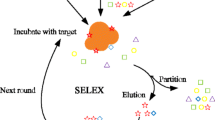
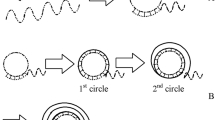
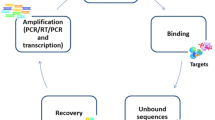
Abstract
Aptamers are short DNA and RNA or protein sequence and have been extensively researched for their application in different field such as therapeutic agents, as delivery vehicles, as analytical tool, and as recognition molecule for sensor and different assay. To better understand the potential of aptamer in food safety and agriculture sector, an overview of the progress in the generation and application in these sectors is discussed in this review. Special attention is paid to the researches which are relatively close to the application for contaminants detection in food material.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Barthelmebs L, Jonca J, Hayat A, Prieto-Simon B, Marty J-L (2011) Enzyme-linked aptamer assays (ELAAs), based on a competition format for a rapid and sensitive detection of ochratoxin a in wine. Food Control 22(5):737–743
Beier R, Pahlke C, Quenzel P, Henseleit A, Boschke E, Cuniberti G, Labudde D (2014) FEMS Microbiology Letters banner. https://doi.org/10.1111/1574-6968.12366
Bell SD, Denu JM, Dixon JE, Ellingtoni AD (1998) RNA molecules that bind to and inhibit the active site of a tyrosine phosphatase. J Biol Chem 273(23):14309–14314
Bourke ATC, Hawes RB (1983) Freshwater cyanobacteria (blue-green algae) and human health. Med J Aust 1(11):491–492
Bruno JG, Carrillo MP, Phillips T, Vail NK, Hanson D (2009) Competitive FRET-aptamer-based detection of methylphosphonic acid: a common nerve agent metabolite. J Fluoresc 18:867–876. https://doi.org/10.1007/s10895-008-0316-3
Bruno JG, Carrillo MP, Phillips T, Hanson D, Bohmann JA (2010) DNA aptamer beacon assay for C-telopeptide and handheld fluorometer to monitor bone resorption. J Fluoresc 21:2021–2033. https://doi.org/10.1007/s10895-011-0903-6
Bruno JG, Richarte AM, Carrillo MP, Edge A (2012) An aptamer beacon responsive to botulinum toxins. Biosens Bioelectron 31(1):240–243
Cao X, Li S, Chen L, Ding H, Xu H, Huang Y, Li J, Liu N, Cao W, Zhu Y, Shen B, Shao N (2009) Combining use of a panel of ssDNA aptamers in the detection of Staphylococcus aureus. Nucleic Acids Res 37(14):4621–4628
Cao F, Lu X, Hu X, Zhang Y, Zeng L, Chen L, Sun M (2016) In vitro selection of DNA aptamers binding pesticide fluoroacetamide. Biosci Biotechnol Biochem 80(5):823–832
Chang GJ, Kuno G, Purdy DE, Davis BS (2004) Recent advancement in flavivirus vaccine development. Expet Rev Vaccine 3:199–220. https://doi.org/10.1586/14760584.3.2.199
Chen A, Yang S (2015) Replacing antibodies with aptamers in lateral flow immunoassay. Biosens Bioelectron 15(71):230–242
Chen F, Zhou J, Luo F, Mohammed AB, Zhang XL (2007) Aptamer from whole-bacterium SELEX as new therapeutic reagent against virulent Mycobacterium tuberculosis. Biochem Biophys Res Commun 357:743–748
Chen Z, Li L, Mu X, Zhao H, Guo L (2012) Electrochemical aptasensor for detection of copper based on a reagentless signal-on architecture and amplification by gold nanoparticles. Talanta 85:730–735
Chen X, Huang Y, Duan N et al (2013) Selection and identification of ssDNA aptamers recognizing zearalenone. Anal Bioanal Chem 405(20):6573–6581
Chen X, Huang Y, Duan N et al (2014) Screening and identification of DNA aptamers against T-2 toxin assisted by graphene oxide. J Agric Food Chem 62(42):10368–10374
Chu S (1991) Laser manipulation of atoms and particles. Science 253(5022):861–866
Cruz-Aguado JA, Penner G (2008a) Determination of ochratoxin a with a DNA aptamer. J Agric Food Chem 56(22):10456–10461
Cruz-Aguado JA, Penner G (2008b) Fluorescence polarization based displacement assay for the determination of small molecules with aptamers. Anal Chem 80(22):8853–8855
Danesh NM, Ramezani M, Sarreshtehdar EA, Abnous K, Taghdisi SM (2016) A novel electrochemical aptasensor based on arch-shape structure of aptamer-complimentary strand conjugate and exonuclease I for sensitive detection of streptomycin. Biosens Bioelectron 15(75):123–128
Dapra J, Lauridsen LH, Nielsen AT, Rozlosnik N (2013) Comparative study on aptamers as recognition elements for antibiotics in a label-free all-polymer biosensor. Biosens Bioelectron 43:315–320
Davydova A, Vorobjeva M, Pyshnyi D, Altman S, Vlassov V, Venyaminova A (2016) Aptamers against pathogenic microorganisms. Crit Rev Microbiol 42(6):847–865
Deeds JR, Landsberg JH, Etheridge SM, Pitcher GC, Longan SW (2008) Non-traditional vectors for paralytic shellfish poisoning. Mar Drugs 6(2):308–348
DeGrasse JA (2012) A single-stranded DNA aptamer that selectively binds to Staphylococcus aureus enterotoxin B. PLoS One 7:e33410
DeRosa MR, Monreal C, Schnitzer M, W alsh R, Sultan Y. (2010) Nanotechnology in fertilizers. Nat Nanotechnol J 5:91
Dhaked RK, Singh MK, Singh P, Gupta P (2010) Botulinum toxin: bioweapon & magic drug. Indian J Med Res 132(11):489–503
Dreisig K, Taxvig C, Birkhøj Kjærstad M, Nellemann C, Hass U, Vinggaard AM (2013) Predictive value of cell assays for developmental toxicity and embryotoxicity. ALTEX:319–330
Duan N, Wu SJ, Chen XJ, Huang YK, Wang ZP (2012) J Agric Food Chem 60:4034–4038
Duan N, Ding XY, He LX, Wu SJ, Wei YX, Wang ZP (2013a) Food Control 33:239–243
Duan N, Wu SJ, Chen XJ, Huang YK, Xia Y, Ma XY, Wang ZP (2013b) J Agric Food Chem 61:3229–3234
Dwivedi HP, Smiley RD, Jaykus LA (2013) Selection of DNA aptamers for capture and detection of Salmonella typhimurium using a whole-cell SELEX approach in conjunction with cell sorting. Appl Microbiol Biotechnol 97:3677–3686
Eissa S, Ng A, Siaj M, Tavares AC, Zourob M (2013) Selection and identification of DNA aptamers against okadaic acid for biosensing application. Anal Chem 85(24):11794–11801
Elshafey R, Siaj M, Zourob M (2014) In vitro selection, characterization, and biosensing application of high-affinity cylindrospermopsin-targeting aptamers. Anal Chem 86(18):9196–9203
Ellington AD, Szostak JW (1990) In vitro selection of RNA molecules that bind specific ligands. Nature 346:818–822
Escudero-Abarca BI, Suh SH, Moore MD, Dwivedi HP, Jaykus L-A (2014) Selection, Characterization and Application of Nucleic Acid Aptamers for the Capture and Detection of Human Norovirus Strains. PLoS One. https://doi.org/10.1371/journal.pone.0106805
Fang Z, Wu W, X Lu, Zeng L (2014) Lateral flow biosensor for DNA extraction-free detection of Salmonella based on aptamer mediated strand displacement amplification. Biosens Bioelectron 56:192–197
Giamberardino A, Labib M, Hassan EM, Tetro J, Springthorpe S et al (2013) Ultrasensitive norovirus detection using DNA aptasensor technology. PLoS One 8:e79087
González-Fernández E, De-los-Santos-Álvarez N, Lobo-Castañón MJ, Miranda-Ordieres AJ, Tuñón-Blanco P (2011) Aptamer-based inhibition assay for the electrochemical detection of tobramycin using magnetic microparticles. Electroanalysis 23:43–49. https://doi.org/10.1002/elan.20100056
Gua W, Sun N, Qin X, Pei M, Wang L (2015) A novel electrochemical aptasensor for ultrasensitive detection of kanamycin based on MWCNTs–HMIMPF6 and nanoporous PtTi alloy. Biosens Bioelectron 74:691–697
Handy SM, Yakes BJ, DeGrasse JA et al (2013) First report of the use of a saxitoxin-protein conjugate to develop a DNA aptamer to a small molecule toxin. Toxicon 61(1):30–37
He J, Liu Y, Fan M, Liu X (2011) Isolation and identification of the DNA aptamer target to acetamiprid. J Agric Food Chem 59:1582–1586. https://doi.org/10.1021/jf104189g
Hu XG, Tulsieram KL, Zhou QX, Mu L, Wen JP (2012) Polymeric nanoparticle-aptamer bioconjugates can diminish the toxicity of mercury in vivo. Toxicol Lett 208(1):69–74
Ikanovic M, Rudzinski WE, Bruno JG, Allman A, Carrillo MP, Dwaraknath S, Bhahdigadi S, Rao P, Kiel JL, Andrews CJ (2007) Fluorescence assay based on aptamer-quantum dot binding to Bacillus thruingiensis spores. J Fluoresc 17(2):193–199
Jahanbani S, Benvidi A (2016) Comparison of two fabricated aptasensors based on modified carbon paste/oleic acid and magnetic bar carbon paste/Fe3O4@oleic acid nanoparticle electrodes for tetracycline detection. Biosens Bioelectron 15(85):553–562
Jones RM, Nicas M, Hubbard A, Sylvester MD, Reingold A (2005) The infectious dose of Francisella Tularensis (Tularemia). Appl Biosafety. https://doi.org/10.1177/153567600501000405
Joshi R, Janagama H, Dwivedi HP et al (2009) Selection, characterization, and application of DNA aptamers for the capture and detection of Salmonella enterica serovars. Mol Cell Probes 23:20–28
Jyoti A, Vajpayee P, Singh G et al (2011) Identification of environmental reservoirs of nontyphoidal salmonellosis: aptamer-assisted bioconcentration and subsequent detection of Salmonella typhimurium by quantitative polymerase chain reaction. Environ Sci Technol 45:8996–9002
Keefe AD, Pai S, Ellington A (2010) Aptamers as therapeutics. Nat Rev Drug Discov 9:537–550
Kim M, Um HJ, Bang S, Lee SH, Oh SJ, Han JH, Kim KW, Min J, Kim YH (2009) Arsenic removal from Vietnamese groundwater using the arsenic-binding DNA aptamer. Environ Sci Technol 43(24):9335–9340
Kolovskaya OS, Zamay TN, Zamay AS, Glazyrin YE, Spivak EA, Zubkova OA, Kadkina AV, Erkaev EN, Zamay GS, Savitskaya AG, Trufanova LV, Petrova LL, Berezovski MV (2014) DNA-aptamer/protein interaction as a cause of apoptosis and arrest of proliferation in Ehrlich ascites adenocarcinoma cells. Biochem Moscow Suppl Ser A 8:60
Labib M, Zamay AS, Kolovskaya OS, Reshetneva IT, Zamay GS, Kibbee RJ, Sattar SA, Zamay TN, Berezovski MV (2012a) Aptamer-based impedimetric sensor for bacterial typing. Anal Chem 84:8114–8117
Labib M, Zamay AS, Kolovskaya OS, Reshetneva IT, Zamay GS, Kibbee RJ, Sattar SA (2012b) Aptamer-based viability impedimetric sensor for bacteria. Anal Chem 84(21):8966–8969
Lee HJ, Kim BC, Kim KW et al (2009) A sensitive method to detect Escherichia coli based on immunomagnetic separation and real-time PCR amplification of aptamers. Biosens Bioelectron 24:3550–3555
Li L, Li B, Qi Y, Jin Y (2009) Label-free aptamer-based colorimetric detection of mercury ions in aqueous media using unmodified gold nanoparticles as colorimetric probe. Anal Bioanal Chem 393(8):2051–2057
Liu X, Zhang X (2015) Aptamer-based technology for food analysis. Appl Biochem Biotechnol 175(1):603–624
Lou X, Qian J, Xiao Y, Viel L, Gerdonb AE, Lagallya ET, Atzbergerc P, Tarasowd TM, Heegera AJ, Soha HT (2009) Micromagnetic selection of aptamers in microfluidic channels. Proc Natl Acad Sci U S A 106(9):2989–2994
Luo YL, Shiao YS, Huang YF (2011) Release of photoactivatable drugs from plasmonic nanoparticles for targeted cancer therapy. ACS Nano 5:7796–7804
Ma X, Wang W, Chen X et al (2014) Selection, identification, and application of aflatoxin B1 aptamer. Eur Food Res Technol 238(6):919–925
Malhotra S, Pandey AK, Rajput YS, Sharma R (2014) Selection of aptamers for aflatoxin M1 and their characterization. J Mol Recognit 27(8):493–500
Marimuthu C, Tang TH, Tominaga J, Tan SC, Gopinath SC (2012) Single-stranded DNA (ssDNA) production in DNA aptamer generation. Analyst 137(6):1307–1315
Marton S, Cleto F, Krieger MA, Cardoso J (2016) Isolation of an Aptamer that binds specifically to E. coli. PLoS One 11(4):e0153637
McKeague M, Bradley CR, de Girolamo A, Visconti A, David Miller J, de Rosa MC (2010) Screening and initial binding assessment of fumonisin B1 aptamers. Int J Mol Sci 11(12):4864–4881
McKeague M, Velu R, Hill K, Bardóczy V, Mészáros T, DeRosa M (2014) Selection and characterization of a novel DNA aptamer for label-free fluorescence biosensing of ochratoxin a. Toxins 6(8):2435–2452
Morton SL, Tindall DR (1996) Determination of okadaic acid content of dinoflagellate cells: a comparison of the HPLC-fluorescent method and two monoclonal antibody ELISA test kits. Toxicon 34(8):947–954
Nakamura C, Kobayashi T, Miyake M, Shirai M, Miyake J (2001) Usage of a DNA aptamer as a ligand targeting microcystin. Mol Cryst Liq Cryst Sci Technol Sect A 371(1):369–374
Nikolaus N, Strehlitz B (2014) DNA-Aptamers binding aminoglycoside antibiotics. Sensors (Basel) 14:3737–3755. https://doi.org/10.3390/s140203737
Nugen SR, Baeumner AJ (2008) Trends and opportunities in food pathogen detection. Anal Bioanal Chem 391(2):451–454
Pan Q, Zhang XL, Wu HY et al (2005) Aptamers that preferentially bind type IVB pili and inhibit human monocytic-cell invasion by Salmonella enterica serovar Typhi. Antimicrob Agents Chemother 49:4052–4060
Pang S, Labuza TP, He L (2014) Development of a single aptamer-based surface enhanced Raman scattering method for rapid detection of multiple pesticides. Analyst 139(8):1895–1901
Peng Z, Ling M, Ning Y, Deng L (2014) Rapid fluorescent detection of Escherichia coli K88 based on DNA aptamer library as direct and specific reporter combined with immuno-magnetic separation. J Fluoresc 24:1159–1168
Petzinger E, Ziegler K (2000) Ochratoxin a from a toxicological perspective. J Vet Pharmacol Ther 23(2):91–98
Qin L, Zheng R, Ma Z et al (2009) The selection and application of ssDNA aptamers against MPT64 protein in Mycobacterium tuberculosis. Clin Chem Lab Med 47:405–411
Rotherham LS, Maserumule C, Dheda K et al (2012) Selection and application of ssDNA aptamers to detect active TB from sputum samples. PLoS One 7:e46862
Sharma R, Barui AK, Rajput YS (2015) Aptamer specific for cefquinome. Indian patent 1775/DEL/2015
Shipley SLS, White E, Kim SK (2010) Selection of aptamers against live E. Coli cells using cell SELEX. FASEB J 24(1): Supplement. 907.14
Singh G, Vajpayee P, Rani N et al (2012) Bio-capture of S. Typhimurium from surface water by aptamer for culture-free quantification. Ecotoxicol Environ Saf 78:320–326
Sinha J, Reyes SJ, Gallivan JP (2010) Reprogramming bacteria to seek and destroy an herbicide. Nat Chem Biol 6(6):464–470
So HM, Park DW, Jeon EK et al (2008) Detection and titer estimation of Escherichia coli using aptamer-functionalized single-walled carbon-nanotube field-effect transistors. Small 4:197–201
Stead SL, Ashwin H, Johnston B et al (2010) An RNA-aptamer-based assay for the detection and analysis of malachite green and leucomalachite green residues in fish tissue. Anal Chem 82(7):2652–2660
Stratis-Cullum DN, McMasters S, Pellegrino PM (2009) Evaluation of relative aptamer binding to campylobacter jejuni bacteria using affinity probe capillary electrophoresis. Anal Lett 42(15):2389–2402
Tombelli S, Minunni M, Luzi E, Mascini M (2007) Aptamer-based biosensors for the detection of HIV-1 tat protein. Bioelectrochemistry 67:135–141
Tuerk C, Gold L (1990) Systematic evolution of ligands by exponential enrichment: RNA ligands to bacteriophage T4 DNA polymerase. Science 249:505–510
Vivekananda J, Kiel JL (2006) Anti-Francisella tularensis DNA aptamers detect tularemia antigen from different subspecies by Aptamer-linked immobilized sorbent assay. Lab Investig 86:610–618
Vivekananda J, Salgado C, Millenbaugh NJ (2014) DNA aptamers as a novel approach to neutralize Staphylococcus aureus α-toxin. Biochem Biophys Res Commun 444:433–438
Wang KY, Zeng YL, Yang XY et al (2011) Utility of aptamer-fluorescence in situ hybridization for rapid detection of Pseudomonas aeruginosa. Eur J Clin Microbiol Infect Dis 30:273–278
Wang R, Zhao J, Jiang T et al (2013) Selection and characterization of DNA aptamers for use in detection of avian influenza virus H5N1. J Virol Methods 189:362–369
Wang S, Liu J, Yong W, Chen Q, Zhang L, Dong Y et al (2015) A direct competitive assay-based aptasensor for sensitive determination of tetracycline residue in honey. Talanta 131:562–569
Wang H, Cheng H, Wang J, Xu L, Chen H, Pei R (2016) Selection and characterization of DNA aptamers for the development of light-up biosensor to detect cd(II). Talanta 154:498–503
Yamamoto C, Sen T (2008) Nucleic acids ligands capable of binding to internalin B or internalin A. Internation Application Published under the patent cooperation treaty, WO2008/011608A2
Yamamoto C, Sen T (2009) Nucleic acids ligands capable of binding to internalin B or internalin A. US Patent Application Publication, (October, 2009) US20090264512A1
Yamamoto C, Sen T (2010) Aptamers that bind to Listeria surface proteins. US Patent Application Publication, (January, 2010) US7645582B2
Yang X, Qian J, Jiang L, Yan Y, Wang K, Liu Q, Wang K (2014) Ultrasensitive electrochemical apta-sensor of ochratoxin a based on two level cascades signal amplification strategy. Bioelectrochemistry 96:7–13
Yan Z, Gan N, Wang D, Cao Y, Chen M, Li T et al (2016) A “signal-on” aptasensor for simultaneous detection of chloramphenicol and polychlorinated biphenyls using multi-metal ions encoded nanospherical brushes as tracers. Biosens Bioelectron 74:718–724
Zelada-Guillén GA, Riu J, Düzgün A, Rius FX (2009) Immediate detection of living bacteria at ultralow concentrations using a carbon nanotube based potentiometric aptasensor. Angew Chem Int Ed 48:7334–7337
Zelada-Guillen GA, Bhosale SV, Riu J, Rius FX (2010) Real-time potentiometric detection of bacteria in complex samples. Anal Chem 82(22):9254–9260
Zheng X, Hu B, Gao SX, Liu DJ, Sun MJ, Wang LH et al (2013) A saxitoxin-binding aptamer with higher affinity and inhibitory activity optimized by rational site-directed mutagenesis and truncation. Toxicon 101:41–47
Zhou J, Neff CP, Swiderski P et al (2012) Functional in vivo delivery of multiplexed anti-HIV-1 siRNAs via a chemically synthesized aptamer with a sticky bridge. Mol Ther 21:192–200
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2019 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Yadav, G.S., Parashar, A., Aggarwal, N.K. (2019). Aptamer: A Next Generation Tool for Application in Agricultural Industry for Food Safety. In: Yadav, G., Kumar, V., Aggarwal, N. (eds) Aptamers. Springer, Singapore. https://doi.org/10.1007/978-981-13-8836-1_12
Download citation
DOI: https://doi.org/10.1007/978-981-13-8836-1_12
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-13-8835-4
Online ISBN: 978-981-13-8836-1
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)