Abstract
Purpose
To determine normative values, intra- and inter-session variability for a range of parameters derived from the photopic negative response (PhNR) using a handheld mini-Ganzfeld stimulator in healthy normal adults.
Methods
Light-adapted flash full-field electroretinograms (ERGs) were recorded from healthy individuals with no visual complaints, visual acuity equal to or better than 0.0 logMAR (20/20 Snellen), and negative family history for visual diseases. ERGs were recorded from both eyes using a DTL® type fiber electrode after dilation of the pupils with instillation of 1 drop of tropicamide eye drops (1%). The full-field PhNR stimulus conditions were produced by a LED-based ColorBurst™ (Diagnosys LLC, Lowell, MA, USA) handheld stimulator. Red flashes of 1, 5 and 7 cd.s/m2 on a blue background of 10 cd/m2 were presented. A-wave, b-wave and PhNR amplitude (determined by both baseline to trough-BT and peak to trough-PT) and peak times were analyzed. Normal limits were determined as 5% percentile for amplitudes and 95% percentile for latencies. Intra- and inter-session variability were assessed with Wilcoxon signed-rank test, intraclass correlation coefficient (ICC) and the coefficient of variability (COV).
Results
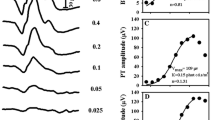
Normative limits for PhNR amplitude (µV) using 1, 5 and 7 cd.s./m2 stimuli were, respectively: 20.81; 18.06 and 19.60 for BT and 69.11; 77.98; 76.51 for PT. Peak times (ms) normative limits for 1, 5 and 7 cd.s/m2 intensities were, respectively, 65.98; 78.20 and 77.96. Overall, intra-session variability assessed by coefficients of variation ranged from 1.35 to 10.28%. Inter-session variability disclosed significant intraclass correlation values for all PhNR parameters only for 1 cd.s/m2 stimuli.
Conclusions
The normative values provided by this study are clinically helpful in the diagnosis of inner retinal disorders, especially those affecting retinal ganglion cells such as glaucoma and other optic neuropathies. Further studies, including a larger sample with variable age range would extend the validity of the current results.

Similar content being viewed by others
References
Binns AM, Mortlock KE, North RV (2011) The relationship between stimulus intensity and response amplitude for the photopic negative response of the flash electroretinogram. Doc Ophthalmol 122:39–52. https://doi.org/10.1007/s10633-010-9257-7
Robson AG, Nilsson J, Shying L, Jalali S, Fulton AB, Tormene AP, Holder GE, Brodie SF (2018) ISCEV guide to visual electrodiagnostic procedures. Doc Ophthalmol 136:1–26. https://doi.org/10.1007/s10633-017-9621-y
Granit R (1933) The components of the retinal action potential in mammals and their relation to the discharge in the optic nerve. J Physiol 77:207–239
Frishman LJ (2006) Origins of the electroretinogram. In: Heckenlively JR, Arden GB (eds) Principles and practice of clinical electrophysiology of vision. The MIT Press, Cambridge, pp 139–183
Penn RD, Hagins WA (1969) Signal transmission along retinal rods and the origin of the electroretinographic a-wave. Nature 223:201–205
Miller RF, Dowling JE (1970) Intracellular responses of Miiller (glial) cells of mudpuppy retina: their relation to the b-wave of the electroretinogram. J Neurophysiol 33:323–341
Newman EA, Odette LL (1984) Model of electroretinogram b-wave generation: a test of the K+ hypothesis. J Neurophysiol 51:164–182
Brown KT, Watanabe K (1962) Isolation and identification of a receptor potential from the pure cone fovea of the monkey retina. Nature 193:958–960
Bush RA, Sieving PA (1994) A proximal retinal component in the primate photopic ERG a-wave. Invest Ophthalmol Vis Sci 35:635–645
Sieving PA, Murayama K, Naarendorp F (1994) Push–pull model of the primate photopic electroretinogram: a role for hyperpolarizing neurons in shaping the b-wave. Vis Neurosci 11:519–532
Bach M, Brigell JG, Hawlina M, Holder GE, Johnson MA, McCulloch DL, Meigen T, Viswanathan S (2013) ISCEV standard for clinical pattern electroretinography (PERG): 2012 update. Doc Ophthalmol. https://doi.org/10.1007/s10633-012-9353-y
Viswanathan S, Frishman LJ, Robson JG, Harwerth RS, Smith EL III (1999) The photopic negative response of the macaque electroretinogram: reduction by experimental glaucoma. Invest Ophthalmol Vis Sci 40:1124–1136
Rangaswamy NV, Frishman LJ, Dorotheo EU, Schiffman JS, Bahrani HM, Tang RA (2004) Photopic ERGs in patients with optic neuropathies: comparison with primate ERGs after pharmacologic blockade of inner retina. Invest Ophthalmol Vis Sci 45:3827–3837. https://doi.org/10.1167/iovs.04-0458
Li B, Barnes G, Holt W (2005) The decline of the photopic negative response (PhNR) in the rat after optic nerve transection. Doc Ophthalmol 111:23–31. https://doi.org/10.1007/s10633-005-2629-8
Machida S, Raz-Prag D, Fariss RN, Sieving PA, Bush RA (2008) Photopic ERG negative response from amacrine cell signaling in RCS rat retinal degeneration. Invest Ophthalmol Vis Sci 49:442–452. https://doi.org/10.1167/iovs.07-0291
McCulloch DM, Marmor MF, Brigell MG, Hamilton R, Holder GE, Tzekov R, Bach M (2015) ISCEV Standard for full-field clinical electroretinography (2015 update). Doc Ophthalmol 130:1–12. https://doi.org/10.1007/s10633-014-9473-7
Karanjia R, Berezovsky A, Sacai PY, Cavascan NN, Liu HY, Nazarali S, Moraes-Filho MN, Anderson K, Tran JS, Watanabe SE, Moraes MN, Sadun F, DeNegri AM, Barboni P, do Val Ferreira Ramos C, La Morgia C, Carelli V, Belfort R Jr, Coupland SG, Salomao SR, Sadun AA (2017) The photopic negative response: an objective measure of retinal ganglion cell function in patients with Leber’s hereditary optic neuropathy. Invest Ophthalmol Vis Sci 58:8527–8533. https://doi.org/10.1167/iovs.17-21773
Tang J, Hui F, Hadoux X, Sarossy M, van Wijngaarden P, Coote M, Crowston JGA (2018) Comparison of the RETeval sensor strip and DTL electrode for recording the photopic negative response. Transl Vis Sci Technol 7(6):27. https://doi.org/10.1167/tvst.7.6.27
Fortune B, Bui BV, Cull G, Wang L, Cioffi GA (2004) Inter-ocular and inter-session reliability of the electroretinogram photopic negative response (PhNR) in non-human primates. Exp Eye Res 78:83–93. https://doi.org/10.1016/j.exer.2003.09.013
Mortlock KE, Binns AM, Aldebasi YH, North RV (2010) Inter-subject, inter-ocular and inter-session repeatability of the photopic negative response of the electroretinogram recorded using DTL and skin electrodes. Doc Ophthalmol 121(2):123–134. https://doi.org/10.1007/s10633-010-9239-9
Tang J, Edwards T, Crowston JG, Sarossy M (2014) The test–retest reliability of the photopic negative response (PhNR). Transl Vis Sci Technol 3(6):1. https://doi.org/10.1167/tvst.3.6.1
Wu Z, Hadoux X, Hui F, Sarossy MG, Crowston JG (2016) Photopic negative response obtained using a handheld electroretinogram device: determining the optimal measure and repeatability. Trans Vis Sci Tech 5(4):8. https://doi.org/10.1167/tvst.5.4.8
Joshi NB, Ly E, Viswanathan S (2017) Intensity response function of the photopic negative response (PhNR): effect of age and test–retest reliability. Doc Ophthalmol 135:1–16. https://doi.org/10.1007/s10633-017-9591-0
Colotto A, Falsini B, Salgarello T, Iarossi G, Galan ME, Scullica L (2000) Photopic negative response of the human ERG: losses associated with glaucomatous damage. Invest Ophthalmol Vis Sci 41:2205–2211
Viswanathan S, Frishman LJ, Robson JG, Walters JW (2001) The photopic negative response of the flash electroretinogram in primary open angle glaucoma. Invest Ophthalmol Vis Sci 42:514–522
Machida S, Gotoh Y, Toba Y, Ohtaki A, Kaneko M, Kurosaka D (2008) Correlation between photopic negative response and retinal nerve fiber layer thickness and optic disc topography in glaucomatous eyes. Invest Ophthalmol Vis Sci 49:2201–2207. https://doi.org/10.1167/iovs.07-0887
Machida S, Tamada K, Oikawa T, Yokoyama D, Kaneko M, Kurosaka D (2010) Sensitivity and specificity of photopic negative response of focal electroretinogram to detect glaucomatous eyes. Br J Ophthalmol 94:202–208. https://doi.org/10.1136/bjo.2009.161166
North RV, Jones AL, Drasdo N, Wild JM, Morgan JE (2010) Electrophysiological evidence of early functional damage in glaucoma and ocular hypertension. Invest Ophthalmol Vis Sci 51:1216–1222. https://doi.org/10.1167/iovs.09-3409
Nakamura H, Hangai M, Mori S, Hirose F, Yoshimura N (2011) Hemispherical focal macular photopic negative response and macular inner retinal thickness in open-angle glaucoma. Am J Ophthalmol 151(494–506):e1. https://doi.org/10.1016/j.ajo.2010.09.018
Preiser D, Lagreze WA, Bach M, Poloschek CM (2013) Photopic negative response versus pattern electroretinogram in early glaucoma. Invest Ophthalmol Vis Sci 54:1182–1191. https://doi.org/10.1167/iovs.12-11201
Machida S, Kaneko M, Kurosaka D (2014) Regional variations in correlation between photopic negative response of focal electoretinograms and ganglion cell complex in glaucoma. Curr Eye Res 40:439–449. https://doi.org/10.3109/02713683.2014.922196
Cvenkel B, Sustar M, Perovsek D (2017) Ganglion cell loss in early glaucoma, as assessed by photopic negative response, pattern electroretinogram, and spectral domain optical coherence tomography. Doc Ophthalmol 135:17–28. https://doi.org/10.1007/s10633-017-9595-9
Banerjee A, Khurana M, Sachidanandam R, Sen P (2019) Comparison between broadband and monochromatic photopic negative response in full-field electroretinogram in controls and subjects with primary open-angle glaucoma. Doc Ophthalmol 138:21–33
Hara Y, Machida S, Ebihara S, Ishizuka M, Tada A, Nishimura T (2020) Comparisons of photopic negative responses elicited by different conditions from glaucomatous eyes. Jpn J Ophthalmol. https://doi.org/10.1007/s10384-019-00711-5
Machida S, Gotoh Y, Tanaka M, Tazawa Y (2004) Predominant loss of the photopic negative response in central retinal artery occlusion. Am J Ophthalmol 137(5):938–940. https://doi.org/10.1016/j.ajo.2003.10.023
Chen H, Wu D, Huang S, Yan H (2006) The photopic negative response of the flash electroretinogram in retinal vein occlusion. Doc Ophthalmol 113:53–59. https://doi.org/10.1007/s10633-006-9015-z
Park JC, Chau FY, Lim JI, McAnany J (2019) Electrophysiological and pupillometric measures of inner retina function in nonproliferative diabetic retinopathy. Doc Ophthalmol 139:99–111. https://doi.org/10.1007/s10633-019-09699-2
Ortiz G, Drucker D, Hyde C, Staffetti J, Kremers J, Tzekov R (2019) The photopic negative response of the Light-adapted 3.0 ERG in clinical settings. Doc Ophthalmol. https://doi.org/10.1007/s10633-019-09723-5
Gotoh Y, Machida S, Tazawa Y (2004) Selective loss of the photopic negative response in patients with optic nerve atrophy. Arch Ophthalmol 122(3):341–346. https://doi.org/10.1001/archopht.122.3.341
Miyata K, Nakamura M, Kondo M, Lin J, Ueno S, Miyake Y, Terasaki H (2007) Reduction of oscillatory potentials and photopic negative response in patients with autosomal dominant optic atrophy with OPA1 mutations. Invest Ophthalmol Vis Sci 48(2):820–824. https://doi.org/10.1167/iovs.06-0845
Moon CH, Hwang SC, Kim BT, Ohn YH, Park TK (2011) Visual prognostic value of optical coherence tomography and photopic negative response in chiasmal compression. Invest Ophthalmol Vis Sci 52:8527–8533. https://doi.org/10.1167/iovs.11-8034
Wang J, Cheng H, Hu YS, Tang RA, Frishman LJ (2012) The photopic negative response of the flash electroretinogram in multiple sclerosis. Invest Ophthalmol Vis Sci 53:1315–1323. https://doi.org/10.1167/iovs.11-8461
Abed E, Piccardi M, Rizzo D, Chiarett A, Ambrosio L, Petroni S, Parrilla R, Dickmann A, Riccardi R, Falsini B (2015) Functional loss of the inner retina in childhood optic gliomas detected by photopic negative response. Invest Ophthalmol Vis Sci 56:2469–2474. https://doi.org/10.1167/iovs.14-16235
Abed E, Placidi G, Campagna F, Federici M, Minnella A, Guerri G, Bertelli M, Piccardi M, Galli-Resta L, Falsini B (2018) Early impairment of the full-field photopic negative response in patients with Stargardt disease and pathogenic variants of the ABCA4 gene. Clin Exp Ophthalmol 46:519–530. https://doi.org/10.1111/ceo.13115
Akiyama G, Matsumoto CS, Shinoda K, Terauchi G, Matsumoto H, Watanabe E, Iwata T, Mizota A, Miyake Y (2016) Intraoperative electrophysiological evaluations of macular function during peripheral scleral indentation. Sci Rep 6:35164. https://doi.org/10.1038/srep35164
Ueno S, Kondo M, Piao CH, Ikenoya K, Miyake Y, Terasaki H (2006) Selective amplitude reduction of the PhNR after macular hole surgery: ganglion cell damage related to ICG-assisted ILM peeling and gas tamponade. Invest Ophthalmol Vis Sci 47:3545–3549. https://doi.org/10.1167/iovs.05-1481
Frishman L, Sustar M, Kremers J, McAnany JJ, Sarossy M, Tzekov R, Viswanathan S (2018) ISCEV extended protocol for the photopic negative response (PhNR) of the full-field electroretinogram. Doc Ophthalmol 136:207–211. https://doi.org/10.1007/s10633-018-9638-x
Alstine AWV, Viswanathan S (2017) Test–retest reliability of the multifocal photopic negative response. Doc Ophthalmol 134:25–36. https://doi.org/10.1007/s10633-016-9569-3
Stokes M (1985) Reliability and repeatability of methods for measuring muscle in physiotherapy. Physioth Pract 1:71–76
Atkinson G, Nevill AM (1998) Statistical methods for assessing measurement error (reliability) in variables relevant to sports medicine. Sports Med 26:217–238
Wali N, Leguire LE (1991) Dark-adapted luminance-response functions with skin and corneal electrodes. Doc Ophthalmol 76:367–375
Westall CA, Dhaliwal HS, Panton CM, Sigesmun D, Levin AV, Nischal KK, Heon E (2001) Values of electroretinogram responses according to axial length. Doc Ophthalmol 102:115–130
Hebert M, Vaegan Lachapelle P (1999) Reproducibility of ERG responses obtained with the DTL electrode. Vis Res 39:1069–1070. https://doi.org/10.1016/S0042-6989(98)00210-7
Kremers J, Jertila M, Link B, Pangeni G, Horn FK (2012) Spectral characteristics of the PhNR in the full-field flash electroretinogram of normals and glaucoma patients. Doc Ophthalmol 124(2):79–90. https://doi.org/10.1007/s10633-011-9304-z
Funding
Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP), research grant #2018/05869-9 to AB; Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES, Brasília, Brasil) Finance Code 001 to GISB; International Foundation for Optic Nerve Disease (IFOND) to SRS.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
No Conflicts of Interest: Adriana Berezovsky, Rustum Karanjia, Arthur Gustavo Fernandes, Gabriel Izan Santos Botelho, Tatiane Luana Novele Bueno, Nívea Nunes Ferraz, Paula Yuri Sacai, Alfredo Arrigo Sadun and Solange Rios Salomão certify that they have no affiliations with or involvement in any organization or entity with any financial interest (such as honoraria; educational grants; participation in speakers’ bureaus; membership, employment, consultancies, stock ownership, or other equity interest; and expert testimony or patent-licensing arrangements), or non-financial interest (such as personal or professional relationships, affiliations, knowledge or beliefs) in the subject matter or materials discussed in this manuscript. Potential Conflict of Interest: Stuart G. Coupland functions as a paid consultant to Diagnosys LLC, the equipment manufacturer.
Statement of human rights
All procedures performed in studies involving human participants were in accordance with the ethical standards of the Federal University of São Paulo ethics committee on research and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Statement on the welfare of animals
No animals were used in this study.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Berezovsky, A., Karanjia, R., Fernandes, A.G. et al. Photopic negative response using a handheld mini-ganzfeld stimulator in healthy adults: normative values, intra- and inter-session variability. Doc Ophthalmol 142, 153–163 (2021). https://doi.org/10.1007/s10633-020-09784-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10633-020-09784-x