Abstract
Purpose
To compare measurements of the full-field photopic negative response (PhNR), as well as intra-subject variation in the PhNR, using time and time–frequency domain analyses.
Methods
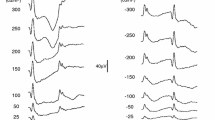
Full-field ERGs were recorded from 20 normally sighted subjects (aged 24–65 years) elicited by a long-wavelength pulse (3 cd s m−2) presented against a short-wavelength adapting field (12.5 cd m−2). Three to 10 waveforms were obtained from each subject, and each waveform was analyzed using standard time domain analyses of the PhNR, as well as a discrete wavelet transform (DWT) to extract time–frequency components that correspond to the PhNR. Three different measures of the PhNR were derived and compared: (1) amplitude at the PhNR trough; (2) amplitude at 72 ms following stimulus onset; (3) energy in the 11 Hz, 60–120 ms DWT frequency bin that corresponds to the PhNR. In addition, the effect of normalizing the PhNR by the b-wave was evaluated for each of the measures. Coefficients of variation (CVs) were computed for each definition to evaluate intra-subject variation.
Results
PhNR amplitudes measured at the trough and at 72 ms were significantly correlated (r = 0.88, p < 0.001). Additionally, PhNR energy derived by DWT was significantly correlated with the amplitude measured at the trough (r = 0.64, p = 0.002) and at 72 ms (r = 0.60, p = 0.005). Mean (±SD) intra-subject CVs were 26 % (15 %), 49 % (26 %), and 30 % (15 %), for measures at the trough, 72 ms, and DWT, respectively. Normalization by the b-wave amplitude (i.e., PhNR/b) had minimal effect on the intra-subject CVs, whereas normalization by the sum of the b-wave and PhNR amplitudes (i.e., PhNR/[b + PhNR]) substantially reduced the CVs for all three measures (mean CVs were less than 17 % for all conditions).
Conclusions
Although each PhNR definition has advantages and disadvantages, all three metrics provide similar estimates of the PhNR. Intra-subject CVs, however, were relatively high for measurements made at 72 ms, indicating that definitions based on a fixed time point may introduce variability. The substantial decrease in intra-subject variation after normalization by the sum of the PhNR and b-wave amplitudes may be advantageous under some conditions.




Similar content being viewed by others
References
Rangaswamy NV, Frishman LJ, Dorotheo EU, Schiffman JS, Bahrani HM, Tang RA (2004) Photopic ERGs in patients with optic neuropathies: comparison with primate ERGs after pharmacologic blockade of inner retina. Invest Ophthalmol Vis Sci 45(10):3827–3837
Viswanathan S, Frishman LJ, Robson JG, Harwerth RS, Smith EL 3rd (1999) The photopic negative response of the macaque electroretinogram: reduction by experimental glaucoma. Invest Ophthalmol Vis Sci 40(6):1124–1136
Viswanathan S, Frishman LJ, Robson JG, Walters JW (2001) The photopic negative response of the flash electroretinogram in primary open angle glaucoma. Invest Ophthalmol Vis Sci 42(2):514–522
Rangaswamy NV, Shirato S, Kaneko M, Digby BI, Robson JG, Frishman LJ (2007) Effects of spectral characteristics of ganzfeld stimuli on the photopic negative response (PhNR) of the ERG. Invest Ophthalmol Vis Sci 48(10):4818–4828
Colotto A, Falsini B, Salgarello T, Iarossi G, Galan ME, Scullica L (2000) Photopic negative response of the human ERG: losses associated with glaucomatous damage. Invest Ophthalmol Vis Sci 41(8):2205–2211
Machida S, Gotoh Y, Toba Y, Ohtaki A, Kaneko M, Kurosaka D (2008) Correlation between photopic negative response and retinal nerve fiber layer thickness and optic disc topography in glaucomatous eyes. Invest Ophthalmol Vis Sci 49(5):2201–2207
Preiser D, Lagreze WA, Bach M, Poloschek CM (2013) Photopic negative response versus pattern electroretinogram in early glaucoma. Invest Ophthalmol Vis Sci 54(2):1182–1191
Wilsey LJ, Fortune B (2016) Electroretinography in glaucoma diagnosis. Curr Opin Ophthalmol 27(2):118–124
Gotoh Y, Machida S, Tazawa Y (2004) Selective loss of the photopic negative response in patients with optic nerve atrophy. Arch Ophthalmol 122(3):341–346
Gowrisankaran S, Genead MA, Anastasakis A, Alexander KR (2013) Characteristics of late negative ERG responses elicited by sawtooth flicker. Doc Ophthalmol 126(1):9–19
Moss HE, Park JC, McAnany JJ (2015) The photopic negative response in idiopathic intracranial hypertension. Invest Ophthalmol Vis Sci 56(6):3709–3714
Abed E, Piccardi M, Rizzo D, Chiaretti A, Ambrosio L, Petroni S, Parrilla R, Dickmann A, Riccardi R, Falsini B (2015) Functional loss of the inner retina in childhood optic gliomas detected by photopic negative response. Invest Ophthalmol Vis Sci 56(4):2469–2474
Mortlock KE, Binns AM, Aldebasi YH, North RV (2010) Inter-subject, inter-ocular and inter-session repeatability of the photopic negative response of the electroretinogram recorded using DTL and skin electrodes. Doc Ophthalmol 121(2):123–134
Fortune B, Bui BV, Cull G, Wang L, Cioffi GA (2004) Inter-ocular and inter-session reliability of the electroretinogram photopic negative response (PhNR) in non-human primates. Exp Eye Res 78(1):83–93
Gauvin M, Lina JM, Lachapelle P (2014) Advance in ERG analysis: from peak time and amplitude to frequency, power, and energy. Biomed Res Int. doi:10.1155/2014/246096
Gauvin M, Little JM, Lina JM, Lachapelle P (2015) Functional decomposition of the human ERG based on the discrete wavelet transform. J Vis 15(16):14
Mallat SG (2009) A wavelet tour of signal processing the sparse way, 3rd edn. Academic Press, Houston
Acknowledgments
This research was supported by National Institutes of Health research Grants R01EY026004 (JM) and P30EY001792 (UIC core grant), an unrestricted departmental grant and a Dolly Green Special Scholar Award (JM) from Research to Prevent Blindness.
Funding
The National Institutes of Health and Research to Prevent Blindness provided financial support in the form of funding. The sponsors had no role in the design or conduct of this research.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest (such as honoraria; educational grants; participation in speakers’ bureaus; membership, employment, consultancies, stock ownership, or other equity interest; and expert testimony or patent-licensing arrangements), or non-financial interest (such as personal or professional relationships, affiliations, knowledge or beliefs) in the subject matter or materials discussed in this manuscript.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Electronic supplementary material
Below is the link to the electronic supplementary material.
10633_2016_9558_MOESM1_ESM.tif
Supplementary Figure 1: The DWT of the mean waveform is shown in the top panel (replotted from Fig. 1). The middle panel shows the effects on DWT energy of setting the mean response voltage to 0 μV after 60 ms; the PhNR component is absent. The bottom panel shows the effects on DWT energy of setting the mean response voltage to 0 μV from 0 ms to 60 ms; the PhNR component is present, but the early a-wave and b-wave components are strongly attenuated. (TIFF 922 kb)
Rights and permissions
About this article
Cite this article
Kundra, H., Park, J.C. & McAnany, J.J. Comparison of photopic negative response measurements in the time and time–frequency domains. Doc Ophthalmol 133, 91–98 (2016). https://doi.org/10.1007/s10633-016-9558-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10633-016-9558-6