Abstract
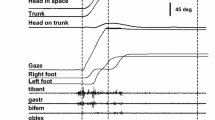
Displacements of the visual axis and multi-segmental (eye-to-foot) coordination in the yaw plane were studied in ten human subjects (Ss) during voluntary reorientations to illuminated targets of eccentricities up to 180°. We also investigated how knowledge of target location modifies the movement pattern. Eccentric targets (outbound trials) elicited eye, head, trunk and foot movements at latencies ca. 0.5, 0.6, 0.7 and 1.1 s, respectively. Knowledge of target location (return trials) reduced latencies for foot and trunk (but not eye and head) thus eye, head and trunk moved more en bloc. In most trials, the initial gaze shift fell short of the target and more than 50% of the visual angle was covered by the sum of vestibular nystagmic fast phases and head-in-space displacement, until target fixation. This indicates that during large gaze shifts the ‘anticompensatory’ role of the vestibulo-ocular reflex in target acquisition is prominent. During some predictable trials Ss acquired targets with a single large gaze shift, shortening target acquisition time by more than 200 ms. In these, gaze velocity (trunk-in-space + head-on-trunk + eye-in-orbit) remained often fairly constant for durations of up to 500 ms, suggesting that gaze velocity is a controlled parameter. Such pattern occurred during trunk mobilization, thus eye velocity co-varied with head-in-space rather than head-on-trunk velocity. Foot rotations were stereotyped and of constant frequency, suggesting they are generated by locomotor pattern generators. However, knowledge of target location reduced foot latencies indicating that local and supraspinal mechanisms interact for foot control. We propose that a single controller is responsible for the coupling of the multiple body segments and gaze velocity control during gaze shifts.










Similar content being viewed by others
Abbreviations
- PC:
-
Principal component
- PCA:
-
Principal component analysis
- Ss:
-
Subjects
- VOR:
-
Vestibulo-ocular reflex
References
Aruin AS, Latash ML (1995) The role of motor action in anticipatory postural adjustments studied with self-induced and externally triggered perturbations. Exp Brain Res 106:291–300
Barnes G (1979) Vestibulo-ocular function during co-ordinated head and eye movements to acquire visual targets. J Physiol 287:127–147
Bizzi E, Kallil RE, Tagliasco V (1971) Eye–head coordination in monkeys: evidence for centrally patterned organization. Science 173:452–454
Bronstein AM, Hood JD (1986) The cervico-ocular reflex in normal subjects and patients with absent vestibular function. Brain Res 373:399–408
Chatfield C, Collins AJ (1980) Introduction to multivariate analysis. Chapman & Hall, New York, pp 55–79
Collewijn H (1977) Gaze in freely moving subjects. In: Baker R, Berthoz A (eds) Control of gaze by brain stem neuron. Elsevier, North-Holland, Amsterdam, pp 13–22
Courtine G, Schieppati M (2003) Human walking along a curved path. II. Gait features and EMG patterns. Eur J NeuroSci 18:191–205
Dietz V, Baaken B, Colombo G (2001) Proprioceptive input overrides vestibulo-spinal drive during human locomotion. Neuro Report 12:2743–2746
Dietz V, Müller R, Colombo G (2002) Locomotor activity in spinal man: significance of afferent input from joint and load receptors. Brain 125:2626–2634
Freedman E (2001) Interactions between eye and head control signals can account for movement kinematics. Biol Cyber 84:453–462
Freedman E, Sparks D (1997) Eye–head coordination during head-unrestrained gaze shifts in rhesus monkeys. J Neurophysiol 77:2328–2348
Fuller JH (1996) Eye position and target amplitude effects on human visual saccadic latencies. Exp Brain Res 109:457–466
Goossens H, Van Opstal A (1997) Human eye–head coordination in two dimensions under different sensorimotor conditions. Exp Brain Res 114:542–560
Guitton D, Volle M (1987) Gaze control in head free humans during orienting movements to targets within and beyond the oculomotor range. J Neurophysiol 58:427–459
Hiebert GW, Whelan PJ, Prochazka A, Pearson KG (1996) Contribution of hind limb flexor muscle afferents to the timing of phase transitions in the cat step cycle. J Neurophysiol 75:1126–1137
Hollands M, Ziavra N, Bronstein A (2004) A new paradigm to investigate the roles of head and eye movements in the coordination of whole-body movements. Exp Brain Res 154:161–166
Isotalo E, Zee DS, Lasker AG (2005) Cognitive influencies on predictive saccadic tracking. Exp Brain Res 165:461–469
Land M (2004) The coordination of rotations of the eyes, head and trunk in saccadic turns produced in natural situations. Exp Brain Res 159:151–160
Laurutis V, Robinson D (1986) The vestibulo-ocular reflex during human saccadic eye movements. J Physiol 373:209–233
Lefèvre Ph, Bottemanne I, Roucoux A (1992) Experimental study and modelling of vestibulo-ocular reflex modulation during large shifts of gaze in humans. Exp Brain Res 91:496–508
Massion J (1984) Postural changes accompanying voluntary movements: normal and pathological aspects. Hum Neurobiol 2:261–267
McCluskey M, Cullen K (2007) Eye, head, and body coordination during large gaze shifts in rhesus monkeys: movement kinematics and the influence of posture. J Neurophysiol 97:2976–2991
Mishkin S, Mellvil Jones G (1966) Predominant direction of gaze during slow head rotation. Aerospace Med 37:897–901
Morasso P, Bizzi E, Dichgans J (1973) Adjustment of saccadic characteristics during head movements. Exp Brain Res 16:492–500
Moschner C, Zangemeister WH (1993) Preview control of gaze saccades: efficacy of prediction modulates eye–head interaction during human gaze saccades. Neurol Res 15:417–432
Pelisson D, Prablanc C, Urquizar C (1988) Vestibuloocular reflex inhibition and gaze saccade control characteristics during eye–head orientation in humans. J Neurophysiol 59:997–1013
Phillips J, Ling L, Fuchs A (1999) Action of the brain stem saccade generator during horizontal gaze shifts. I. Discharge patterns of omnidirectional pause neurons. J Neurophysiol 81:1284–1295
Roucoux A, Crommelinck M, Guerit JM, Meulders M (1981) Two modes of eye–head coordination and the role of the vestibule-ocular reflex in these two strategies. In: Fuchs A, Becker W (eds) Progress in oculomotor research. Elsevier, North Holland, pp 309–315
Roucoux A, Crommelinck M (1988) Control of head movement during visual orientation. In: Peterson BW, Richmond F (eds) Control of head movement. Oxford University Press, London, pp 208–223
Roy J, Cullen K (1998) A neural correlate for vestibulo-ocular reflex suppression during voluntary eye–head gaze shifts. Nat Neurosci 1:404–410
Roy J, Cullen K (2002) Vestibuloocular reflex signal modulation during voluntary and passive head movements. J Neurophysiol 87:2337–2357
Scholz JP, Schöner G (1999) The uncontrolled manifold concept: identifying control variables for a functional task. Exp Brain Res 126:289–306
Siegler I, Israël I, Berthoz A (1998) Shift of the beating field of vestibular nystagmus: an orientation strategy? Neurosci Lett 254:93–96
Solomon D, Kumar V, Jenkins RA, Jewell J (2006) Head control strategies during whole-body turns. Exp Brain Res 173:475–486
Tomlinson R, Bahra P (1986) Combined eye–head gaze shifts in the primate II. Interactions between saccades and the vestibuloocular reflex. J Neurophysiol 56:1558–1570
Weber KD, Fletcher WA, Melvill Jones G, Block EW (2000) Oculomotor responses to on-axis rotational stepping in normal and adaptively altered podokinetic states. Exp Brain Res 135:527–534
Zangemeister WH, Stark L (1982) Types of gaze movement: variable interactions of eye and head movements. Exp Neurol 77:563–577
Acknowledgments
We thank Sokratis Sklavos who helped with PCA. Financial support from the MRC is gratefully acknowledeged.
Author information
Authors and Affiliations
Corresponding author
Appendix
Appendix
PCA and artificial data simulations
Generally, PCA explores whether a set of variable data (herewith eye or head-in-space velocity, c.f. “Results”), stemming from the repetition of an experimental condition upon an event (‘trial’), can be thought to represent variations of an underlying function (‘system’). Variability levels might be maximal in case of a random pattern generating function while low variability levels might reflect stability of the process. Thus, a high degree of correlation among the output reiterations implies the existence of common patterns. PCA computes these patterns (called ‘principal components’, PCs) and the percentage of variation they account for (Chatfield and Collins 1980). Having a set of k-correlated traces X 1,…,X k , all assigned to the same channel, PCA creates a new set of orthogonal vectors Y 1,…,Y k called PCs). PCs are derived in decreasing order of importance so that the first PC (Y 1) explains most of the variance of the original data. Percentage of variance explained by the jth PC \( Y_{j} = X \cdot z_{j} \) (j = 1,…,k) is equal to the ω j eigenvalue \( \left( {\omega_{1} > \omega_{2} > \cdots > \omega_{k} > 0,\quad \sum\limits_{j = 1}^{k} {\omega_{j} = 1} } \right) \) of the covariance matrix \( \sigma = X^{\text{T}} \cdot X \) with corresponding eigenvector z j : \( \left( {\sigma - \omega_{j} \cdot I} \right)z_{j} = 0 \) and \( \left| {\sigma - \omega_{i} \cdot I} \right| = 0 \), where I is the k × k unique matrix, X = [X 1,…,X k ] and T is for transpose. Eigenvalues and eigenvectors were computed by using the MATLAB™ function eig.
The idea of working with PCA is: having a set of traces derived from repetitive trials, i.e., head velocity, we expect similarities in the trajectories, if we assume that the same function produces them. However, each trajectory may still be perturbed by randomly time-varying external sources. These sources produce non-systematic infrequent disturbances which appear in PCs of lower order of importance (with percentage of variance explained less than 1%). In contrast, the systematic, recurring parts of the trajectory are contained into the first and second PCs. Two useful properties of PCA as applied to our experiments with a multi-segmental control system are that: (1) the system’s degree of linearity is positively related to the proportion of the variability accounted for by the first PC and (2) the system’s degrees of freedom (dimensions) are the number of PCs that account for more than 1% of the system’s variability. One function is enough to describe all trials if the proportion of variability accounted for by the first PC approaches 100%; two functions (signals) would be necessary if the variability accounted for by the second PC and third PC is greater and less than 1%, respectively. Accordingly, a totally random configuration of a signal from trial to trial would require a system of n dimensions, equal to the number n of trials used for analysis (Chatfield and Collins 1980).
A disadvantage of PCA is that it requires data curves of equal length, and since trial duration may be variable, the number of trials included in the analysis will depend on the selection of an appropriate time interval. Simulations with artificial data were carried out to test these issues, before applying this analysis to our raw velocity data from the single-step gaze shifts. Specifically, we considered a time-varying Markov process for generating a red noise signal r: \( r_{i} = \xi \cdot r_{i - 1} + g_{i} ,\quad r_{0} = 0,\;i = 1, \ldots ,n \) where 0 < ξ < 1 is the Markov index of variability and g i is taken from a Gaussian white noise distribution. m = 27 different red noise signals r (1),…,r (m) were thus generated and each was added to a ramp-step function x (Fig. 11) producing m correlated traces\( X_{j} = x + a \cdot r^{(j)} ,\quad j = 1, \ldots ,m,\;0 < a < 1 \), of n = 100 points each. PCA was then applied on signals X 1,…,X m , showing that the first PC, can in fact reveal the pattern of the original ramp-step signal, in spite of the biasing effects of the red noise (Fig. 11).
Artificial data simulations. With dashes is represented the ramp-step signal (thick gray) when biased by red noise (in a ξ = 0.97 and α = 0.05; in b ξ = 0.75 and α = 0.05). The variance of the system (spanned by the m = 27 correlated traces) accounted for by the first PC (thin solid) is 97.3% in a and 99.4% in b (Euclidean error distance from the ramp-step signal is 0.53 and 0.30, respectively). In both examples all other PCs account for less than 1% of variability. Note that the ramp-step signal is very well approximated by the first PC
Rights and permissions
About this article
Cite this article
Anastasopoulos, D., Ziavra, N., Hollands, M. et al. Gaze displacement and inter-segmental coordination during large whole body voluntary rotations. Exp Brain Res 193, 323–336 (2009). https://doi.org/10.1007/s00221-008-1627-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-008-1627-y