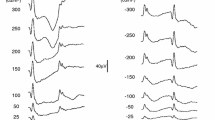
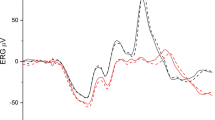
Abstract
Purpose
To investigate how light stimulus conditions of varying spatial sizes affect components of the flash and long-flash electroretinogram (ERG) in normal subjects.
Method
Three stimulus conditions were generated by a Ganzfeld stimulator: a white flash on white background (WoW), a red flash on a blue background (RoB) and an L+M-cone isolating on–off (long flash) stimulus (Cone Iso). ERGs were recorded from six subjects (5 M, 1 F) with DTL electrodes to full-field (FF), 70°, 60°, 50°, 40°, 30° and 20° diameter circular stimuli. Amplitudes and peak times for a-, b-, d- and i-wave, and PhNR were examined. PhNR amplitudes were estimated in two different ways: from baseline (fB) and from preceding b-wave peak (fP).
Results
With decreasing stimulus size, amplitudes for all ERG waveform components attenuated and peak times increased, although the effect varied across different components. An exponential fit described the relationship between amplitudes and size of stimulated retinal area well for most components and conditions (R2= 0.75-0.99), except for PhNR(fB) (R2= − 0.16–0.88). For peak times, an exponential decay function also fitted the data well (R2= 0.81–0.97), except in a few cases where the exponential constant was too small and a linear regression function was applied instead (a-wave Cone Iso, b- and i-wave WoW). The exponential constants for RoB amplitudes (b-wave, PhNR(fB), PhNR(fP)) were larger compared to their counterparts under WoW (p < 0.05), while there was no difference between the constants for a-wave amplitudes and peak times and for PhNR peak times. The exponential constants of amplitudes vs. area under WoW and Cone Iso were remarkably similar, while under RoB PhNR(fB) showed larger constants compared to either a- or b-wave (p < 0.05).
Conclusion
ERG components change in a predictable way with stimulus size and spectral characteristics of the stimulus under these conditions. This predictability could allow a modified version of these sets of stimuli to be tested for clinical applicability.









Similar content being viewed by others
References
Robson JG, Saszik SM, Ahmed J et al (2003) Rod and cone contributions to the a-wave of the electroretinogram of the macaque. J Physiol 547:509–530. https://doi.org/10.1113/jphysiol.2002.030304
Bush RA, Sieving PA (1994) A proximal retinal component in the primate photopic ERG a-wave. Invest Ophthalmol Vis Sci 35:635–645
Ueno S, Kondo M, Niwa Y et al (2004) Luminance dependence of neural components that underlies the primate photopic electroretinogram. Invest Ophthalmol Vis Sci 45:1033–1040. https://doi.org/10.1167/iovs.03-0657
Dong CJ, Hare WA (2000) Contribution to the kinetics and amplitude of the electroretinogram b-wave by third-order retinal neurons in the rabbit retina. Vision Res 40:579–589. https://doi.org/10.1016/s0042-6989(99)00203-5
Rufiange M, Rousseau S, Dembinska O et al (2002) Cone-dominated ERG luminance-response function: the Photopic Hill revisited. Documenta Ophthalmol Adv Ophthalmol 104:231–248
Rosolen SG, Rigaudière F, LeGargasson J-F et al (2004) Comparing the photopic ERG i-wave in different species. Veterinary Ophthalmol 7:189–192. https://doi.org/10.1111/j.1463-5224.2004.04022.x
Rangaswamy NV, Frishman LJ, Dorotheo EU et al (2004) Photopic ERGs in patients with optic neuropathies: comparison with primate ERGs after pharmacologic blockade of inner retina. Invest Ophthalmol Vis Sci 45:3827–3837. https://doi.org/10.1167/iovs.04-0458
Viswanathan S, Frishman LJ, Robson JG et al (2001) The photopic negative response of the flash electroretinogram in primary open angle glaucoma. Invest Ophthalmol Vis Sci 42:514–522
Colotto A, Falsini B, Salgarello T et al (2000) Photopic negative response of the human ERG: losses associated with glaucomatous damage. Invest Ophthalmol Vis Sci 41:2205–2211
Gotoh Y, Machida S, Tazawa Y (2004) Selective loss of the photopic negative response in patients with opticnerve atrophy. Arch Ophthalmol 122:341–346. https://doi.org/10.1001/archopht.122.3.341
McCulloch DL, Marmor MF, Brigell MG et al (2015) ISCEV standard for full-field clinical electroretinography (2015 update). Doc Ophthalmol 130:1–12. https://doi.org/10.1007/s10633-014-9473-7
Rangaswamy NV, Shirato S, Kaneko M et al (2007) Effects of spectral characteristics of ganzfeld stimuli on the photopic negative response (PhNR) of the ERG. Invest Ophthalmol Vis Sci 48:4818–4828. https://doi.org/10.1167/iovs.07-0218
Frishman L, Sustar M, Kremers J et al (2018) ISCEV extended protocol for the photopic negative response (PhNR) of the full-field electroretinogram. Doc Ophthalmol 136:207–211. https://doi.org/10.1007/s10633-018-9638-x
Kremers J, Jertila M, Link B et al (2012) Spectral characteristics of the PhNR in the full-field flash electroretinogram of normals and glaucoma patients. Doc Ophthalmol 124:79–90. https://doi.org/10.1007/s10633-011-9304-z
Tsai TI, Jacob MM, McKeefry D et al (2016) Spatial properties of L- and M-cone driven incremental (On-) and decremental (Off-) electroretinograms: evidence for the involvement of multiple post-receptoral mechanisms. J Opt Soc Am A Opt Image Sci Vis 33:A1–A11. https://doi.org/10.1364/josaa.33.0000a1
de Graaf B, Wertheim AH (1988) The perception of object motion during smooth pursuit eye movements: adjacency is not a factor contributing to the Filehne illusion. Vision Res 28:497–502. https://doi.org/10.1016/0042-6989(88)90172-1
Miyake Y (1998) Focal macular electroretinography. Nagoya J Med Sci 61:79–84
Machida S, Toba Y, Ohtaki A et al (2008) Photopic negative response of focal electoretinograms in glaucomatous eyes. Invest Ophthalmol Vis Sci 49:5636–5644. https://doi.org/10.1167/iovs.08-1946
Machida S, Tamada K, Oikawa T et al (2010) Sensitivity and specificity of photopic negative response of focal electoretinogram to detect glaucomatous eyes. Br J Ophthalmol 94:202–208. https://doi.org/10.1136/bjo.2009.161166
Kinoshita J, Takada S, Iwata N et al (2016) Comparison of photopic negative response (PhNR) between focal macular and full-field electroretinograms in monkeys. Documenta Ophthalmologica. Adv Ophthalmol 132:177–187. https://doi.org/10.1007/s10633-016-9538-x
Martin PR, Grünert U (1992) Spatial density and immunoreactivity of bipolar cells in the macaque monkey retina. J Compar Neurol 323:269–287. https://doi.org/10.1002/cne.903230210
Dawson WW, Trick GL, Litzkow CA (1979) Improved electrode for electroretinography. Invest Ophthalmol Vis Sci 18:988–991
Kremers J (2003) The assessment of L- and M-cone specific electroretinographical signals in the normal and abnormal human retina. Prog Retinal Eye Res 22:579–605
Estévez O, Spekreijse H (1982) The “silent substitution” method in visual research. Vision Res 22:681–691. https://doi.org/10.1016/0042-6989(82)90104-3
Donner KO, Rushton WA (1959) Retinal stimulation by light substitution. J Physiol 149:288–302. https://doi.org/10.1113/jphysiol.1959.sp006340
Shapiro AG, Pokorny J, Smith VC (1996) Cone-rod receptor spaces with illustrations that use CRT phosphor and light-emitting-diode spectra. J Opt Soc Am A Opt Image Sci Vision 13:2319–2328
Aher AJ, McKeefry DJ, Parry NRA et al (2018) Rod- versus cone-driven ERGs at different stimulus sizes in normal subjects and retinitis pigmentosa patients. Documenta Ophthalmologica. Adv Ophthalmol 136:27–43. https://doi.org/10.1007/s10633-017-9619-5
Meigen T, Bach M (1999) On the statistical significance of electrophysiological steady-state responses. Documenta Ophthalmologica. Adv Ophthalmol 98:207–232
Ortiz G, Drucker D, Hyde C et al (2020) The photopic negative response of the Light-adapted 3.0 ERG in clinical settings. Doc Ophthalmol 140:115–128. https://doi.org/10.1007/s10633-019-09723-5
Nagra M, Gilmartin B, Thai NJ et al (2017) Determination of retinal surface area. J Anat 231:319–324. https://doi.org/10.1111/joa.12641
Drasdo N, Fowler CW (1974) Non-linear projection of the retinal image in a wide-angle schematic eye. Br J Ophthalmol 58:709–714. https://doi.org/10.1136/bjo.58.8.709
Dacey DM (1993) The mosaic of midget ganglion cells in the human retina. J Neurosci 13:5334–5355
Hoffmann EM, Zangwill LM, Crowston JG et al (2007) Optic disk size and glaucoma. Survey Ophthalmol 52:32–49. https://doi.org/10.1016/j.survophthal.2006
Victor JD, Shapley RM (1979) The nonlinear pathway of Y ganglion cells in the cat retina. J Gen Physiol 74:671–689. https://doi.org/10.1085/jgp.74.6.671
Yeh T, Lee BB, Kremers J (1995) Temporal response of ganglion cells of the macaque retina to cone-specific modulation. J Opt Soc Am A Opt Image Sci Vis 12:456–464. https://doi.org/10.1364/josaa.12.000456
Curcio CA, Allen KA (1990) Topography of ganglion cells in human retina. J Comp Neurol 300:5–25. https://doi.org/10.1002/cne.903000103
Curcio CA, Millican CL, Allen KA et al (1993) Aging of the human photoreceptor mosaic: evidence for selective vulnerability of rods in central retina. Invest Ophthalmol Vis Sci 34:3278–3296
Jacob MM, Pangeni G, Gomes BD et al (2015) The spatial properties of L- and M-cone inputs to electroretinograms that reflect different types of post-receptoral processing. PLoS ONE 10:e0121218. https://doi.org/10.1371/journal.pone.0121218
Murray IJ, Parry NR, Kremers J et al (2004) Photoreceptor topography and cone-specific electroretinograms. Vis Neurosci 21:231–235. https://doi.org/10.1017/s0952523804213268
Rodrigues AR, FilhoMda S, Silveira LC et al (2010) Spatial distributions of on- and off-responses determined with the multifocal ERG. Doc Ophthalmol 120:145–158. https://doi.org/10.1007/s10633-009-9205-6
Martins CMG, Tsai T, Barboni MTS et al (2016) The influence of stimulus size on heterochromatic modulation electroretinograms. J Vision 16:13. https://doi.org/10.1167/16.8.13
Masri R (2019) Neurons of the primate retina: a qualitative and quantitative analysis, in save sight institute and discipline of ophthalmology. University of Sydney, Sydney
Grunert U, Martin PR (2020) Cell types and cell circuits in human and non-human primate retina. Prog Retin Eye Res. https://doi.org/10.1016/j.preteyeres.2020.100844
Porciatti V (2015) Electrophysiological assessment of retinal ganglion cell function. Exp Eye Res 141:164–170. https://doi.org/10.1016/j.exer.2015.05.008
Shapley RM, Victor JD (1978) The effect of contrast on the transfer properties of cat retinal ganglion cells. J Physiol 285:275–298. https://doi.org/10.1113/jphysiol.1978.sp012571
Lee BB, Wehrhahn C, Westheimer G et al (1995) The spatial precision of macaque ganglion cell responses in relation to vernier acuity of human observers. Vision Res 35:2743–2758. https://doi.org/10.1016/0042-6989(95)00015-r
Viswanathan S, Frishman LJ, Robson JG et al (1999) The photopic negative response of the macaque electroretinogram: reduction by experimental glaucoma. Invest Ophthalmol Vis Sci 40:1124–1136
Morny EKA, Patel K, Votruba M et al (2019) The relationship between the photopic negative response and retinal ganglion cell topography. Invest Ophthalmol Vis Sci 60:1879–1887. https://doi.org/10.1167/iovs.18-25272
Kondo M, Piao CH, Tanikawa A et al (2000) Amplitude decrease of photopic ERG b-wave at higher stimulus intensities in humans. Jpn J Ophthalmol 44:20–28. https://doi.org/10.1016/s0021-5155(99)00172-0
Sustar M, Hawlina M, Brecelj J (2006) ON- and OFF-response of the photopic electroretinogram in relation to stimulus characteristics. Documenta Ophthalmologica. Adv Ophthalmol 113:43–52. https://doi.org/10.1007/s10633-006-9013-1
Kondo M, Kurimoto Y, Sakai T et al (2008) Recording focal macular photopic negative response (PhNR) from monkeys. Invest Ophthalmol Vis Sci 49:3544–3550. https://doi.org/10.1167/iovs.08-1798
Kondo M, Miyake Y, Horiguchi M et al (1998) Recording multifocal electroretinogram on and off responses in humans. Invest Ophthalmol Vis Sci 39:574–580
Hood DC, Bach M, Brigell M et al (2012) ISCEV standard for clinical multifocal electroretinography (mfERG) (2011 edition). Doc Ophthalmol 124:1–13. https://doi.org/10.1007/s10633-011-9296-8
Hood DC, Seiple W, Holopigian K et al (1997) A comparison of the components of the multifocal and full-field ERGs. Vis Neurosci 14:533–544. https://doi.org/10.1017/s0952523800012190
Acknowledgements
We would like to thank Brian Vohnsen for careful reading and comments on the manuscript.
Funding
M.N. was supported by the Research, Innovation, and Scholarly Endeavors Program’s Scholarly Student Excellent Award, at the University of South Florida Morsani College of Medicine. J.K. and A.A. are supported by the German Research Council (DFG; Grant#: KR1317/13-2). The sponsors had no role in the design or conduct of this research.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest (such as honoraria; educational grants; participation in speakers’ bureaus; membership, employment, consultancies, stock ownership, or other equity interest; and expert testimony or patent-licensing arrangements), or non-financial interest (such as personal or professional relationships, affiliations, knowledge or beliefs) in the subject matter or materials discussed in this manuscript.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the University Hospital Erlangen and University of South Florida and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Nittmann, M.G., Aher, A.J., Kremers, J. et al. Relationship between stimulus size and different components of the electroretinogram (ERG) elicited by flashed stimuli. Doc Ophthalmol 142, 213–231 (2021). https://doi.org/10.1007/s10633-020-09797-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10633-020-09797-6