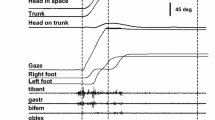
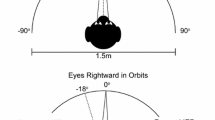
Abstract
Coordinating the movements of different body parts is a challenging process for the central nervous system because of several problems. Four of these main difficulties are: first, moving one part can move others; second, the parts can have different dynamics; third, some parts can have different motor goals; and fourth, some parts may be perturbed by outside forces. Here, we propose a novel approach for the control of linked systems with feedback loops for each part. The proximal parts have separate goals, but critically the most distal part has only the common goal. We apply this new control policy to eye-head coordination in two-dimensions, specifically head-unrestrained gaze saccades. Paradoxically, the hierarchical structure has controllers for the gaze and the head, but not for the eye (the most distal part). Our simulations demonstrate that the proposed control structure reproduces much of the published empirical data about gaze movements, e.g., it compensates for perturbations, accurately reaches goals for gaze and head from arbitrary initial positions, simulates the nine relationships of the head-unrestrained main sequence, and reproduces observations from lesion and single-unit recording experiments. We conclude by showing how our model can be easily extended to control structures with more linked segments, such as the control of coordinated eye on head on trunk movements.











Similar content being viewed by others
Notes
One simplification here is that we ignore internal noise in the system, e.g., Harris and Wolpert (1998) showed that optimal control of a system with signal-dependent noise can reproduce the speed-accuracy trade-off for saccades. As we are dealing with rejection of perturbations in linked systems, internal noise is not our primary concern.
To cancel its VOR, the monkey looked at a head-fixed target while sitting on a rotating chair that oscillated sinusoidally
References
Aizawa, H., & Wurtz, R. (1998). Reversible inactivation of monkey superior colliculus. I. Curvature of saccadic trajectory. Journal of Neurophysiology, 79(4), 2082–2096.
Anastasopoulos, D., Ziavra, N., Hollands, M., Bronstein, A. (2009). Gaze displacement and inter-segmental coordination during large whole body voluntary rotations. Experimental Brain Research, 193(3), 323–336.
Bahill, A., Clark, M., Stark, L. (1975). The main sequence, a tool for studying human eye movements. Mathematical Biosciences, 24(3–4), 191–204.
Bechara, B., & Gandhi, N. (2010). Matching the oculomotor drive during head-restrained and head-unrestrained gaze shifts in monkey. Journal of Neurophysiology, 104(2), 811–828.
Bizzi, E. (1979). Strategies of eye-head coordination. Progress in Brain Research, 50, 795–803.
Bizzi, E., Kalil, R., Tagliasco, V. (1971). Eye-head coordination in monkeys: evidence for centrally patterned organization. Science, 173(3995), 452–454.
Boulanger, M., Galiana, H., Guitton, D. (2012). Human eye-head gaze shifts preserve their accuracy and spatiotemporal trajectory profiles despite long-duration torque perturbations that assist or oppose head motion. Journal of Neurophysiology, 108(1), 39–56.
Cannon, S., & Robinson, D. (1987). Loss of the neural integrator of the oculomotor system from brain stem lesions in monkey. Journal of Neurophysiology, 57(5), 1383–1409.
Cheron, G., & Godaux, E. (1987). Disabling of the oculomotor neural integrator by kainic acid injections in the prepositus-vestibular complex of the cat. The Journal of physiology, 394(1), 267–290.
Choi, W., & Guitton, D. (2006). Responses of collicular fixation neurons to gaze shift perturbations in head-unrestrained monkey reveal gaze feedback control. Neuron, 50(3), 491–505.
Chun, K.S., & Robinson, D. (1978). A model of quick phase generation in the vestibuloocular reflex. Biological Cybernetics, 28(4), 209–221.
Collins, C., & Barnes, G. (1999). Independent control of head and gaze movements during head-free pursuit in humans. The Journal of Physiology, 515(1), 299–314.
Corneil, B., Olivier, E., Munoz, D. (2002). Neck muscle responses to stimulation of monkey superior colliculus. II. Gaze shift initiation and volitional head movements. Journal of Neurophysiology, 88(4), 2000–2018.
Corriou, J. (2004). Process control: theory and applications. London: Springer-Verlag. ISBN:1-85233-776-1.
Cullen, K., & Roy, J. (2004). Signal processing in the vestibular system during active versus passive head movements. Journal of Neurophysiology, 91(5), 1919–1933.
Cullen, K., Huterer, M., Braidwood, D., Sylvestre, P. (2004). Time course of vestibuloocular reflex suppression during gaze shifts. Journal of Neurophysiology, 92(6), 3408–3422.
Dale, A., & Cullen, K.E. (2013). The nucleus prepositus predominantly outputs eye movement-related information during passive and active self-motion. Journal of Neurophysiology, 109(7), 1900–1911.
Duhamel, J.R., Colby, C., Goldberg, M. (1992). The updating of the representation of visual space in parietal cortex by intended eye movements. Science, 255(5040), 90–92.
Farshadmanesh, F., Klier, E., Chang, P., Wang, H., Crawford, J. (2007). Three-dimensional eye–head coordination after injection of muscimol into the interstitial nucleus of cajal (inc). Journal of Neurophysiology, 97(3), 2322–2338.
Freedman, E. (2001). Interactions between eye and head control signals can account for movement kinematics. Biological Cybernetics, 84(6), 453–462.
Freedman, E. (2008). Coordination of the eyes and head during visual orienting. Experimental Brain Research, 190(4), 369–387.
Freedman, E., & Sparks, D. (1997). Eye-head coordination during head-unrestrained gaze shifts in rhesus monkeys. Journal of Neurophysiology, 77(5), 2328–2348.
Freedman, E., & Sparks, D. (2000). Coordination of the eyes and head: movement kinematics. Experimental Brain Research, 131(1), 22–32.
Fujita, M. (2005). Feed-forward associative learning for volitional movement control. Neuroscience Research, 52(2), 153–165.
Fuller, J. (1992). Head movement propensity. Experimental Brain Research, 92(1), 152–164.
Galiana, H., & Guitton, D. (1992). Central organization and modeling of eye-head coordination during orienting gaze shifts. Annals of the New York Academy of Sciences, 656(1), 452–471.
Galiana, H., & Outerbridge, J. (1984). A bilateral model for central neural pathways in vestibuloocular reflex. Journal of Neurophysiology, 51(2), 210–241.
Gandhi, N.J. (2012). Interactions between gaze-evoked blinks and gaze shifts in monkeys. Experimental Brain Research, 216(3), 321–339.
Gandhi, N., & Sparks, D. (2007). Dissociation of eye and head components of gaze shifts by stimulation of the omnipause neuron region. Journal of Neurophysiology, 98(1), 360–373.
Gilchrist, I., Brown, V., Findlay, J. (1997). Saccades without eye movements. Nature, 390(6656), 130–131.
Gilchrist, I., Brown, V., Findlay J, Clarke M. (1998). Using the eye–movement system to control the head. Proceedings of the Royal Society of London Series B: Biological Sciences, 265, 1831–1836.
Goffart, L., & Pélisson, D. (1998). Orienting gaze shifts during muscimol inactivation of caudal fastigial nucleus in the cat. I. Gaze dysmetria. Journal of Neurophysiology, 79(4), 1942–1958.
Goffart, L., Guillaume, A., Pélisson, D. (1998a). Compensation for gaze perturbation during inactivation of the caudal fastigial nucleus in the head-unrestrained cat. Journal of Neurophysiology, 80(3), 1552–1557.
Goffart, L., Pélisson, D., Guillaume, A. (1998b). Orienting gaze shifts during muscimol inactivation of caudal fastigial nucleus in the cat. II. Dynamics and eye-head coupling. Journal of Neurophysiology, 79(4), 1959–1976.
Goossens, H., & van Opstal, A. (1997). Human eye-head coordination in two dimensions under different sensorimotor conditions. Experimental Brain Research, 114(3), 542–560.
Grantyn, A., & Berthoz, A. (1985). Burst activity of identified tecto-reticulo-spinal neurons in the alert cat. Experimental Brain Research, 57(2), 417–421.
Guillaume, A., & Pélisson, D. (2001). Gaze shifts evoked by electrical stimulation of the superior colliculus in the head-unrestrained cat. II. Effect of muscimol inactivation of the caudal fastigial nucleus. European Journal of Neuroscience, 14(8), 1345–1359.
Guitton, D. (1992). Control of eye–head coordination during orienting gaze shifts. Trends in Neurosciences, 15(5), 174–179.
Guitton, D., & Volle, M. (1987a). Gaze control in humans: eye-head coordination during orienting movements to targets within and beyond the oculomotor range. Journal of Neurophysiology, 58(3), 427–459.
Guitton, D., & Volle, M. (1987b). Gaze control in humans: eye-head coordination during orienting movements to targets within and beyond the oculomotor range. Journal of Neurophysiology, 58(3), 427–459.
Guitton, D., Munoz, D., Galiana, H. (1990). Gaze control in the cat: studies and modeling of the coupling between orienting eye and head movements in different behavioral tasks. Journal of Neurophysiology, 64(2), 509–531.
Hanes, D., Smith, M., Optican, L., Wurtz, R. (2005). Recovery of saccadic dysmetria following localized lesions in monkey superior colliculus. Experimental Brain Research, 160(3), 312–325.
Harris, C., & Wolpert, D. (1998). Signal-dependent noise determines motor planning. Nature, 394(6695), 780–784.
Haustein, W. (1989). Considerations on listing’s law and the primary position by means of a matrix description of eye position control. Biological Cybernetics, 60(6), 411–420.
Isa, T., & Sasaki, S. (2002). Brainstem control of head movements during orienting; organization of the premotor circuits. Progress in Neurobiology, 66(4), 205–242.
Jürgens, R., Becker, W., Kornhuber, H. (1981). Natural and drug-induced variations of velocity and duration of human saccadic eye movements: evidence for a control of the neural pulse generator by local feedback. Biological Cybernetics, 39(2), 87–96.
Kardamakis, A., & Moschovakis, A. (2009). Optimal control of gaze shifts. The Journal of Neuroscience, 29(24), 7723–7730.
Kardamakis, A., Grantyn, A., Moschovakis, A. (2010). Neural network simulations of the primate oculomotor system. v. eye–head gaze shifts. Biological Cybernetics, 102(3), 209–225.
Kase, M., Miller, D., Noda, H. (1980). Discharges of purkinje cells and mossy fibres in the cerebellar vermis of the monkey during saccadic eye movements and fixation. The Journal of Physiology, 300(1), 539–555.
Kato, R., Grantyn, A., Dalezios, Y., Moschovakis, A. (2006). The local loop of the saccadic system closes downstream of the superior colliculus. Neuroscience, 143(1), 319–337.
Keller, E., et al. (1974). Participation of medial pontine reticular formation in eye movement generation in monkey. Journal of Neurophysiology, 37(2), 316–332.
Klier, E., Wang, H., Constantin, A., Crawford, J. (2002). Midbrain control of three-dimensional head orientation. Science, 295(5558), 1314–1316.
Land, M. (2004). The coordination of rotations of the eyes, head and trunk in saccadic turns produced in natural situations. Experimental Brain Research, 159(2), 151–160.
Land, M. (2009). Vision, eye movements, and natural behavior. Visual Neuroscience, 26(1), 51–62.
Laurutis, V., & Robinson, D. (1986). The vestibulo-ocular reflex during human saccadic eye movements. The Journal of Physiology, 373(1), 209–233.
Lefèvre, P., & Galiana, H. (1992). Dynamic feedback to the superior colliculus in a neural network model of the gaze control system. Neural Networks, 5(6), 871–890.
Lefèvre, P., Bottemanne, I., Roucoux, A. (1992). Experimental study and modeling of vestibulo-ocular reflex modulation during large shifts of gaze in humans. Experimental Brain Research, 91(3), 496–508.
Lefèvre, P., Quaia, C., Optican, L. (1998). Distributed model of control of saccades by superior colliculus and cerebellum. Neural Networks, 11(7–8), 1175–1190.
Liao, K., Kumar, A., Han, Y., Grammer, V., Gedeon, B., Leigh, R. (2005). Comparison of velocity waveforms of eye and head saccades. Annals of the New York Academy of Sciences, 1039(1), 477–479.
Luschei, E.S., & Fuchs, A.F. (1972). Activity of brain stem neurons during eye movements of alert monkeys. Journal of Neurophysiology, 35(4), 445-461.
Mays, L., & Sparks, D. (1980). Saccades are spatially, not retinocentrically, coded. Science, 208, 1163–1165.
McFarland, J., & Fuchs, A. (1992). Discharge patterns in nucleus prepositus hypoglossi and adjacent medial vestibular nucleus during horizontal eye movement in behaving macaques. Journal of Neurophysiology, 68(1), 319–332.
Mottolese, C., Richard, N., Harquel, S., Szathmari, A., Sirigu, A., Desmurget, M. (2013). Mapping motor representations in the human cerebellum. Brain, 136, 330–342.
Munoz, D., & Guitton, D. (1985). Tectospinal neurons in the cat have discharges coding gaze position error. Brain Research, 341(1), 184–188.
Munoz, D., & Guitton, D. (1986). Presaccadic burst discharges of tecto-reticulo-spinal neurons in the alert head-free and-fixed cat. Brain Research, 398(1), 185–190.
Optican, L. (2005). Sensorimotor transformation for visually guided saccades. Annals of the New York Academy of Sciences, 1039(1), 132–148.
Optican, L. (2009). Oculomotor system: models. In Encyclopedia of neuroscience (pp. 25–34). Oxford: Academic.
Optican, L., & Quaia, C. (2002). Distributed model of collicular and cerebellar function during saccades. Annals of the New York Academy of Sciences, 956(1), 164–177.
Optican, L., & Robinson, D. (1980). Cerebellar-dependent adaptive control of primate saccadic system. Journal of Neurophysiology, 44(6), 1058–1076.
Paré, M., & Guitton, D. (1998). Brain stem omnipause neurons and the control of combinedeye-head gaze saccades in the alert cat. Journal of Neurophysiology, 79(6), 3060–3076.
Pélisson, D., Guitton, D., Munoz, D. (1989). Compensatory eye and head movements generated by the cat following stimulation-induced perturbations in gaze position. Experimental Brain Research, 78(3), 654–658.
Pélisson, D., Goffart, L., Guitton, D. (1995). On-line compensation of gaze shifts perturbed by micro-stimulation of the superior colliculus in the cat with unrestrained head. Experimental Brain Research, 106(2), 196–204.
Pélisson, D., Goffart, L., Guillaume, A. (1998). Contribution of the rostral fastigial nucleus to the control of orienting gaze shifts in the head-unrestrained cat. Journal of Neurophysiology, 80(3), 1180–1196.
Pélisson, D., Goffart, L., Guillaume, A., Quinet, J. (2003). Visuo-motor deficits induced by fastigial nucleus inactivation. The Cerebellum, 2(1), 71–76.
Peng, G., Hain, T., Peterson, B. (1996). A. dynamical model for reflex activated head movements in the horizontal plane. Biological Cybernetics, 75(4), 309–319.
Prsa, M., & Galiana, H. (2007). Visual-vestibular interaction hypothesis for the control of orienting gaze shifts by brain stem omnipause neurons. Journal of Neurophysiology, 97(2), 1149–1162.
Quaia, C., & Optican, L. (1998). Commutative saccadic generator is sufficient to control a 3-d ocular plant with pulleys. Journal of Neurophysiology, 79(6), 3197–3215.
Quaia, C., Aizawa, H., Optican, L., Wurtz, R. (1998). Reversible inactivation of monkey superior colliculus. II. Maps of saccadic deficits. Journal of Neurophysiology, 79(4), 2097–2110.
Quaia, C., Lefèvre, P., Optican, L. (1999). Model of the control of saccades by superior colliculus and cerebellum. Journal of Neurophysiology, 82(2), 999–1018.
Robinson, D.A. (1975). Oculomotor control signals. In G. Lennerstand & P. Bach-y-Rita (Eds.), Basic mechanisms of ocular motility and their clinical implications (Vol. 24, pp. 337–374). Pergamon Press.
Robinson, F., Straube, A., Fuchs, A. (1993). Role of the caudal fastigial nucleus in saccade generation. II. Effects of muscimol inactivation. Journal of Neurophysiology, 70(5), 1741–1758.
Rottach, K., Das, V., Wohlgemuth, W., Zivotofsky, A., Leigh, R. (1998). Properties of horizontal saccades accompanied by blinks. Journal of Neurophysiology, 79(6), 2895–2902.
Roy, J., & Cullen, K. (1998). A neural correlate for vestibulo-ocular reflex suppression during voluntary eye-head gaze shifts. Nature Neuroscience, 1(5), 404–10.
Schiller, P., True, S., Conway, J. (1979). Effects of frontal eye field and superior colliculus ablations on eye movements. Science, 206(4418), 590–592.
Schiller, P., True, S., Conway, J. (1980). Deficits in eye movements following frontal eye-field and superior colliculus ablations. Journal of Neurophysiology, 44(6), 1175–1189.
Schweighofer, N., Arbib, M., Dominey, P. (1996). A model of the cerebellum in adaptive control of saccadic gain. I. The model and its biological substrate. Biological Cybernetics, 75(1), 19–28.
Scudder, C., Kaneko, C., Fuchs, A. (2002). The brainstem burst generator for saccadic eye movements. A modern synthesis. Experimental Brain Research, 142(4), 439–462.
Sparks, D., & Travis, R. (1971). Firing patterns of reticular formation neurons during horizontal eye movements. Brain Research, 33(2), 477.
Sylvestre, P., & Cullen, K. (2006). Premotor correlates of integrated feedback control for eye–head gaze shifts. The Journal of Neuroscience, 26(18), 4922–4929.
Tomlinson, R., & Bahra, P. (1986a). Combined eye-head gaze shifts in the primate. I. Metrics. Journal of Neurophysiology, 56(6), 1542–1557.
Tomlinson, R., & Bahra, P. (1986b). Combined eye-head gaze shifts in the primate. II. Interactions between saccades and the vestibuloocular reflex. Journal of Neurophysiology, 56(6), 1558–1570.
Tweed, D. (1997). Three-dimensional model of the human eye-head saccadic system. Journal of Neurophysiology, 77(2), 654–666.
Viviani, P., & Berthoz, A. (1975). Dynamics of the head-neck system in response to small perturbations: analysis and modeling in the frequency domain. Biological Cybernetics, 19(1), 19–37.
Walton, M., Bechara, B., Gandhi, N. (2008). Effect of reversible inactivation of superior colliculus on head movements. Journal of Neurophysiology, 99(5), 2479–2495.
Zangemeister, W., Lehman, S., Stark, L. (1981). Simulation of head movement trajectories: model and fit to main sequence. Biological Cybernetics, 41(1), 19–32.
Acknowledgments
We are especially grateful to Dr. N. J. Gandhi for kindly providing the data points used to make our Fig. 5 and 6.
Drs. Optican and Daye were supported by the Intramural Research Program of the National Eye Institute.
Dr. Blohm has been supported by the National Science and Engineering Research Council (Canada), the Ontario Research Fund (Canada), the Canadian Foundation for Innovation (Canada) and the Botterell Foundation (Queens University, Kingston, ON, Canada).
Dr. Lefevre has been supported by Fonds National de la Recherche Scientifique, Action de Recherche Concertée (Belgium). This paper presents research results of the Belgian Network Dynamical Systems, Control and Optimization, funded by the Interuniversity Attraction Poles Programmes, initiated by the Belgian State, Science Policy Office.
Conflict of interests
The authors declare that they have no conflict of interest
Author information
Authors and Affiliations
Corresponding author
Additional information
Action Editor: Simon R Schultz
Rights and permissions
About this article
Cite this article
Daye, P.M., Optican, L.M., Blohm, G. et al. Hierarchical control of two-dimensional gaze saccades. J Comput Neurosci 36, 355–382 (2014). https://doi.org/10.1007/s10827-013-0477-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10827-013-0477-1