Summary
Skeletal muscle tissue is sensitive to the acute and chronic stresses associated with resistance training. These responses are influenced by the structure of resistance activity (i.e. frequency, load and recovery) as well as the training history of the individuals involved. There are histochemical and biochemical data which suggest that resistance training alters the expression of myosin heavy chains (MHCs). Specifically, chronic exposure to bodybuilding and power lifting type activity produces shifts towards the MHC I and IIb isoforms, respectively. However, it is not yet clear which training parameters trigger these differential expressions of MHC isoforms. Interestingly, many programmes undertaken by athletes appear to cause a shift towards the MHC I isoform. Increments in the cross-sectional area of muscle after resistance training can be primarily attributed to fibre hypertrophy. However, there may be an upper limit to this hypertrophy. Furthermore, significant fibre hypertrophy appears to follow the sequence of fast twitch fibre hypertrophy preceding slow twitch fibre hypertrophy. Whilst some indirect measures of fibre number in living humans suggest that there is no interindividual variation, postmortem evidence suggests that there is. There are also animal data arising from investigations using resistance training protocols which suggest that chronic exercise can increase fibre number. Furthermore, satellite cell activity has been linked to myotube formation in the human.
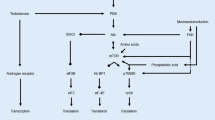
However, other animal models (i.e. compensatory hypertrophy) do not support the notion of fibre hyperplasia. Even if hyperplasia does occur, its effect on the cross-sectional area of muscle appears to be small. Phosphagen and glycogen metabolism, whilst important during resistance activity appear not to normally limit the performance of resistance activity. Phosphagen and related enzyme adaptations are affected by the type, structure and duration of resistance training. Whilst endogenous glycogen reserves may be increased with prolonged training, typical isotonic training for less than 6 months does not seem to increase glycolytic enzyme activity. Lipid metabolism may be of some significance in bodybuilding type activity. Thus, not surprisingly, oxidative enzyme adaptations appear to be affected by the structure and perhaps the modality of resistance training. The dilution of mitochondrial volume and endogenous lipid densities appears mainly because of fibre hypertrophy.
Similar content being viewed by others
References
Abernethy PJ. Interactions between strength and endurance training. PhD dissertation, The University of Queensland, Australia, 1991
Abernethy PJ. Ammonia and lactate response to leg press work at different repetition maxima. Abstract. 1992 NSCA Australia National Conference. Journal of Strength and Conditioning Research 7: 187, 1993a
Abernethy PJ. Influence of acute endurance activity on isokinetic strength. Journal of Strength and Conditioning Research 7: 141–146, 1993b
Abernethy PJ, Quigley BM. Concurrent strength and endurance training of the elbow extensors. Journal of Strength and Conditioning Research, in press, 1994
Alway SE, Grumbt WH, Stray-Gundersen J, Gonyea WJ. Effects of resistance training on elbow flexors of highly competitive bodybuilders. Journal of Applied Physiology 72: 1512–1521, 1992
Appell H-J. Satellite cell activation in human skeletal muscle after training: evidence for muscle fibre neoformation. International Journal of Sports Medicine 9: 297–299, 1988
Appell H-J. Muscle morphology: effects of use and disuse. In Hargreaves (Ed.) Fatigue in sport and exercise, pp. 86–95, Footscray, 1990
Asmussen E, Nausen K, Nielson L, Techow OSA, Tonder P. Lactate production and anaerobic work capacity after prolonged exercise. Acta Physiologica Scandinavica 90: 731–742, 1974
Bandy WD, Lovelace-Chandler V, McKitrick-Bandy B. Adaptation of skeletal muscle to resistance training. Journal of Orthopaedic and Sports Physical Therapy 12: 248–255, 1990
Bar A, Pette D. Three fast myosin heavy chains in adult rat skeletal muscle. FEBS Letters 235: 153–155, 1988
Barany M. ATPase activity of myosin correlated with speed of muscle shortening. Journal of General Physiology 50: 197–216, 1967
Bell D, Jacobs I. Muscle fibre-specific glycogen utilisation in strength-trained males and females. Medicine and Science in Sports and Exercise 21: 649–654, 1989
Bell D, Jacobs I. Muscle fibre area, fibre type and capillarization in male and female body builders. Canadian Journal of Sports Sciences 15: 115–119, 1990
Billeter J, Heizmann CW, Howald H, Jenny E. Analysis of myosin light and heavy chain types in single human skeletal muscle fibers. European Journal of Biochemistry 116: 389–395, 1981
Biral D, Betto R, Danieli-Betto D, Salviati G. Myosin heavy chain composition of single fibres from normal human muscle. Biochemical Journal 250: 307–308, 1988
Bodine SC, Roy RR, Eldred E, Edgerton VR. Maximal force as a function of anatomical features of motor units in the cat tibialis anterior. Journal of Neurophysiology 57: 1730–1745, 1987
Boobis L, Williams C, Wooton SA. Influence of sprint training on muscle metabolism during brief maximal exercise in man. Journal of Physiology 342: 36p–37p, 1983a
Boobis L, Williams C, Wooton SA. Human muscle metabolism during brief maximal exercise. Journal of Physiology 338: 21p–22p, 1983b
Booth FW, Thomason DB. Molecular and cellular adaptation of muscle in response to exercise: perspectives of various models. Physiological Reviews 71: 541–585, 1991
Bottinelli R, Schiaffino S, Reggiani C. Force-velocity relations and myosin heavy chain isoform compositions of skinned fibres from rat skeletal muscle. Journal of Physiology 437: 655–672, 1991
Bottinelli R, Sandoli D, Canepari M, Reggiani C. Maximal isometric force and myosin heavy chain composition in single skinned skeletal muscle fibres from the rat. Pflugers Archiv — European Journal of Physiology 420: r193, 1992
Brooke M, Kaiser K. Muscle fiber types. How many and what kind? Archives of Neurology 23: 369–379, 1970a
Brooke M, Kaiser K. Three ‘myosin ATPase’ systems. The nature of their pH lability and sulflhydryl dependence. Journal of Histochemistry and Cytochemistry 18: 670–672, 1970b
Buchthal F, Schmalbruch H. Motor unit of mammalian muscle. Physiological Review 60: 90–142, 1980
Cabric M, Appell H-J. Effect of electrical stimulation of high and low frequency on maximum isometric force and some morphological characteristics in men. International Journal of Sports Medicine 8: 256–260, 1987
Carson JA, Roman WJ, Alway SE. Modulation of native fast myosin isoforms in the biceps brachii muscle after stretch-overloaded. Medicine and Science in Sports and Exercise 24: S107, 1992
Chasiotis D, Sahlin K, Hultman E. Regulation of glycogenolysis in human muscle at rest and during exercise. Journal of Applied Physiology 53: 708–715, 1982
Cheetham ME, Boobis LH, Brooks S, Williams C. Human muscle metabolism during sprint running. Journal of Applied Physiology 61: 54–60, 1986
Clarkson PM, Kroll W, McBride TC. Maximal isometric strength and fiber type composition in power and endurance athletes. European Journal of Applied Physiology 44: 35–42, 1980
Colliander EB, Tesch PA. Effects of eccentric and concentric muscle actions in resistance training. Acta Physiologica Scandinavica 140: 31–39, 1990
Costill DL, Coyle EF, Fink WF, Lesmes GR, Witzmann S. Adaptations in skeletal muscle following strength training. Journal of Applied Physiology 46: 96–99, 1979
Coyle EF, Feiring DC, Rotkis TC, Cote RW, Roby FB, et al. Specificity of power improvements through slow and fast isokinetic training. Journal of Applied Physiology 51: 1437–1442, 1981
Craig BW, Lucas J, Pohlman R, Stelling H. The effects of running, weightlifting and a combination of both on growth hormone release. Journal of Applied Sports Science Research 5: 198–203, 1991
Danieli-Betto D, Zerbato E, Betto R. Type 1, 2A and 2B myosin heavy chain electrophoretic analysis of rat muscle fibres. Biochemical and Biophysical Research Communications 138: 981–987, 1986
Danieli-Betto D, Betto R, Midrio M. Calcium sensitivity and myofibrillar protein isoforms of rat skinned skeletal muscle fibres. Pflugers Archiv — European Journal of Physiology 417: 303–308, 1990
Darr KC, Schultz E. Exercise-induced satellite cell activation in growing and mature skeletal muscle. Journal of Applied Physiology 63: 1816–1821, 1987
Dons B, Bollerup K, Bonde-Peterson F, Hancke S. The effect of weight-lifting exercise related to muscle fiber composition and muscle cross-sectional area in humans. European Journal of Applied Physiology and Occupational Physiology 40: 95–106, 1979
Dudley GA, Djamil R. Incompatibility of endurance- and strength-training modes of exercise. Journal of Applied Physiology 54: 582–586, 1985
Dudley GA. Metabolic consequences of restive-type exercise. Medicine and Science in Sports and Exercise 20 (Suppl. 5): s158–s161, 1988
Etemadi AA, Husseini F. Frequency and size of muscle fibres in athletic body builders. Anatomical Record 162: 269–274, 1968
Fitts RH, McDonald KS, Schluter JM. The determinants of skeletal muscle force and power: their adaptability with changes in activity pattern. Journal of Biomechanics 24 (Suppl): 111–122, 1991
Frontera WR, Meredith CN, O’Reilly KP, Knuttgen HG, Evans WJ. Strength conditioning in older men: skeletal muscle hypertrophy and improved function. Journal of Applied Physiology 64: 1038–1044, 1988
Gollnick PD, Karlsson J, Piehl K. Selective glycogen depletion in skeletal muscle fibres of man following sustained contractions. Journal of Physiology 241: 59–67, 1974a
Gollnick PD, Piehl K, Saltin B. Selective glycogen depletion pattern in human muscle fibres after exercise of varying intensity and at varying pedalling rates. Journal of Physiology 241: 45–57, 1974b
Gollnick PD, Timson BF, Moore RL, Riedy M. Muscular enlargement and number of fibers in skeletal muscles of rats. Journal of Applied Physiology 50: 936–943, 1981a
Gollnick PD, Pernow B, Essen B, Jansson E, Saltin B. Availability of glycogen and plasma FFA for subtrate utilization in leg muscle of man during exercise. Clinical Physiology 1: 27–42, 1981b
Gollnick PD, Parsons D, Riedy M, Moore RL, Timson DJ. An evaluation of mechanisms modulating muscle size in response to varying perturbations. In Borer et al. (Eds) Frontiers of exercise biology, pp. 27–50, Human Kinetics Publishers, Champaign 1983a
Gollnick PD, Parsons D, Riedy M, Moore RL. Fibre number and size in overloaded chicken anterior latissimus dorsi muscle. Journal of Applied Physiology 54: 1292–1297, 1983b
Gonyea WJ, Ericson GC. An experimental model for the study of exercise-induced skeletal muscle hypertrophy. Journal of Applied Physiology 40: 630–633, 1976
Gonyea WJ, Ericson GC, Bonde-Petersen F. Skeletal muscle fiber splitting induced by weight-lifting exercise in cats. Acta Physiologica Scandinavica 99: 105–109, 1977
Gonyea WJ. Role of exercise in inducing increases in skeletal muscle fiber number. Journal of Applied Physiolology 48: 421–426, 1980
Gonyea WJ. Skeletal muscle growth induced by strength training. In Borer et al. (Eds) Frontiers of exercise biology, pp. 15–26, Human Kinetics Publisher, Champaign, 1983
Gonyea WJ, Sale DG, Gonyea FB, Mikesky A. Exercise induced increases in muscle fiber number. European Journal of Applied Physiology and Occupational Physiology 55: 137–141, 1986
Greaser ML, Moss RL, Reiser PJ. Variations in contractile properties of rabbit single muscle fibres in relation to troponin and myosin light chains. Journal of Physiology 406: 85–98, 1988
Green HJ. Myofibrillar composition and mechanical function in mammalian skeletal muscle. Sciences Reviews 1: 43–64, 1992
Gregory P, Low RB, Stirewalt WS. Changes in skeletal-muscle myosin isoenzymes with hypertrophy and exercise. Biochemical Journal 238: 55–63, 1986
Grimby G, Bjorntorp P, Fahlen M, Hoskins TA, Hook O, et al. Metabolic effects of isometric training. Scandinavian Journal of Clinical Laboratory Investigation 31: 301–305, 1973
Haggmark T, Jansson E, Svane B. Cross-sectional area of the thick muscle in man measured by computed tomography. Scandinavian Journal of Clinical Laboratory Investigation 38: 355–360, 1978
Hakkinen K, Komi PV. Muscle hypertrophy in body builders. European Journal of Applied Physiology and Occupational Physiology 44: 35–42, 1980
Hakkinen K, Komi PV, Tesch PA. Effect of combined concentric and eccentric strength training and detraining on force-time, muscle fibre, and metabolic characteristics of leg extensor muscles. Scandinavian Journal of Sports Sciences 3: 50–58, 1981
Harris RC, Edwards RHT, Hultman E, Nordsjo LO, Nyland B, et al. The time course of phosphoryl creatine resynthesis during recovery of the quadriceps muscle in men. Pflugers Archiv — European Journal of Physiology 367: 137–142, 1976
Hather B, Tesch P, Buchanan P, Dudley G. Eccentric actions and skeletal muscle adaptations to resistance training. Medicine and Science in Sports and Exercise 23: S130, 1991a
Hather BM, Tesch PA, Buchanan P, Dudley GA. Influence of eccentric actions on skeletal muscle adaptations to resistance training. Acta Physiologica Scandinavica 143: 177–185, 1991b
Hather B, Adams G, Baldwin K, Dudley G. Biochemical confirmation of histochemically determined fiber type transformation in human skeletal muscle. Medicine and Science in Sports and Exercise 24: S126, 1992
Hickson RC. Interference of strength development by simultaneously training for strength and endurance. Journal of Applied Physiology 45: 255–263, 1980
Hirvonen J, Rehunen S, Rusko H, Harkonen M. Breakdown of high-energy phosphate compounds and lactate accumulation during short suprarnaximal exercise. European Journal of Applied Physiology and Occupational Physiology 56: 253–259, 1987
Ho KW, Roy RR, Tweedle CD, Heusner WW, Van Huss WD, et al. Skeletal muscle fibre splitting with weight lifting exercise in rats. American Journal of Anatomy 157: 433–440, 1980
Houston ME, Froese EA, Valeriote SP, Green HJ, Ranney DA. Muscle performance, morphology and metabolic capacity during strength training and detraining: a one leg model. European Journal of Applied Physiology 51: 25–35, 1983
Jacobs I, Kaiser P, Tesch P. Muscle strength and fatigue after selective glycogen depletion in human skeletal muscle fibres. European Journal of Applied Physiology 46: 47–53, 1981
Jacobs I, Bar-Or O, Karlsson J, Dotan R, Tesch P. Changes in muscle metabolism in females with 30-s exhaustive exercise. Medicine and Science in Sports and Exercise 14: 457–460, 1982
Jansson E, Dudley GA, Norman B, Tesch PA. ATP and IMP in single muscle fibres after high intensity exercise. Clinical Physiology 7: 337–345, 1987
Jenny E, Weber H, Lutz H, Billeter R. Fiber populations in rabbit skeletal muscles from birth to old age. In Pette (Ed.) Plasticity of Muscle, pp. 97–109, Walter de Gruyter, Berlin, 1980
Jolesz F, Streter FA. Development, innervation, and activity-pattern induced changes in skeletal muscle. Annual Reviews of Physiology 43: 531–552, 1981
Karapondo D, Staron R, Hagerman F. The time course for fast-twitch fiber type conversions in resistance trained men and women. Medicine and Science in Sports and Exercise 23: S130, 1991
Karlsson J, Nordesco LO, Jorfeldt L, Saltin B. Muscle lactate, ATP and CP levels during exercise after physical training in man. Journal of Applied Physiology 33: 194–203, 1972
Karlsson J, SjodinB, Thorstensson A, Hulten B, Frith K. LDH isoenzymes in skeltal muscles of endurance and strength trained athletes. Acta Physiologica Scandinavica 93: 150–156, 1975
Katz A, Sahlin K, Henriksson J. Muscle ammonia metabolism during isometric contraction in humans. American Journal of Physiology 250: c834–c840, 1986
Keul J, Haralambie G, Bruder M, Gottstein H-J. The effect of weight lifting exercise on heart rate and metabolism in experienced weight lifters. Medicine and Science in Sports and Exercise 10: 13–15, 1978
Klitgaard H, Bergman O, Betto R, Salviati G, Schiaffino S, et al. Co-existence of myosin heavy chain I and IIa isoforms in human skeletal muscle fibres with endurance training. Pflugers Archiv— European Journal of Physiology 416: 470–472, 1990a
Klitgaard H, Zhou M, Schiaffino S, Betto R, Salviati G, et al. Aging alters the myosin heavy chain composition of single fibres from human skeletal muscle. Acta Physiologica Scandinavica 140: 55–62, 1990b
Klitgaard H, Zhou M, Richter EA. Myosin heavy chain compostion of single fibres from m. biceps brachii of male bodybuiders. Acta Physiologica Scandinavica 140: 175–180, 1990c
Komi PV, Viitasalo JHT, Havu M, Thorstensson SA, Sjodin B, et al. Skeletal muscle fibers and muscle enzyme activities in monozygous and dizygous twins of both sexes. Acta Physiologica Scandinavica 100: 385–392, 1977
Komi PV, Viitasalo J, Rauramaa R, Vihko V. Effect of isometric strength training on mechanical, electrical and metabolic aspects of muscle function. European Journal of Applied Physiology and Occupational Physiology 52: 104–106, 1978
Krotkiewski M, Aniansson G, Grimby P, Bjorntorp P, Sjostrom L. The effect of unilateral isokinetic strength training on local adipose and muscle tissue morphology, thickness and enzymes. European Journal of Applied Physiology and Occupational Physiology 42: 181–271, 1979
LaFramboise WA, Daod M, Guthrie RD, Moretti P, Schiaffino S, et al. Electrophoretic separation and immunological identification of type 2X myosin heavy chain in rat skeletal muscle. Biochemica et Biophysica Acta 1035: 109–112, 1990
Larsson L, Tesch PA. Motor unit fibre density in extremely hypertrophied skeletal muscle in men: muscle electrophysiological signs of fibre hyperplasia. European Journal of Applied Physiology and Occupational Physiology 55: 130–136, 1986
Larsson L, Edstrom L, Lindegren B, Gorza L, Schiaffino S. MHC composition and enzyme-histochemical and physiological properties of a novel fast-twitch motor unit type. American Journal of Physiology 261: c93–cl01, 1991
Lesmes GR, Costill DL, Coyle EF, Fink WJ. Muscle strength and power changes during maximal isokietic training. Medicine and Science in Sports and Exercise 10: 266–269, 1978
Lexell J, Henriksson-Larsen K, Sjostrom M. Distribution of different fibre types in human skeletal muscles. Acta Physiologica Scandinavica 117: 115–122, 1983
Lovind-Andersen J, Klitgaard H, Bangsbo J, Saltin B. Myosin heavy chain expression in human skeletal muscle of elite soccer players: effect of strength training. Acta Physiologica Scandinavica 143: 24a, 1991
MacDougall JD, Ward GR, Sale DG, Sutton JR. Biochemical adaptation of human skeletal muscle to heavy resistance training and immobilization. Journal of Applied Physiology 43: 700–703, 1977
MacDougall JD, Sale DG, Moroz JR, Elder GCB, Sutton JR, et al. Mitochondrial volume density in human skeletal muscle following heavy resistance training. Medicine and Science in Sports and Exercise 11: 164–166, 1979
MacDougall JD, Elder GCB, Sale DG, Moroz JR, Sutton JR. Effects of strength training and immobilization on human muscle fibres. European Journal of Applied Physiology and Occupational Physiology 43: 25–34, 1980
MacDougall JD, Sale DG, Elder GCB, Sutton JR. Muscle ultrastructural characteristics of elite powerlifters and bodybuilders. European Journal of Applied Physiology and Occupational Physiology 48: 117–126, 1982
MacDougall JD, Sale DG, Alway SE, Sutton JR. Muscle fiber number in biceps brachii in bodybuilders and control subjects. Journal of Applied Physiology 57: 1399–1403, 1984
MacDougall JD. Morphological changes in human skeletal muscle following strength training and immobilization. In Jones & McComas (Eds) Human muscle power, pp. 269–288, Human Kinetics, Champaign, 1986
Maughan RJ. Relationship between muscle strength and muscle cross-sectional area. Implications for training. Sports Medicine 1: 263–269, 1984
Miller AEJ, MacDougall JD, Tarnopolsky MA, Sale DG. Gender differences in strength in muscle fiber characteristics. European Journal of Applied Physiology 66: 254–262, 1993
Nelson AG, Arnall DA, Loy SF, Silvester LJ, Conlee RK. Consequences of combining strength and endurance training regimens. Physical Therapy 70: 287–294, 1990
Nygaard E, Houston M, Suzuki Y, Jorgensen K, Saltin B. Morphology of the brachial biceps muscle and elbow flexion in men. Acta Physiologica Scandinavica 117: 287–292, 1983
Oakley CR, Gollnick PD. Conversion of rat muscle fiber types. Histochemistry 83: 555–560, 1985
Periasamy M, Gregory P, Martin BJ, Stirewalt WS. Regulation of myosin heavy chain gene expression during skeletal muscle hypertrophy. Biochemical Journal 257: 691–698, 1989
Pette D. Activity-induced fast to slow transitions in mammalian muscle. Medicine and Science in Sports and Exercise 16: 517–528, 1984
Pettigrew FP, Noble EG. Shifts in rat plantaris motor unit characteristics with aging and compensatory overload. Journal of Applied Physiology 71: 2363–2368, 1991
Prince FP, Hikida RS, Hagerman FC. Human muscle fiber types in power lifters, distance runners and untrained subjects. Pflugers Archiv — European Journal of Physiology 363: 19–26, 1976
Reiser PJ, Moss RL, Giulian GG, Greaser ML. Shortening velocity in single fibers from adult rabbit soleus muscles is correlated with myosin heavy chain composition. Journal of Biological Chemistry 260: 9077–9080, 1985
Reitsma W. Skeletal muscle hypertrophy after heavy exercise in rats with surgically reduced muscle function. American Journal of Physical Medicine 48: 237–258, 1969
Robergs RA, Pearson DR, Costill DL, Pascoe DD, Fink WJ, et al. Glycogen metabolism during and following weight-resistance exercise of different intensities. Journal of Applied Sport Sciences Research 4: 106, 1990
Sale DG, MacDougall JD, Jacobs I, Garner S. Interaction between concurrent strength and endurance training. Journal of Applied Physiology 68: 260–270, 1990
Sale DG, Marten JE, Moroz DE. Hypertrophy without increased isometric strength after weight training. European Journal of Applied Physiology and Occupational Physiology 64: 51–55, 1992
Saltin B, Henriksson J, Nygaard E, Anderson P, Jansson E. Fibre types and metabolic potentials of skeletal muscle in sedentary men and endurance runners. Annals of the New York Academy of Sciences 301: 3–29, 1977
Salviati G, Betto R, Danieli-Betto D. Polymorphism of myofibrillar proteins of rabbit skeletal muscle fibres. Biochemical Journal 207: 261–272, 1982
Schantz P. Capillary supply in hypertrophied human skeletal musle. Acta Physiologica Scandinavica 114: 635–637, 1982
Schantz P, Randall-Fox E, Hutchinson W, Tyden A, Astrand PO. Muscle fibre type distribution, muscle cross-sectional area and maximal voluntary strength in humans. Acta Physiologica Scandinavica 117: 219–226, 1983
Schiaffino S, Ausoni S, Gorza L, Saggin L, Gundersen K, et al. Myosin heavy chain isoforms and velocity of shortening of type 2 skeletal muscle fibres. Acta Physiologica Scandinavica 134: 575–576, 1988
Shenkman BS, Saraeva LA. Relationship of various types of fibres in skeletal muscle as a factor affecting the efficacy of endurance training sports. Training, Medicine and Rehabilitation 1: 101–105, 1989
Sherman WM, Armstrong LE, Murray TM, Hagerman FC, Costill DL, et al. Effect of a 42.2 km footrace and subsequent rest or exercise on muscular strength and work capacity. Journal of Applied Physiology 57: 1668–1673, 1984
Sjostrom M, Lexell J, Eriksson A, Taylor CC. Evidence of fibre hyperplasia in human skeletal muscles from healthy young men? European Journal of Applied Physiology and Occupational Physiology 62: 301–304, 1991
Staron RS, Hagerman FC, Hikida RS. The effects of detraining on an elite power lifter. Journal of Neurological Sciences 51: 247–257, 1981
Staron RS, Hikida RS, Hagerman FC. Myofibrillar ATPase activity in human muscle fast-twitch subtypes. Histochemistry 78: 405–408, 1983a
Staron RS, Hikida RS, Hagerman FC. Re-evaluation of human muscle fast-twitch subtypes: evidence for a continuum. Histochemistry 78: 33–39, 1983b
Staron RS, Hikida RS, Hagerman FC, Dudley GA, Murray TF. Human skeletal muscle fiber type adaptability to various workloads. Journal of Histochemical Cytochemistry 32: 146–154, 1984
Staron RS, Pette D. Correlation between myofibrillar ATPase activity and myosin heavy chain composition in rabbit muscle fibers. Histochemistry 86: 19–23, 1986
Staron RS, Pette D. The multiplicity of combinations of myosin light chains and heavy chains in histochemically typed single fibres. Rabbit soleus muscle. Biochemical Journal 243: 687–693, 1987a
Staron RS, Pette D. The multiplicity of combinations of myosin light chains and heavy chains in histochemically typed single fibres. Rabbit anterior muscle. Biochemical Journal 243: 695–699, 1987b
Staron RS, Malicky ES, Leonardi MJ, Falkel JE, Hagerman FC et al. Muscle hypertrophy and foot fibre type conversions in heavy resistance-trained women. European Journal of Applied Physiology 60: 71–79, 1990
Staron RS, Leonardi MJ, Karapondo DL, Malicky ES, Falkel JE, et al. Strength and skeletal muscle adaptations in heavy-resistance-trained women after detraining and retraining. Journal of Applied Physiology 70: 631–640, 1991
Staron RS, Hikida RS. Histochemical, biochemical, and ultra-structural analyses of single human muscle fibres, with special reference to the C-fibre population. Journal of Histochemical Cytochemistry 40: 563–568, 1992
Sugiura T, Morimoto A, Murakami N. Effects of endurance training on myosin heavy chain isoforms and enzyme activity in the rat diaphragm. Pflugers Archiv — European Journal of Physiology 421: 77–81, 1992
Sweeney HL, Kushmerick MJ, Maberchi K, Sreter FA, Gergely J. Myosin alkali light chain and heavy chain variations correlate with altered shortening velocity of isolated skeletal muscle fibers. Journal of Biological Chemistry 263: 9034–9039, 1988
Swynghedauw B. Developmental and functional adaptation of contractile proteins in cardiac and skeletal muscles. Physiological Reviews 66: 710–771, 1986
Tamaki T, Uchiyama S, Nakano S. A weight-lifting exercise model for inducing hypertrophy in the hindlimb muscles of rats. Medicine and Science in Sports and Exercise 24: 881–886, 1992
Taylor AW, Essen B, Saltin B. Myosin ATPase in skeletal muscle of healthy men. Acta Physiologica Scandinavica 91: 568–570, 1974
Taylor NAS, Wilkinson JG. Exercise-induced skeletal muscle growth. Hypertrophy or hyperplasia? Sports Medicine 3: 190–200, 1986
Termin A, Staron RS, Pette D. Myosin heavy chain isoforms in histochemically defined fiber types of rat muscle. Histochemistry 92: 453–457, 1989
Tesch PA, Larsson L. Muscle hypertrophy in bodybuilders. European Journal of Applied Physiology and Occupational Physiology 49: 301–306, 1982
Tesch PA, Wright JE, Daniels WL, Sjodin B. Physical performance and muscle metabolic characteristics. In Knuttgen et al. (Eds) Biochemistry of exercise, pp. 258–263, Human Kinetics Publishers, Champaign, 1983 a
Tesch PA, Komi PV, Jacobs I, Karlsson J, Viitasalo JT. Influence of lactate accumulation on EMG frequency spectrum during repeated concentric contractions. Acta Physiologica Scandinavica 119: 61–67, 1983b
Tesch PA, Lindberg S. Blood lactate accumulation during arm exercise in world class kayak paddlers and strength trained athletes. European Journal of Applied Physiology and Occupational Physiology 52: 441–445, 1984
Tesch PA, Karlsson J. Muscle fiber types and size in trained and untrained muscles of elite athletes. Journal of Applied Physiology and Occupational Physiology 59: 1716–1720, 1985
Tesch PA, Colliander EB, Kaiser P. Muscle metabolism during intense, heavy-resistance exercise. European Journal of Applied Physiology and Occupational Physiology 55: 362–366, 1986
Tesch PA, Komi PV, Hakkinen K. Enzymatic adaptations consequent to long-term strength training. International Journal of Sports Medicine 8: 66–69, 1987
Tesch PA. Skeletal muscle adaptations consequent to long-term heavy resistance exercise. Medicine and Science in Sports and Exercise 20: s132–s134, 1988
Tesch PA, Thorsson A, Essen-Gustavsson B. Enzyme activities of FT and ST muscle fibres in heavy-resistance trained athletes. Journal of Applied Physiology 67: 83–87, 1989
Thorstensson A, Hulten B, Dobeln W, Karlsson J. Effect of strength training on enzyme activities and fibre characteristics in human skeletal muscle. Acta Physiologica Scandinavica 96: 392–398, 1976
Thorstensson A, Karlsson J. Fatiguability and fibre composition of human skeletal muscle. Acta Physiologica Scandinavica 98: 318–322, 1976
Timson BF. Evaluation of animal models for the study of exercise-induced muscle enlargement. Journal of Applied Physiology 69: 1935–1945, 1990
Tsika RW, Herrick RE, Baldwin KM. Interaction of compensatory overload and hindlimb suspension on myosin isoform expression. Journal of Applied Physiolgy 62: 2180–2186, 1987a
Tsika RW, Herrick RE, Baldwin KM. Time course adaptations in rat skeletal muscle isomyosins during compensatory growth and regression. Journal of Appied Physiology 63: 2111–2121, 1987b
Viitasalo JT, Komi PV. Force time characteristics and fibre composition in human extensor muscles. European Journal of Applied Physiology and Occupational Physiology 40: 7–15, 1978
Wagner PD, Giniger E. Hydrolysis of ATP and reversible binding to F-actin by myosin heavy chains free of all light chains. Nature 292: 560–562, 1981
Weeds A. Myosin light chains, polymorphism and fibre types in skeletal muscles. In Pette (Ed.) Plasticity of muscle, pp. 55–68, Walter de Gruyter, Berlin, 1980
Whalen RG. Myosin isoenzymes as molecular markers for muscle physiology. Journal of Experimental Biology 115: 43–53, 1985
Yarasheski KE, Lemon PWR, Gilloteaux J. Effect of heavy-resistance exercise training on muscle fiber composition in young rats. Journal of Applied Physiology 69: 434–437, 1990
Young A. The relative isometric strength of type I and type II muscle fibres in the human quadriceps. Clinical Physiology 4: 23–32, 1984
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Abernethy, P.J., Jürimäe, J., Logan, P.A. et al. Acute and Chronic Response of Skeletal Muscle to Resistance Exercise. Sports Med. 17, 22–38 (1994). https://doi.org/10.2165/00007256-199417010-00003
Published:
Issue Date:
DOI: https://doi.org/10.2165/00007256-199417010-00003