, Karlsson P, Helminen J, Rissanen P. 2002. Climatological statistics of Finland 1971–2000. Clim Stat Finland 1:1–99
Efron B, Tibshirani RJ. 1993. An introduction to the bootstrap. Chapman & Hall, New York
Gaberscik A, Martincic A. 1987. Seasonal dynamics of net photosynthesis and productivity of Sphagnum papillosum. Lindbergia 13:105–110
Gifford RM. 2003. Plant respiration in productivity models: conceptualisation, representation and issues for global terrestrial carbon-cycle research. Funct Plant Biol 30:171–186
Gorham E. 1991. Northern peatlands—role in the carbon-cycle and probable responses to climatic warming. Ecol Appl 1:182–195
Griffis TJ, Rouse WR, Waddington JM. 2000. Interannual variability of net ecosystem CO2 exchange at a subarctic fen. Global Biogeochem Cycles 14:1109–1121
Harley PC, Tenhunen JD, Murray KJ, Beyers J. 1989. Irradiance and temperature effects on photosynthesis of tussock tundra Sphagnum mosses from the foothills of the Philip Smith Mountains, Alaska. Oecologia 79:251–259
Heikkinen JEP, Virtanen T, Huttunen JT, Elsakov V, Martikainen PJ. 2004. Carbon balance in East European tundra. Global Biogeochem Cycles 18:GB1023. doi:10.1029/2003GB002054
Hurlbert SH. 1984. Pseudoreplication and the design of ecological field experiments. Ecol Monogr 54:187–211
Karlsson PS. 1989. In situ photosynthetic performance of four coexisting dwarf shrubs in relation to light in a subarctic woodland. Funct Ecol 3:481–487
Klanderud K, Totland O. 2005. Simulated climate change altered dominance hierarchies and diversity of an alpine biodiversity hotspot. Ecology 86:2047–2054
Lafleur PM, Roulet NT, Bubier JL, Frolking S, Moore TR. 2003. Interannual variability in the peatland-atmosphere carbon dioxide exchange at an ombrotrophic bog. Global Biogeochem Cycles 17:1036. doi:10.1029/2002GB001983
Laiho R, Vasander H, Penttila T, Laine J. 2003. Dynamics of plant-mediated organic matter and nutrient cycling following water-level drawdown in boreal peatlands. Global Biogeochem Cycles 17:1053. doi:10.1029/2002GB002015
Lloyd J, Taylor JA. 1994. On the temperature dependence of soil respiration. Funct Ecol 8:315–323
Lovelock CE, Feller IC. 2003. Photosynthetic performance and resource utilization of two mangrove species coexisting in a hypersaline scrub forest. Oecologia 134:455–462
Lumiala OV. 1944. Über die Beziehung einiger Moorpflanzen zu der Grundwasserhöhe. Bull Comiss geol Finlande No 132
Martikainen PJ, Nykänen H, Alm J, Silvola J. 1995. Change in fluxes of carbon dioxide, methane and nitrous-oxide due to forest drainage of mire sites of different trophy. Plant Soil 169:571–577
Moore PD. 2002. The future of cool temperate bogs. Environ Conserv 29:3–20
Moore TR, Dalva M. 1993. The influence of temperature and water-table position on carbon dioxide and methane emissions from laboratory columns of peatland soils. J Soil Sci 44:651–664
Nykänen H, Heikkinen JEP, Pirinen L, Tiilikainen K, Martikainen PJ. 2003. Annual CO2 exchange and CH4 fluxes on a subarctic palsa mire during climatically different years. Global Biogeochem Cycles 17:1018. doi:10.1029/2002GB001861
Parsons AN, Welker JM, Wookey PA, Press MC, Callaghan TV, Lee JA. 1994. Growth responses of 4 sub-arctic dwarf shrubs to simulated environmental change. J Ecol 82:307–318
Richardson AD, Hollinger DY. 2005. Statistical modeling of ecosystem respiration using eddy covariance data: maximum likelihood parameter estimation, and Monte Carlo simulation of model and parameter uncertainty, applied to three simple models. Agric Forest Meteorol 131:191–208
Roulet N, Moore T, Bubier J, Lafleur P. 1992. Northern fens—methane flux and climatic change. Tellus Ser B Chem Phys Meteorol 44:100–105
Schipperges B, Rydin H. 1998. Response of photosynthesis of Sphagnum species from contrasting microhabitats to tissue water content and repeated desiccation. New Phytologist 140:677–684
Shevtsova A, Ojala A, Neuvonen S, Vieno M, Haukioja E. 1995. Growth and reproduction of dwarf shrubs in a sub-arctic plant community—annual variation and aboveground interactions with neighbors. J Ecol 83:263–275
Silvola J, Aaltonen H. 1984. Water content and photosynthesis in the peat mosses Sphagnum fuscum and Sphagnum angustifolium. Ann Bot Fenn 21:1–6
Soegaard H, Nordstroem C. 1999. Carbon dioxide exchange in a high-arctic fen estimated by eddy covariance measurements and modelling. Global Change Biol 5:547–562
Tuittila ES, Vasander H, Laine J. 2004. Sensitivity of C sequestration in reintroduced Sphagnum to water-level variation in a cutaway peatland. Restor Ecol 12:483–493
Turunen J, Tomppo E, Tolonen K, Reinikainen A. 2002. Estimating carbon accumulation rates of undrained mires in Finland—application to boreal and subarctic regions. Holocene 12:69–80
Waddington JM, Roulet NT. 2000. Carbon balance of a boreal patterned peatland. Global Change Biol 6:87–97
Wan S, Luo Y. 2003. Substrate regulation of soil respiration in a tallgrass prairie: results of a clipping and shading experiment. Global Biogeochem Cycles 17:1054. doi:10.1029/2002GB001971
Wijesinghe DK, John EA, Hutchings MJ. 2005. Does pattern of soil resource heterogeneity determine plant community structure? An experimental investigation. J Ecol 93:99–112
Wilson D, Alm J, Riutta T, Laine J, Byrne KA, Farrell EP, Tuittila E-S. 2007. A high resolution green area index for modelling the seasonal dynamics of CO2 exchange in peatland. Plan Ecol 190:37–51. doi:10.1007/s11258-006-9189-1
Acknowledgments
We are grateful to Jouni Meronen for technical assistance, to David Wilson for revising the language and to numerous Master’s students for helping with the field measurements. The comments of the two anonymous reviewers and Gaius Shaver greatly improved the manuscript. The study was funded by the Academy of Finland (SA50707, SA201623, SA202424), by the Helsinki University Environmental Research Centre (HERC) and by Jenny and Antti Wihuri Foundation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Riutta, T., Laine, J. & Tuittila, ES. Sensitivity of CO2 Exchange of Fen Ecosystem Components to Water Level Variation. Ecosystems 10, 718–733 (2007). https://doi.org/10.1007/s10021-007-9046-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10021-007-9046-7