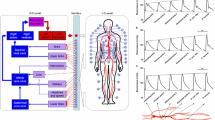
Abstract
Blood vessels exhibit a remarkable ability to adapt in response to sustained alterations in hemodynamic loads and diverse disease processes. Although such adaptations typically manifest at the tissue level, underlying mechanisms exist at cellular and molecular levels. Dramatic technological advances in recent years, including sophisticated theoretical and computational modeling, have enabled significantly increased understanding at tissue, cellular, and molecular levels, yet there has been little attempt to integrate the associated models across these length and time scales. In this chapter, we suggest a new paradigm for identifying strengths and weaknesses of models at different scales and for establishing congruent models that more completely predict vascular adaptations. Specifically, we show the importance of linking intracellular with cellular models and cellular models with tissue level models. In this way, we propose a new approach for incorporating events across these three levels, thus providing a means to predict phenomena that can only emerge from a system of integrated interactions.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Abbott, R.G., Forrest, S., Pienta, K.J.: Simulating the hallmarks of cancer. Artif. Life 12, 617 (2006)
Alon, U.: Network motifs: theory and experimental approaches. Nat. Rev. Genet. 8, 450 (2007)
An, G.: Concepts for developing a collaborative in silico model of the acute inflammatory response using agent-based modeling. J. Crit. Care 21, 105 (2006)
An, G.: In silico experiments of existing and hypothetical cytokine-directed clinical trials using agent-based modeling. Crit. Care Med. 32, 2050 (2004)
An, G.: Mathematical modeling in medicine: a means, not an end. Crit. Care Med. 33, 253 (2005)
Baek, S., Rajagopal, K.R., Humphrey, J.D.: A theoretical model of enlarging intracranial fusiform aneurysms. J. Biomech. Eng. 128, 142 (2006)
Bailey, A.M., Thorne, B.C., Peirce, S.M.: Multi-cell agent-based simulation of the microvasculature to study the dynamics of circulating inflammatory cell trafficking. Ann. Biomed. Eng. 35, 916 (2007)
Bauer, A.L., Jackson, T.L., Jiang, Y.: A cell-based model exhibiting branching and anastomosis during tumor-induced angiogenesis. Biophys. J. 92, 3105 (2007)
Bauer, A.L., Jackson, T.L., Jiang, Y., Rohlf, T.: Receptor cross-talk in angiogenesis: mapping environmental cues to cell phenotype using a stochastic, Boolean signaling network model. J. Theor. Biol. 264, 838 (2010)
Benest, A.V., Stone, O.A., Miller, W.H., Glover, C.P., Uney, J.B., Baker, A.H., Harper, S.J., Bates, D.O.: Arteriolar genesis and angiogenesis induced by endothelial nitric oxide synthase overexpression results in a mature vasculature. Arterioscler. Thromb. Vasc. Biol. 28, 1462 (2008)
Bhalla, U.S., Iyengar, R.: Emergent properties of networks of biological signaling pathways. Science 283, 381 (1999)
Bian, K., Doursout, M.F., Murad, F.: Vascular system: role of nitric oxide in cardiovascular diseases. J. Clin. Hypertens (Greenwich) 10, 304 (2008)
Bouvet, C., Moreau, S., Blanchette, J., de Blois, D., Moreau, P.: Sequential activation of matrix metalloproteinase 9 and transforming growth factor beta in arterial elastocalcinosis. Arterioscler. Thromb. Vasc. Biol. 28, 856 (2008)
Casal, A., Sumen, C., Reddy, T.E., Alber, M.S., Lee, P.P.: Agent-based modeling of the context dependency in T cell recognition. J. Theor. Biol. 236, 376 (2005)
Castro, M.M., Tanus-Santos, J.E., Gerlach, R.F.: Matrix metalloproteinases: targets for doxycycline to prevent the vascular alterations of hypertension. Pharmacol. Res. 64, 567 (2011)
Chaturvedi, R., et al.: On multiscale approaches to three-dimensional modelling of morphogenesis. J. R. Soc. Interface 2, 237 (2005)
Chen, N., Glazier, J.A., Izaguirre, J.A., Alber, M.S.: A parallel implementation of the cellular potts model for simulation of cell-based morphogenesis. Comput. Phys. Commun. 176, 670 (2007)
Christ, G.J., Spray, D.C., el-Sabban, M., Moore, L.K., Brink, P.R.: Gap junctions in vascular tissues. Evaluating the role of intercellular communication in the modulation of vasomotor tone. Circ. Res. 79, 631 (1996)
Davies, P.F.: Flow-mediated endothelial mechanotransduction. Physiol. Rev. 75, 519 (1995)
DiCorleto, P.E., Bowen-Pope, D.F.: Cultured endothelial cells produce a platelet-derived growth factor-like protein. Proc. Natl. Acad. Sci. USA. 80, 1919 (1983)
Drasdo, D., Forgacs, G.: Modeling the interplay of generic and genetic mechanisms in cleavage, blastulation, and gastrulation. Dev. Dyn. 219, 182 (2000)
Duran, W.N., Breslin, J.W., Sanchez, F.A.: The NO cascade, eNOS location, and microvascular permeability. Cardiovasc. Res. 87, 254 (2010)
Fameli, N., Kuo, K.H., van Breemen, C.: A model for the generation of localized transient [Na+] elevations in vascular smooth muscle. Biochem. Biophys. Res. Commun. 389, 461 (2009)
Galkina, E., Ley, K.: Immune and inflammatory mechanisms of atherosclerosis (*). Annu. Rev. Immunol. 27, 165 (2009)
Graner, F., Glazier, J.A.: Simulation of biological cell sorting using a two dimensional extended potts model. Phys. Rev. Lett. 69, 2013–2016 (1992)
Grant, M.R., Mostov, K.E., Tlsty, T.D., Hunt, C.A.: Simulating properties of in vitro epithelial cell morphogenesis. PLoS Comput. Biol. 2, e129 (2006)
Grimm, V., Railsback, S.F.: Individual-based modeling and ecology. Princeton University Press, Princeton (2005)
Hastings, N.E., Simmers, M.B., McDonald, O.G., Wamhoff, B.R., Blackman, B.R.: Atherosclerosis-prone hemodynamics differentially regulates endothelial and smooth muscle cell phenotypes and promotes pro-inflammatory priming. Am. J. Physiol. Cell Physiol. 293, C1824 (2007)
Hayenga, H.N., Thorne, B.C., Peirce, S.M., Humphrey, J.D.: Ensuring congruency in multiscale modeling: towards linking agent based and continuum biomechanical models of arterial adaptation. Ann. Biomed. Eng. 39, 2669 (2011)
Humphrey, J.D.: Cardiovascular solid mechanics: cells, tissues, and organs. Springer, New York (2002)
Humphrey, J.D.: Stress, strain, and mechanotransduction in cells. J. Biomech. Eng. 123, 638 (2001)
Humphrey, J.D.: Vascular adaptation and mechanical homeostasis at tissue, cellular, and sub-cellular levels. Cell Biochem. Biophys. 50, 53 (2008)
Humphrey, J.D., Rajagopal, K.R.: A constrained mixture model for arterial adaptations to a sustained step change in blood flow. Biomech. Model. Mechanobiol. 2, 109 (2003)
Izaguirre, J.A., et al.: CompuCell, a multi-model framework for simulation of morphogenesis. Bioinformatics 20, 1129 (2004)
Kapela, A., Bezerianos, A., Tsoukias, N.M.: A mathematical model of vasoreactivity in rat mesenteric arterioles: I. Myoendothelial communication. Microcirculation 16, 694 (2009)
Kawai-Kowase, K., Owens, G.K.: Multiple repressor pathways contribute to phenotypic switching of vascular smooth muscle cells. Am. J. Physiol. Cell Physiol. 292, C59 (2007)
Kohn-Luque, A., de Back, W., Starruss, J., Mattiotti, A., Deutsch, A., Perez-Pomares, J.M., Herrero, M.A.: Early embryonic vascular patterning by matrix-mediated paracrine signalling: a mathematical model study. PLoS One 6, e24175 (2011)
Kuwai, T., et al.: Targeting the EGFR, VEGFR, and PDGFR on colon cancer cells and stromal cells is required for therapy. Clin. Exp. Metastasis 25, 477 (2008)
Longo, D., Peirce, S.M., Skalak, T.C., Davidson, L., Marsden, M., Dzamba, B., DeSimone, D.W.: Multicellular computer simulation of morphogenesis: blastocoel roof thinning and matrix assembly in Xenopus laevis. Dev. Biol. 271, 210 (2004)
Merks, R.M., Brodsky, S.V., Goligorksy, M.S., Newman, S.A., Glazier, J.A.: Cell elongation is key to in silico replication of in vitro vasculogenesis and subsequent remodeling. Dev. Biol. 289, 44 (2006)
Nakagawa, T., Inoue, H., Sasahara, M.: Platelet-derived growth factor and renal disease. Curr. Opin. Nephrol. Hypertens 21, 80 (2012)
Osol, G.: Mechanotransduction by vascular smooth muscle. J. Vasc. Res. 32, 275 (1995)
Peirce, S.M., Skalak, T.C., Papin, J.A.: Coupling intracellular networks with tissue-level physiology. IBM J. Res. 50, 1 (2006)
Peirce, S.M., Van Gieson, E.J., Skalak, T.C.: Multicellular simulation predicts microvascular patterning and in silico tissue assembly. Faseb. J. 18, 731 (2004)
Pries, A.R., Reglin, B., Secomb, T.W.: Modeling of angioadaptation: insights for vascular development. Int. J. Dev. Biol. 55, 399 (2011)
Raffetto, J.D., Khalil, R.A.: Matrix metalloproteinases and their inhibitors in vascular remodeling and vascular disease. Biochem. Pharmacol. 75, 346 (2008)
Ramli, J., CalderonArtero, P., Block, R.C., Mousa, S.A.: Novel therapeutic targets for preserving a healthy endothelium: strategies for reducing the risk of vascular and cardiovascular disease. Cardiol. J. 18, 352 (2011)
Ridnour, L.A., Isenberg, J.S., Espey, M.G., Thomas, D.D., Roberts, D.D., Wink, D.A.: Nitric oxide regulates angiogenesis through a functional switch involving thrombospondin-1. Proc. Natl. Acad. Sci. USA 102, 13147 (2005)
Robertson, S.H. et al.: Multiscale computational analysis of Xenopus laevis morphogenesis reveals key insights of systems-level behavior. BMC Syst. Biol. 1, 46 (2008)
Rudic, R.D., Shesely, E.G., Maeda, N., Smithies, O., Segal, S.S., Sessa, W.C.: Direct evidence for the importance of endothelium-derived nitric oxide in vascular remodeling. J. Clin. Invest. 101, 731 (1998)
Schermuly, R.T., et al.: Reversal of experimental pulmonary hypertension by PDGF inhibition. J. Clin. Invest. 115, 2811 (2005)
Scianna, M., Munaron, L., Preziosi, L.: A multiscale hybrid approach for vasculogenesis and related potential blocking therapies. Prog. Biophys. Mol. Biol. 106, 450 (2011)
Segovia-Juarez, J.L., Ganguli, S., Kirschner, D.: Identifying control mechanisms of granuloma formation during M. tuberculosis infection using an agent-based model. J. Theor. Biol. 231, 357 (2004)
Sessa, W.C.: eNOS at a glance. J. Cell Sci. 117, 2427 (2004)
Simpson, M.J., Merrifield, A., Landman, K.A., Hughes, B.D.: Simulating invasion with cellular automata: connecting cell-scale and population-scale properties. Phys. Rev. E Stat. Nonlin. Soft Matter Phys. 76, 021918 (2007)
Smith Jr., R.S., Lin, K.F., Agata, J., Chao, L., Chao, J.: Human endothelial nitric oxide synthase gene delivery promotes angiogenesis in a rat model of hindlimb ischemia. Arterioscler. Thromb. Vasc. Biol. 22, 1279 (2002)
Thorne, B.C., Bailey, A.M., Peirce, S.M.: Combining experiments with multi-cell agent-based modeling to study biological tissue patterning. Br. Bioinf. 8, 245 (2007)
Thorne, B.C., Hayenga, H.N., Humphrey, J.D., Peirce, S.M.: Toward a multi-scale computational model of arterial adaptation in hypertension: verification of a multi-cell agent based model. Front. Physiol. 2, 20 (2011)
Tomasek, J.J., Gabbiani, G., Hinz, B., Chaponnier, C., Brown, R.A.: Myofibroblasts and mechano-regulation of connective tissue remodelling. Nat. Rev. Mol. Cell Biol. 3, 349 (2002)
Tsoukias, N.M.: Nitric oxide bioavailability in the microcirculation: insights from mathematical models. Microcirculation 15, 813 (2008)
Valentin, A., Cardamone, L., Baek, S., Humphrey, J.D.: Complementary vasoactivity and matrix remodelling in arterial adaptations to altered flow and pressure. J. R. Soc. Interface 6, 293 (2009)
Wagenseil, J.E., Mecham, R.P.: Vascular extracellular matrix and arterial mechanics. Physiol. Rev. 89, 957 (2009)
Wilensky, U.: NetLogo—http://ccl.northwestern.edu/netlogo. Center for Connected Learning and Computer-Based Modeling. Northwestern University, Evanston (1999)
Yamamoto, K., Ikeda, U., Shimada, K.: Role of mechanical stress in monocytes/macrophages: implications for atherosclerosis. Curr. Vasc. Pharmacol. 1, 315 (2003)
Yang, J., Clark, J.W., Bryan, R.M., Robertson, C.S.: Mathematical modeling of the nitric oxide/cGMP pathway in the vascular smooth muscle cell. Am. J. Physiol. Heart Circ. Physiol. 289, H886 (2005)
Yokota, T., Ma, R.C., Park, J.Y., Isshiki, K., Sotiropoulos, K.B., Rauniyar, R.K., Bornfeldt, K.E., King, G.L.: Role of protein kinase C on the expression of platelet-derived growth factor and endothelin-1 in the retina of diabetic rats and cultured retinal capillary pericytes. Diabetes 52, 838 (2003)
Yoshida, T., Gan, Q., Shang, Y., Owens, G.K.: Platelet-derived growth factor-BB represses smooth muscle cell marker genes via changes in binding of MKL factors and histone deacetylases to their promoters. Am. J. Physiol. Cell Physiol. 292, C886 (2007)
Yoshida, T., Owens, G.K.: Molecular determinants of vascular smooth muscle cell diversity. Circ. Res. 96, 280 (2005)
Yu, J., Zhang, Y., Zhang, X., Rudic, R.D., Bauer, P.M., Altieri, D.C., Sessa, W.C.: Endothelium derived nitric oxide synthase negatively regulates the PDGF-survivin pathway during flow-dependent vascular remodeling. PLoS One 7, e31495 (2012)
Zajac, M., Jones, G.L., Glazier, J.A.: Simulating convergent extension by way of anisotropic differential adhesion. J. Theor. Biol. 222, 247 (2003)
Zhang, L., Strouthos, C.G., Wang, Z., Deisboeck, T.S.: Simulating brain tumor heterogeneity with a multiscale agent-based model: linking molecular signatures, phenotypes and expansion rate. Math. Comput. Model. 49, 307 (2009)
Zhang, R., Wang, L., Zhang, L., Chen, J., Zhu, Z., Zhang, Z., Chopp, M.: Nitric oxide enhances angiogenesis via the synthesis of vascular endothelial growth factor and cGMP after stroke in the rat. Circ. Res. 92, 308 (2003)
Acknowledgments
This work was supported, in part, via NIH grants HL-86418 to JDH and HL-82838 to SMP.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2013 Springer-Verlag Berlin Heidelberg
About this chapter
Cite this chapter
Hayenga, H.N., Thorne, B.C., Yen, P., Papin, J.A., Peirce, S.M., Humphrey, J.D. (2013). Multiscale Computational Modeling in Vascular Biology: From Molecular Mechanisms to Tissue-Level Structure and Function. In: Gefen, A. (eds) Multiscale Computer Modeling in Biomechanics and Biomedical Engineering. Studies in Mechanobiology, Tissue Engineering and Biomaterials, vol 14. Springer, Berlin, Heidelberg. https://doi.org/10.1007/8415_2012_147
Download citation
DOI: https://doi.org/10.1007/8415_2012_147
Published:
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-642-36481-5
Online ISBN: 978-3-642-36482-2
eBook Packages: EngineeringEngineering (R0)