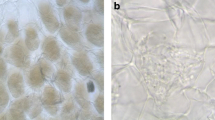
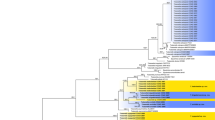
Abstract
We have assessed the identities of fungi associated with Orchis tridentata, an endangered orchid species growing in open woodland and poor grassland of Central and Southern Europe. Fungal diversity in ten O. tridentata adult individuals collected in two protected areas of Central Italy was analysed by means of morphological and molecular methods. Sequencing of the cloned ITS fungal inserts corresponding to the dominant PCR products obtained from amplification of total root DNA with ITS1F and ITS4 primers revealed a variety of fungal species occurring in O. tridentata roots. Among them, members of the basidiomycete families Ceratobasidiaceae, Tulasnellaceae and Hymenogastraceae were recovered, together with ascomycetes belonging to Leptodontidium and Terfezia. The implications of these results in the understanding of O. tridentata biology and for the conservation of this threatened orchid species are discussed.
Similar content being viewed by others
References
Dressler R.L., How many orchid species?, Selbyana, 2005, 26, 155–158
I.U.C.N., IUCN Red List Categories, IUCN: Gland, Switzerland, 1994
Kull T., Hutchings M.J., A comparative analysis of decline in the distribution ranges of orchid species in Estonia and the United Kingdom, Biol. Conserv., 2006, 129, 31–39
Kull T., Kull K., How orchids regulate their numbers, In: Nair H., Arditti J. (Eds.), Proceedings of the 17th world Orchid Conference (24 April–1 May 2002, Shah Alam, Malaysia), Shah Alam, 2002, Natural History Publications (Borneo), Kota Kinabalu, Sabah, Malaysia, 2005, 173–177
Schatz B., Geoffroy A., Dainat B., Bessière J.M., Buatois B., Hossaert-McKey M., Selosse M.A., A case study of modified interactions with symbionts in a hybrid Mediterranean orchid, Am. J. Bot., 2010, 97, 1278–1288
Shefferson R.P., Kull T., Tali K., Mycorrhizal interaction of orchids colonizing Estonian mine tailings hills, Am. J. Bot., 2008, 95, 156–164
Rasmussen H.N., Terrestrial orchids: From seed to mycotrophic plant, Cambridge University Press, Cambridge, UK, 1995
Kull T., Arditti J., Orchid Biology: Reviews and Perspectives, VIII, Kluwer Academic Publishers, Dordrecht, 2002
Leake J.R., The biology of mycoheterotrophic (’saprophytic’) plants, Tansley Review No. 69, New Phytol., 1994, 127, 171–216
Julou T., Burghardt B., Gebauer G., Berveiller D., Damesin C., Selosse M.A., Mixotrophy in Orchids: insights from a comparative study of green individuals and nonphotosynthetic individuals of Cephalantera damasonium, New Phytol., 2005, 166, 639–653
Gebauer G., Meyer M., 15N and 13C natural abundance of autotrophic and mycoheterotrophic orchids provides insight into nitrogen and carbon gain from fungal association, New Phytol., 2003, 160, 209–223
Dearnaley J.D.W., Further advances in orchids mycorrhizal research, Mycorrhiza, 2007, 17, 475–486
Bidartondo M.I., Read D.J., Fungal specificity bottlenecks during orchid germination and development, Mol. Ecol., 2008, 17, 3707–3716
Selosse M.A., Cameron D.D., Introduction to a virtual special issue on mycoheterotrophy: the New Phytologist sheds light on non-green plants, New Phytol., 2010, 185, 591–593
Otero J.T., Ackerman J.D., Bayman P., Diversity and host specificity of endophytic Rhizoctonia-like fungi from tropical orchids, Am. J. Bot., 2002, 89, 1852–1858
Roberts P., Rhizoctonia-forming fungi: A taxonomic guide, Kew Royal Botanic Gardens, Surrey, UK, 1999
Taylor D.L., McCormick M.K., Internal transcribed spacer primers and sequences for improved characterization of basidiomycetous orchid mycorrhizas, New Phytol., 2008, 177, 1020–1033
Pecoraro L., Girlanda M., Kull T., Perini C., Perotto S., Molecular identification of root fungal associates in Orchis pauciflora Tenore, Plant Biosystems, DOI: 10.1080/11263504.2011.634447 (in press)
Girlanda M., Segreto R., Cafasso D., Liebel H.T., Rodda M., Ercole E., et al., Mediterranean meadow photosynthetic orchids feature partial mycoheterotrophy and specific mycorrhizal associations, Am. J. Bot., 2011, 98, 1148–1163
Jacquemyn H., Honnay O., Cammue B.P.A., Brys R., Lievens B., Low specificity and nested subset structure characterize mycorrhizal associations in five closely related species of the genus Orchis, Mol. Ecol., 2010, 19, 4086–4095
Jacquemyn H., Brys R., Cammue B.P.A., Honnay O., Lievens B., Mycorrhizal associations and reproductive isolation in three closely related Orchis species, Ann. Bot., 2011, 107, 347–356
Vendramin E., Gastaldo A., Tondello A., Baldan B., Villani M., Squartini A., Identification of two fungal endophytes associated with the endangered orchid Orchis militaris L., J. Microbiol. Biotechnol., 2010, 20, 630–636
Rossi W., Orchids of Italy [Orchidee d’Italia], Quad. Cons. Natura, 15, Min. Ambiente-Ist. Naz. Fauna Selvatica, 2002
Delforge P., Orchids of Britain & Europe, Harper Collins Publishers, Basingstoke, 1995
Alonzi A., Ercole S., Piccini C., La protezione delle specie della flora e della fauna selvatica: quadro di riferimento legislativo regionale, APAT Rapporti 75/2006, 2006
Doyle J.J., Doyle J.L., Isolation of plant DNA from fresh tissues, Focus, 1990, 12, 13–15
Gardes M., Bruns T.D., ITS primers with enhanced specificity for basidiomycetes — applications to the identification of mycorrhizae and rusts, Mol. Ecol., 1993, 2, 113–118
Abadie J.C., Püttsepp Ü., Gebauer G., Faccio A., Bonfante P., Selosse M.A., Cephalanthera longifolia (Neottieae, Orchidaceae) is mixotrophic: a comparative study between green and nonphotosynthetic individuals, Can. J. Bot., 2006, 84, 1462–1477
Smith S.E., Read D.J., Mycorrhizal Symbiosis, 3rd ed., Academic Press, San Diego, California, 2008
Cameron D.D., Leake J.R., Read D.J., Mutualistic mycorrhiza in orchids: evidence from plantfungus carbon and nitrogen transfers in the green-leaved terrestrial orchid Goodyera repens, New Phytol., 2006, 171, 405–416
Liebel H.T., Bidartondo M.I., Preiss K., Segreto R., Stöckel M., Rodda M., et al., C and N stable isotope signatures reveal constraints to nutritional modes in orchids from the Mediterranean and Macaronesia, Am. J. Bot., 2010, 97, 903–912
Sen R., Hietala A.M., Zelmer C.D., Common anastomosis and internal transcribed spacer RFLP groupings in binucleate Rhizoctonia isolates representing root endophytes of Pinus silvestris, Ceratorhiza spp. from orchid mycorrhizas and a phytopathogenetic anastomosis group, New Phytol., 1999, 144, 331–341
Grönberg H., Kaparakis G., Sen R., Binucleate Rhizoctonia (Ceratorhiza spp.) as non-mycorrhizal endophytes alter Pinus sylvestris L. seedling root architecture and effect growth of rooted cuttings, Scand. J. For. Res., 2006, 21, 450–457
Hadley G., Orchid mycorrhizal, In: Arditti J. (Ed.), Orchid biology: reviews and perspectives, II, Cornell University Press, Ithaca, NY, 1982
McCormick M.K., Whigham D.F., O’Neill J., Mycorrhizal diversity in photosynthetic terrestrial orchids, New Phytol., 2004, 163, 425–438
Girlanda M., Selosse M.A., Cafasso D., Brilli F., Delfine S., Fabbian R., et al., Inefficient photosynthesis in the Mediterranean orchid Limodorum abortivum is mirrored by specific association to ectomycorrhizal Russulaceae, Mol. Ecol., 2006, 15, 491–504
Sharon M., Freeman S., Kuninaga S., Sneh B., Genetic diversity, anamostosis groups and virulence of Rhizoctonia spp. from strawberry, Eur. J. Plant. Pathol., 2007, 117, 247–265
Bidartondo M.I., Burghardt B., Gebauer G., Bruns T.D., Read D.J., Changing partners in the dark: isotopic and molecular evidence of ectomycorrhizal liaisons between forest orchids and trees, Proceedings of the Royal Society of London B, 2004, 271, 1799–1806
Shefferson R.P., Weiss M., Kull T., Taylor D.L., High specificity generally characterizes mycorrhizal association in rare lady’s slipper orchids, genus Cypripedium, Mol. Ecol., 2005, 14, 613–626
Otero J.T., Flanagan N.S., Herre E.A., Ackerman J.D., Bayman P., Widespread mycorrhizal specificity correlates to mycorrhizal function in the neotropical, epiphytic orchid Ionopsis utricularioides (Orchidaceae), Am. J. Bot., 2007, 94, 1944–1950
Irwin M.J., Bougoure J.J., Dearnaley J.D.W., Pterostylis nutans (Orchidaceae) has a specific association with two Ceratobasidium root associated fungi across its range in Eastern Australia, Mycoscience, 2007, 48, 231–239
Otero J.T., Bayman P., Ackerman J.D., Variation in mycorrhizal performance in the epiphytic orchid Tolumnia variegata in vitro: the potential for natural selection, Evol. Ecol., 2005, 19, 29–43
Selosse M.A., Faccio A., Scappaticci P., Bonfante P., Chlorophyllous and achlorophyllous specimens of Epipactis microphylla (Neottieae, Orchidaceae) are associated with ectomycorrhizal septomycetes, including truffles, Microbiol. Ecol., 2004, 47, 416–426
Shimura H., Sadamoto M., Matsuura M., Kawahara T., Naito S., Koda Y., Characterization of mycorrhizal fungi isolated from the threatened Cypripedium macranthos in a northern island of Japan: two phylogenetically distinct fungi associated with the orchid, Mycorrhiza, 2009, 19, 525–534
Lievens B., van Kerckhove S., Juste A., Cammue B.P.A., Honnay O., Jacquemyn H., From extensive clone libraries to comprehensive DNA arrays for the efficient and simultaneous detection and identification of orchid mycorrhizal fungi, J. Microbiol. Methods, 2010, 80, 76–85
Wu L., Guo S., Interaction between an isolate of dark-septate fungi and its host plant Saussurea involucrata, Mycorrhiza, 2008, 18, 79–85
Currah R.S., Smreciu A., Hambleton S., Mycorrhizae and mycorrhizal fungi of boreal species of Platanthera and Coeloglossum (Orchidaceae), Can. J. Bot., 1990, 68, 1171–1181
Morte A., Lovisolo C., Schubert A., Effect of drought stress on growth and water relations of the mycorrhizal association Helianthemum almeriense-Terfezia claveryi, Mycorrhiza, 2000, 10, 115–119
Batty A.L., Dixon K.W., Brundrett M., Sivasithamparam K., Constraints to symbiotic germination of terrestrial orchid seed in a mediterranean bushland, New Phytol., 2001, 152, 511–520
Liu H., Luo Y., Liu H., Studies of Mycorrhizal Fungi of Chinese Orchids and Their Role in Orchid Conservation in China-A Review, Bot. Rev., 2010, 76, 241–262
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Pecoraro, L., Girlanda, M., Kull, T. et al. Analysis of fungal diversity in Orchis tridentata Scopoli. cent.eur.j.biol. 7, 850–857 (2012). https://doi.org/10.2478/s11535-012-0071-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.2478/s11535-012-0071-y