Abstract
Proliferation suppression and apoptosis are the prominent characteristics induced by heat stress (HS) in cells, whereas the effects of HS on cell growth (mass accumulation) are unknown. In this study, Lantang swine (an indigenous breed of China) skeletal muscle satellite cells (SCs) were pre-cultured at 37 °C for 24 h. The HS group was subjected to HS at 41 °C, while the control group was maintained at 37 °C. Heat shock protein 70 (HSP70) expression and SC size are significantly increased (P<0.05) by HS, but cell proliferation is suppressed (P<0.05) and apoptosis is induced (P<0.05). HS led to a lower percentage of SCs in the G0/G1 phase (P<0.05) together with a higher percentage of SCs in the S phase (P<0.05). However, the percentage of SCs in the G2/M phase was decreased (P<0.05) at 48 h but then increased (P<0.05) at 72 h with HS. In addition, the phosphorylation ratios of protein kinase b (Akt), ribosomal protein S6 kinase (S6K), and ribosomal protein S6 were increased (P<0.05) by HS. Nevertheless, the phosphorylation ratios of the 4E binding protein 1 and the eukaryotic initiation factor-4E were indistinguishable (P>0.05) from those of the control group. The phosphorylation ratio of the mammalian target of rapamycin (mTOR) (Ser2448) increased(P<0.05) within 48 h, and apparent differences were abrogated at 72 h (P>0.05). Moreover, cleaved caspase-3 expression was increased at 72 h (P<0.05). These findings indicate that HS induces apoptosis and disrupts cell cycle distribution to decrease the number of cells. Additionally, HS can promote SC growth via an activated Akt/mTOR/S6K signaling pathway.
概要
目的
探究热应激对蓝塘猪骨骼肌卫星细胞增殖与生长的影响, 分析其作用机制。
创新点
通过体外细胞模型研究了热应激对猪骨骼肌卫星细胞增殖与生长的影响,发现热应激通过 Akt/mTOR/S6K 途径调控细胞的生长。
方法
本试验选用1 日龄蓝塘猪背最长肌的骨骼肌卫星细胞, 对照组为37 °C 正常培养, 热应激组培养温度为41 °C, 其它培养条件相同。采用细胞计数、MTT 法、流式细胞术等手段分析比较热应激对蓝塘猪骨骼肌卫星细胞增殖与生长的影响; 利用实时逆转录聚合酶链式反应 (RT-PCR) 和蛋白质印迹法 (Western blot) 等方法, 检测了热休克蛋白HSP70、半胱氨酸蛋白酶-3 (caspase-3) 及哺乳动物雷帕霉素靶蛋白 (mTOR) 信号通路关键基因的表达。
结论
(1) 41 °C 热处理可引起蓝塘猪骨骼肌卫星细胞产生热应激反应; (2) 热应激72 h 会通过改变细胞周期、抑制细胞增殖并诱导细胞凋亡等共同作用减少细胞数目; (3) 热应激通过Akt/mTOR/S6K途径调控细胞的生长。
Similar content being viewed by others
1 Introduction
It has been demonstrated that when cells or organisms are subjected to abnormally high environmental temperatures, they undergo an active heat shock response (Velichko et al., 2013). An increase in heat shock proteins (HSPs) is a well-characterized feature of the heat shock response (Lindquist, 1986), and studies have demonstrated that heat shock protein 70 (HSP70) can be an indicator of heat stress (HS) in different species (Kamanga-Sollo et al., 2011; Sirotkin and Bauer, 2011). It is well established that the effects of HS vary depending on the strength and duration of stress exposure (Li S.Q. et al., 2012; Rezai Rad et al., 2013).
In addition to the decrease in growth rate, feed intake, and reproduction (Pearce et al., 2013; Morales et al., 2014), studies in many species have indicated that muscle growth is also affected by HS-related alterations in muscle physiology (Liu et al., 2009; Locke and Celotti, 2014). Because muscle fiber number is fixed before birth, the rate and extent of postnatal muscle growth is determined by satellite cells (SCs) (Millward et al., 1976). Consequently, the effects of HS on the proliferation, growth, and apoptosis of skeletal muscle SCs could play a crucial role in determining the impact of HS on muscle physiology.
Cell number is dependent on a balance between cell proliferation and cell death, whereas cell size is dependent on cell growth (Tumaneng et al., 2012). Cell fate is reasonable to speculate that HS may influence cell proliferation and apoptosis. Studies have reported that HS can induce cell division arrest, and exposure of cells to acute or chronic HS induces cell death via apoptosis, necrosis, or autophagy (Kühl and Rensing, 2000; Li Y. et al., 2012; Zhang et al., 2012). A number of studies have reported the effects of HS on cell number. However, to our knowledge, there is little known on effects of HS on cell growth. The mammalian target of rapamycin (mTOR) pathway has been reported to regulate cell growth and proliferation via its two well-established substrates, i.e. ribosomal protein S6 kinases (S6Ks) and eukaryotic translation initiation factor 4E-binding protein 1 (4EBP1), respectively (Dowling et al., 2010). Indeed, a recent in vivo study showed that HS activates the Akt/mTOR signaling pathway in rat skeletal muscle (Yoshihara et al., 2013). However, it remains unclear whether the Akt/mTOR signaling pathway participates in the regulation of cell proliferation during HS.
The Langtang pig is an indigenous breed of south China that dominates the market in that region due to its earlier sexual maturity and better meat quality (Wang et al., 2012). Furthermore, compared with most commercial breeds of pigs, the Langtang pig has a stronger adaptive capacity in areas suffering from HS. In light of the crucial role of cell number and growth in determining skeletal muscle mass, the aim of this study was to determine whether the proliferation, apoptosis, and growth of Lantang swine skeletal muscle SCs are altered during HS.
2 Materials and methods
The study was conducted with approval and in accordance with the directives of the Institutional Animal Care and Use Committee of South China Agricultural University, Guangzhou, China.
2.1 Cell culture and experimental design
SCs were isolated from the longissimus dorsi muscles of new-born Lantang swine, so that the majority of the SCs were purified from fast-twitch muscles, and the resulting mononucleated cell preparations were prepared for immunocytochemical analysis using previously described procedures (Wang et al., 2012; Gao et al., 2015). SCs were grown serially in plastic culture flasks in Dulbecco’s modified Eagle medium/nutrient mixture F-12 (DMEM/F-12) containing 10% fetal bovine serum (FBS). At confluence, cells were trypsinized and seeded in 96- or 6-well cell culture plates with approximately 1×104 or 5×104 cells/well, respectively, and maintained at 37 °C in a 5% CO2 incubator. After overnight (24 h) incubation, half of the cell culture plates were transferred to another incubator and maintained at 41 °C sustained 120 h for the duration of the HS research. The medium was changed every 2 d. At least three independent experiments were performed to verify the results and the cells were isolated from a variety of piglets per replicate.
2.2 Cell proliferation activity analysis
SCs were seeded in 96- or 6-well cell culture plates with approximately 1×104 or 5×104 cells/well, respectively. The effects of HS on cell proliferation were determined by cell count assay and 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) method after treatment of 24, 48, 72, 96, and 120 h. For MTT analysis, 20 μl 5 mg/ml MTT (Sigma, St. Louis, MO, USA) solutions were added to each well and incubated for 4 h. The plates were centrifuged at 1400g for 15 min at 25 °C, and the supernatants were carefully discarded. A total of 200 μl DMSO working solution (180 μl DMSO plus 20 μl 1 mol/L HCl) were added to each well. The optical density (OD) value of the yellow reaction product was evaluated with an enzyme-linked immunosorbent assay (ELISA) reader at a wavelength of 490 nm (n=20). For cell counting assay, 6-well cell culture plates were gently washed with phosphate buffered saline (PBS) and viable cells were counted using a hemocytometer under a light microscope (n=6).
2.3 Flow cytometry
SCs were cultured in 6-well cell culture plates and cells were collected at 24, 48, and 72 h, respectively, as previously described (Gao et al., 2015). Collected cells were fixed with 70% ice cold ethanol for cell cycle and cell size analysis, and cells were fixed with binding buffer for cell apoptosis analysis. For cell cycle analysis, the cells were centrifuged at 200g for 10 min at 4 °C, re-suspended in 1 ml PBS, treated with 100 ml 200 mg/ml DNase-free RNase A, and incubated at 37 °C for 30 min. Finally, the cells were treated with 100 μl 50 μg/ml propidium iodide (PI) and incubated at room temperature (25 °C) for 10 min in the dark and subjected to flow cytometry using a Becton Dickinson FACScan (BD, Franklin Lake, NJ, USA). For cell size determination, fixed cells were washed twice with PBS and centrifuged at 200g for 10 min at 4 °C. Cell samples were then run on a Becton Dickinson FACScan. For cell apoptosis analysis, cells were mixed with 5 μl annexin V-FITC before flow cytometric analysis. Then, 5 μl of PI was added to cells and incubated at room temperature (25 °C) for 10 min in the dark. Cell samples were finally run on a Becton Dickinson FACScan (n=6).
2.4 RNA isolation and quantitative reverse transcriptase polymerase chain reaction (qRT-PCR)
Total RNA was isolated using the TRIzol reagent according to the manufacturer’s instructions (Invitrogen, Carlsbad, CA, USA). The concentration and purity of the extracted total RNA were measured by spectrophotometric evaluation at 260 and 280 nm (Thermo Scientific, Waltham, MA, USA), and the RNA integrity was determined using 1% agarose gel electrophoresis. Quantitative real-time RT-PCR was performed using a Stratagene MxPro 3005P thermocycler (Agilent Technologies, Santa Clara, CA, USA) and one-step SYBR Green PCR Mix (TaKaRa, Dalian, China) according to optimized PCR protocols. Cycling conditions were: 95 °C for 5 min followed by 40 cycles of 95 °C for 30 s, 60 °C for 30 s, and 72 °C for 40 s. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was amplified in parallel with the target genes as a control, and each sample was analyzed 12 times. The relative mRNA expression level was calculated using
(ΔCT=CT of target gene-CT of GAPDH gene) method with a temperature of 37 °C as the control (n=6).
The primer sequences (5' to 3') were as follows: HSP70: GTG GCT CTA CCC GCA TCC C (forward) and GCA CAG CAG CAC CAT AGG C (reverse) and GAPDH: GGT CGG AGT GAA CGG ATT TG (forward) and CCT TGA CTG TGC CGT GGA AT (reverse).
2.5 Western blot
Cells were washed twice with PBS and then lysed on ice with lysis buffer (50 mmol/ml Tris-HCl, pH 7.5, 150 mmol/L NaCl, 1 mmol/L ethylenediaminetetraacetic acid (EDTA), 0.5% Triton X-100, 1 mmol/L phenylmethanesulfonyl fluoride (PMSF), and 1 mmol/L Na3VO4) containing complete protease inhibitors (Invitrogen, Carlsbad, CA, USA). Subsequently, the samples were centrifuged at 12000g for 5 min at 4 °C to remove insoluble debris, and the protein concentration was determined with the BCA Protein Assay Reagent Kit (Thermo Fisher Scientific, San Jose, CA, USA). The protein samples were boiled for 10 min and 15 μg of lysates were subjected to 10% sodium dodecyl sulfate (SDS) gel electrophoresis following the manufacturer’s instructions (SDS-PAGE gel kit; Beyotime, Jiangsu, China). Proteins were separated by electrophoresis at 80 V for 15 min and 110 V for 90 min using Tris-glycine running buffer (0.025 mmol/L Tris base, 0.192 mol/L glycine, and 0.1% SDS, pH 8.3), as described previously (Gao et al., 2015). Prestained molecular weight markers (Invitrogen, Carlsbad, CA, USA) were used to determine the molecular weight of proteins. After the electrophoresis was complete, the samples were transferred to polyvinylidene fluoride (PVDF) membranes at 100 V for 70 min using a transfer buffer containing 25 mmol/L Tris, 192 mmol/L glycine, and 10% methanol. The membranes were blocked for 2 h with Tris buffer solution (TBS) (20 mmol/L Tris and 500 mmol/L NaCl, pH 7.6) containing 5% bovine serum albumin (BSA) at room temperature, and then incubated overnight at 4 °C with primary antibodies. The membranes were washed 6 times for 5 min each with TBST buffer (TBS-buffered saline with Tween-20), and then incubated with the appropriate horseradish peroxidase-labeled secondary antibody for 2 h at room temperature. Finally, the proteins were visualized with the electrochemiluminescence (ECL)-plus chemiluminescence detection kit (Beyotime Institute of Biotechnology, Haimen, Nantong, China) in a FluorChem M system (Cell Biosciences, San Leandro, CA, USA), and the density of the bands was analyzed using ImageJ (<http://rsb.info.nih.gov/ij>). The protein values of HSP70, cleaved caspase-3, and eukaryotic initiation factor-4E (eIF4E) are presented as expression relative to the β-actin level in each lane, while the other protein values are presented with phosphorylation levels relative to the total level. The primary antibodies 1:1000 rabbit polyclonal antibody against Akt, 1:1000 rabbit polyclonal antibody against phospho-Akt (Ser473), 1:1000 rabbit polyclonal antibody against mTOR, 1:1000 rabbit monoclonal antibody against phospho-mTOR (Ser2448), 1:1000 mouse monoclonal antibodies against S6K, 1:1000 rabbit polyclonal antibody against phospho-S6K (Thr389), 1:1000 rabbit polyclonal antibody anti-ribosomal protein S6 (rpS6), 1:1000 rabbit polyclonal antibody against phospho-rpS6 (Ser235/236), 1:1000 rabbit polyclonal antibody against 4EBP1, and 1:1000 rabbit polyclonal antibody against phospho-4EBP1 (Thr70) were purchased from Cell Signaling Technology (Beverly, MA, USA). 1:800 mouse monoclonal antibody against eIF4E (sc-9976) and 1:800 rabbit polyclonal antibody against cleaved caspase-3 (sc-7148) were purchased from Santa Cruz (Dallas, TX, USA). 1:1000 mouse monoclonal antibodies against HSP70 (MB0105) and anti-β-actin were purchased from Bioworld (Louis Park, MN, USA). The second antibodies, anti-rabbit and anti-mouse IgG, were purchased from Beijing Biosynthesis Biotechnology (Tongzhou, Beijing, China) (n=4).
2.6 Statistical analysis
Values are expressed as mean±standard error of mean (SEM). Comparisons between groups were performed using an independent samples t-test using SAS Version 9.2 (SAS Institute Inc., Cary, NC, USA) with statistical significance assumed when P<0.05.
3 Results
3.1 Heat shock response
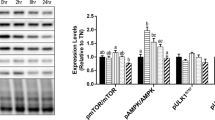
To determine whether the SCs cultured at 41 °C suffered from HS, the HSP70 mRNA and protein levels were assessed using qRT-PCR and Western blotting, respectively. As predicted, the HSP70 mRNA and protein expression levels were significantly increased (P<0.05) at 41 °C (Fig. 1). These results indicate that the temperature used is sufficient to induce a heat shock response in Lantang swine SCs.
Heat shock protein 70 (HSP70) messenger RNA (mRNA) and protein expression level in Lantang swine satellite cells with or without heat stress (HS)
After pre-incubation at 37 °C for 24 h, the incubator temperature of the HS group was increased to 41 °C and that of the control group was maintained at 37 °C. (a) Relative quantity of HSP70 mRNA (n=6). The vertical axis represents the target gene threshold cycle (CT) corrected for GAPDH. (b) The expression level of HSP70 protein was measured by Western blot, and β-actin was used as an internal control (n=4). The data represent the means±SEM. * P<0.05, HS treatment vs. the control. The results are representative of three separate experiments
3.2 Cell proliferation and cell growth
To evaluate the effects of HS on the proliferation of Lantang swine SCs, cell counting and MTT assays were conducted, and the results are shown in Figs. 2a and 2b. Our results indicate that HS stimulated swine SC proliferation (P<0.05) within 24 h, and there was no difference between the control and HS groups at 48 h (P>0.05). Compared with the control group, the SC number was decreased in the HS group beginning at 72 h after treatment (P<0.05); the results of the MTT assay were the same as those of cell counting, which reveal that continuous HS (72 h) effectively inhibits the proliferation of SCs. According to the proliferation results, we selected a heating time of 24 to 72 h to elucidate the effect of HS on SC growth. Cell size distribution was measured by flow cytometry (FCM; Fig. 2c), which show that HS unexpectedly increased the SC size (P<0.05) compared with the control group (Fig. 2d). A similar result was also found by hematoxylin-eosin staining (data not shown). These results demonstrated that HS suppresses SC proliferation and promotes SC growth.
Effect of heat stress (HS) on the proliferation and growth of Lantang swine satellite cells (SCs)
After pre-incubation at 37 °C for 24 h, the incubator temperature of HS group was increased to 41 °C and that of the control group was maintained at 37 °C. Cell numbers were determined using the cell count (n=6) (a) and MTT (n=20) (b) assays at the indicated time after HS. (c, d) Cell size was determined with flow cytometry at the indicated time (n=6). The data represent the means±SEM. * P<0.05, HS treatment vs. the control. The results are representative of three separate experiments
3.3 Cell cycle and apoptosis
It has been well established that cell division is regulated by the cell cycle, and accumulating evidence has revealed that HS inhibits cell proliferation mainly via G0/G1 and G2/M arrest (Kühl and Rensing, 2000). Therefore, we reasoned that the effect of heat on the proliferation of Lantang swine SCs is cell-cycle related. The phase distribution was monitored with FCM, and the DNA content distribution of the cells consisted of two predominant peaks corresponding to cells in the G0/G1 and G2/M phases and an intermediate region between both peaks contained cells in the S phase of the cell cycle (Fig. 3a). From our results, we observed a decrease in the percentage of cells in the G0/G1 phase (P<0.05; Fig. 3b) and an increase in the percentage of cells in the S phases (P<0.05; Fig. 3c). Furthermore, the percentage of cells in the G2/M phase (Fig. 3d) decreased at 48 h (P<0.05), whereas the percentage of cells increased at 72 h (P<0.05). In addition to cell cycle arrest, apoptosis is another factor that affects cell number; thus, to investigate this further, our apoptosis analysis revealed that HS significantly increased (P<0.05) apoptosis and the expression of cleaved caspase-3 (P<0.05) in Lantang swine SCs at 72 h (Fig. 4).
Analysis of cell cycle distribution in Lantang swine satellite cells (SCs)
After pre-incubation at 37 °C for 24 h, the incubator temperature of the heat stress (HS) group was increased to 41 °C and that of the control group was maintained at 37 °C. (a) The distribution of phase was monitored using flow cytometry (n=6). The two prominent peaks represent cells in the G0/G1 and G2/M phases, and the intermediate region between peaks represents the S phase cells. Percentages of cells in G0/G1 (b), S (c), and G2/M (d) phases were detected in flow cytometry analyses at the indicated time. The data represent the means±SEM. * P<0.05, HS treatment vs. the control. The results are representative of three separate experiments
3.4 Signaling responses
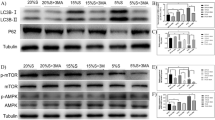
Considering the importance of the mTOR pathway for regulating cell proliferation, growth, and apoptosis, the phosphorylation ratios of mTOR pathway components are shown in Fig. 5. The total protein expression levels, with the exception of Akt, were decreased at 72 h, and there was no statistically significant change in mTOR, S6K, rpS6, or 4EBP1 after HS (data not shown). We found that the phosphorylation ratios of Akt (Ser473), S6K (Thr389), and rpS6 (Ser235/236) were significantly higher (P<0.05) in the HS than in the control group within 72 h, but no significant difference (P>0.05) was found for p-4EBP1 (Thr70) and eIF4E. HS significantly (P<0.05) up-regulated the p-mTOR (Ser2448) phosphorylation ratio within 48 h, but there was no significant difference (P>0.05) at 72 h.
4 Discussion
An HS response will be induced in organisms that have been exposed to HS. Studies of a number of species, tissues, and cells have shown that the HS response results in the accumulation of HSPs (Lindquist, 1986; Welch, 1992). The effects of HS are affected by the intensity and duration of HS since the long processing HS time or the high setting HS temperature can easily led to death of cells. Therefore, given that our treatment lasted for 120 h, we have chosen 41 °C for the HS-inducing temperature, which is approximately 1 °C above the core body temperature of swine (39.3–39.6 °C) and within the range of rectal temperatures observed in studies of heat-stressed swine (Liu et al., 2009). Increases in the HSP70 mRNA and protein level indicated that the temperature used in this study was sufficient to induce an HS response in swine SCs, which is consistent with Kamanga-Sollo et al. (2011).
Induction of apoptosis in Lantang swine satellite cell (SC) cultures after heat stress (HS)
After pre-incubation at 37 °C for 24 h, the incubator temperature of the HS group was increased to 41 °C and that of the control group was maintained at 37 °C. Typical histograms (a) and profiles (b) of HS-induced apoptosis was determined by AnV-FITC/PI staining after HS exposure and FCM analysis (n=6). (c) The expression of cleaved caspase-3 was determined by Western blot and β-actin was used as an internal control. The data represent the means±SEM. * P<0.05, HS treatment vs. the control. The results are representative of three separate experiments
The proliferative ability of SCs was measured using cell counting and MTT assay over a short period of 120 h. Our results showed that HS could inhibit SC proliferation and the inhibition was much more severe when the treating time was prolonged. These results are consistent with the view that effects of HS are determined by the duration and intensity of the temperature. Furthermore, cell cycle progression and cell growth interact with each other to sustain the proliferative response, and sustained proliferative responses are supported by continuous cell division (Li et al., 2014) and stress response pathways often evoke cell cycle arrest to avoid the distribution of damaged macromolecules, leading to proliferation inhibition (Costa et al., 2011). In addition, DNA damage is caused by both exogenous and endogenous agents resulting in different types of lesion. These lesions are then recognized by components of the different repair mechanisms. Cells always change the cell cycle distribution to deal with the stresses because these repair processes can take some time to complete. To understand how proliferation is controlled under HS, SCs were tested for DNA content after 24, 48, and 72 h of proliferation to determine the distribution of cells in the G0/G1, S, and G2/M phases of the cell cycle. The results showed that the percentage of cells in the G0/G1 phase decreased significantly but the percentage of cells in the S phase became significantly higher after HS than that observed in the control group. Interestingly, although the percentage of cells in the G2/M phase was significantly lower at 48 h, it was higher at 72 h after HS. These results are in accordance with Bang et al. (2000)’s observation that 15 min of HS increases the percentage of cells in the G2/M phase. We speculate that HS induced the DNA damage and ultimately led to cell cycle progress arrest. Heat shock sensitivity itself is cell cycle phase dependent, and various studies demonstrated that cells in S phase or mitosis are more sensitive to high temperatures than those in G1 or G2 phases (Velichko et al., 2013). There is also evidence that heat stress leads to a transient arrest of cells at mainly two cell cycle checkpoints, the G1/S and G2/M transitions (Furusawa et al., 2012). Because the PI3K/Akt/mTOR pathway has been proven to play a predominant role in cell cycle progression, we surmised that the change in the cell cycle distribution might be due to a change in the activation of the PI3K/Akt/mTOR pathway. Li Y. et al. (2012) have shown that decreases in the activity of the Akt/mTOR pathway inhibit cell proliferation by inducing G2/M arrest. Our data showed that although HS increased the Akt and mTOR phosphorylation ratio, the discrepancy in the mTOR phosphorylation ratio disappeared at 72 h. In combination with the increase in the G2/M phase, these results support the hypothesis that HS disrupts the cell cycle. In contrast with the recent report that 4EBPs control mTORC1-mediated cell proliferation (Dowling et al., 2010), the 4EBP1 phosphorylation ratio was indistinguishable from that of the control cells, and these results are supported by the study of rat skeletal muscle under HS (Yoshihara et al., 2013), which showed that HS activates the Akt/mTOR signaling pathway while the phosphorylation of 4EBP1 was shown to be similar among groups of rats in both the soleus and plantaris muscles. Thus, our data might indicate that the HS-mediated perturbation of SC cycle kinetics and reduction in SC proliferation are independent of 4EBP1 status. Furthermore, the SC proliferation may be dependent upon several other factors, including paired box 7, myogenic factor 5, myogenin, myostatin, etc. However, more studies are needed to support this speculation.
Expression of mammalian target of rapamycin (mTOR) pathway regulators in Lantang swine satellite cell (SC) cultures after heat stress (HS) (n=4)
After pre-incubation at 37 °C for 24 h, the incubator temperature of the HS group was increased to 41 °C and that of the control group maintained at 37 °C. The values of protein kinase b (Akt) (a), mTOR (b), ribosomal protein S6 kinase (S6K) (c), ribosomal protein S6 (rpS6) (d), and 4E binding protein 1 (4EBP1) (e) are presented with phosphorylation levels relative to the total level in each line, while the values of eukaryotic initiation factor-4E (eIF4E) (f) are presented as expression relative to the β-actin level. The data represent the means±SEM. * P<0.05, HS treatment vs. the control. The results are representative of three separate experiments
Cell growth and cell proliferation are clearly separable processes. Cell growth defines the mass accumulation or the size increase, and how cells control their size under various conditions is a fundamental open question (Taheri-Araghi et al., 2015). Highly dynamic cells can maintain their size for life but can also grow or shrink, requiring robust but adaptable controls, and the loss of the control can lead to a series of diseases (Lloyd, 2013). Our results demonstrate that HS significantly increased the SC size. As far as we know, this is the first study of HS effects on SCs growth. The PI3K/Akt/mTORC1 pathway has been shown to be a major regulator of cell growth through its downstream S6K1 rather than its S6K2 (Pende et al., 2004). Our results illustrate that HS altered the mTOR phosphorylation ratio of Ser2448 within 48 h. However, the phosphorylation ratio was indistinguishable from that of the control cells at 72 h. Combined with the change in the phosphorylation ratio of Akt, our results indicate that HS activated the Akt/mTOR signaling pathway. Despite the fact that there was no significant difference in 4EBP1, another downstream target of mTOR, the S6K phosphorylation ratio, increased after HS and S6K activation and this was confirmed by the phosphorylation of its substrate S6 ribosomal protein. In fact, Uehara et al. (2004) has shown that HS induced a significant increase in p-S6K expression after heat exposure at 41 °C. Thus, the results suggest that HS promotes SC growth via the Akt/mTOR/S6K pathway.
In addition to cell cycle arrest, apoptosis is another factor that determines cell number. Kühl and Rensing (2000) have indicated that cellular sensitivity to hyperthermia depends on the cell cycle stage and that cells in the S phase are more sensitive to death-inducing conditions. Our results showed that HS increased cell number in 24 h and decreased the cell number in 72 h. Therefore, we speculate that apoptosis may play an important role in the decrease of the number of SCs. As expected, the results reveal increases in the apoptosis percentage and expression of cleaved caspase-3 at 72 h, which is consistent with Shelton et al. (2010). Although Akt has been shown to prevent HS-induced cell death, the HS-mediated Akt phosphorylation ratio increased in a way that is consistent with that found in the study of Yoshihara et al. (2013) rather than that found in the study of Bang et al. (2000). In contrast, the increase in the Akt phosphorylation ratio was inconsistent with the fact that HS induced apoptosis at 72 h. There may be several factors other than Akt involved in SC apoptosis. Furthermore, it is possible that the increase in the Akt phosphorylation ratio in the HS treatment group may have been, at least partly, responsible for limiting massive SC death during this period. However, the relationship between Akt expression and SC death is pending investigation. In addition to indicating an HS response, HSP70 plays a crucial role in adjusting the proliferation of cells that have been exposed to stress, which may explain the increase in cell number at 24 h. However, in conditions of continuous stress (HS for 72 h), cellular defense mechanisms are insufficient to cope with the excessive stress, leading to induced apoptosis and a decrease in cell number. Taken together, this study indicates that short-term HS assists significantly in the promotion of SC proliferation and long-term HS decreases the number of SCs by suppressing proliferation and inducing apoptosis.
5 Conclusions
This study demonstrate that HS suppresses SC proliferation and induces apoptosis to decrease the number of cells. The main finding of this study is that HS promotes SC growth via the Akt/mTOR/S6K pathway.
Compliance with ethics guidelines
Chun-qi GAO, Yin-ling ZHAO, Hai-chang LI, Wei-guo SUI, Hui-chao YAN, and Xiu-qi WANG declare that they have no conflict of interest.
All institutional and national guidelines for the care and use of laboratory animals were followed.
References
Bang, O.S., Ha, B.G., Park, E.K., et al., 2000. Activation ofAkt is induced by heat shock and involved in suppressionof heat-shock-induced apoptosis of NIH3T3 cells. Biochem. Biophys. Res. Commun., 278(2):306–311. [doi:10.1006/bbrc.2000.3805]
Costa, M.R., Ortega, F., Brill, M.S., et al., 2011. Continuouslive imaging of adult neural stem cell division and lineageprogression in vitro. Development, 138(6):1057–1068.[doi:10.1242/dev.061663]
Dowling, R.J., Topisirovic, I., Alain, T., et al., 2010.mTORC1-mediated cell proliferation, but not cell growth, controlled by the 4E-BPs. Science, 328(5982):1172–1176.[doi:10.1126/science.1187532]
Furusawa, Y., Iizumi, T., Fujiwara, Y., et al., 2012. Inhibitionof checkpoint kinase 1 abrogates G2/M checkpoint activationand promotes apoptosis under heat stress. Apoptosis,17(1):102–122. [doi:10.1007/s10495-011-0660-7]
Gao, C.Q., Zhi, R., Yang, Z., et al., 2015. Low dose of IGF-Iincreases cell size of skeletal muscle satellite cells viaAkt/S6K signaling pathway. J. Cell Biochem., in press.[doi:10.1002/jcb.25212]
Kamanga-Sollo, E., Pampusch, M.S., White, M.E., et al., 2011. Effects of heat stress on proliferation, protein turnover,and abundance of heat shock protein messenger ribonucleicacid in cultured porcine muscle satellite cells. J.Anim. Sci., 89(11):3473–3480. [doi:10.2527/jas.2011-4123]
Kühl, N.M., Rensing, L., 2000. Heat shock effects on cell cycle progression. Cell Mol. Life Sci., 57(3):450–463. [doi:10.1007/PL00000707]
Li, C.M., Yan, H.C., Fu, H.L., et al., 2014. Molecular cloning,sequence analysis, and function of the intestinal epithelialstem cell marker Bmi1 in pig intestinal epithelial cells. J.Anim. Sci., 92(1):85–94. [doi:10.2527/jas.2013-7048]
Li, S.Q., Li, R.F., Xi, S.M., et al., 2012. Systematical analysisof impacts of heat stress on the proliferation, apoptosisand metabolism of mouse hepatocyte. J. Physiol. Sci.,62(1):29–43. [doi:10.1007/s12576-011-0183-6]
Li, Y., Zhang, P., Qiu, F., et al., 2012. Inactivation of PI3K/Akt signaling mediates proliferation inhibition and G2/Mphase arrest induced by andrographolide in human glioblastomacells. Life Sci., 90(25-26):962–967. [doi:10.1016/j.lfs.2012.04.044]
Lindquist, S., 1986. The heat-shock response. Annu. Rev. Biochem., 55:1151–1191. [doi:10.1146/annurev.bi.55.070186.005443]
Liu, F., Yin, J., Du, M., et al., 2009. Heat-stress-induceddamage to porcine small intestinal epithelium associatedwith down regulation of epithelial growth factor signaling. J. Anim. Sci., 87(6):1941–1949. [doi:10.2527/jas.2008-1624]
Lloyd, A.C., 2013. The regulation of cell size. Cell, 154(6):1194–1205. [doi:10.1016/j.cell.2013.08.053]
Locke, M., Celotti, C., 2014. The effect of heat stress on skeletalmuscle contractile properties. Cell Stress Chaperones, 19(4):519–527. [doi:10.1007/s12192-013-0478-z]
Millward, D., Garlick, P., Nnanyelugo, D., et al., 1976. The relative importance of muscle protein synthesis and breakdown in the regulation of muscle mass. Biochem. J.,156(1):185–188.
Morales, A., Grageola, F., Garcia, H., et al., 2014. Performance,serum amino acid concentrations and expressionof selected genes in pair-fed growing pigs exposed to highambient temperatures. J. Anim. Physiol. Anim. Nutr.,98(5):928–935. [doi:10.1111/jpn.12161]
Pearce, S.C., Gabler, N.K., Ross, J.W., et al., 2013. The effectsof heat stress and plane of nutrition on metabolism ingrowing pigs, J. Anim. Sci., 91(5):2108–2118. [doi:10.2527/jas.2012-5738]
Pende, M., Um, S.H., Mieulet, V., et al., 2004. S6K1(-/-)/S6K2(-/-) mice exhibit perinatal lethality and rapamycinsensitive5'-terminal oligopyrimidine mRNA translationand reveal a mitogen-activated protein kinase-dependentS6 kinase pathway. Mol. Cell Biol., 24(8):3112–3124.[doi:10.1128/mcb.24.8.3112-3124.2004]
Rezai Rad, M., Wise, G.E., Brooks, H., et al., 2013. Activationof proliferation and differentiation of dental follicle stemcells (DFSCs) by heat stress. Cell Prolif., 46(1):58–66.[doi:10.1111/cpr.12004]
Shelton, S.N., Dillard, C.D., Robertson, J.D., 2010. Activation of caspase-9, but not caspase-2 or caspase-8, is essentialfor heat-induced apoptosis in Jurkat cells. J. Biol. Chem.,285(52):40525–40533. [doi:10.1074/jbc.M110.167635]
Sirotkin, A.V., Bauer, M., 2011. Heat shock proteins in porcineovary: synthesis, accumulation and regulation bystress and hormones. Cell Stress Chaperones, 16(4):379–387. [doi:10.1007/s12192-010-0252-4]
Taheri-Araghi, S., Bradde, S., Sauls, J.T., et al., 2015. Cell sizecontrol and homeostasis in bacteria. Curr. Biol.,25(3):385–391. [doi:10.1016/j.cub.2014.12.009]
Tumaneng, K., Russell, R.C., Guan, K.L., 2012. Organ sizecontrol by hippo and tor pathways. Curr. Biol., 22(9):R368-R379. [doi:10.1016/j.cub.2012.03.003]
Uehara, K., Goto, K., Kobayashi, T., et al., 2004. Heat-stress enhances proliferative potential in rat soleus muscle. Jpn.J. Physiol., 54(3):263–271. [doi:10.2170/jjphysiol.54.263]
Velichko, A.K., Markova, E.N., Petrova, N.V., et al., 2013.Mechanisms of heat shock response in mammals. Cell Mol. Life Sci., 70(22):4229–4241. [doi:10.1007/s00018-013-1348-7]
Wang, X.Q., Yang, W.J., Yang, Z., et al., 2012. The differentialproliferative ability of satellite cells in Lantang andlandrace pigs. PLoS ONE, 7(3):e32537. [doi:10.1371/journal.pone.0032537]
Welch, W.J., 1992. Mammalian stress response: cell physiology,structure/function of stress proteins, and implicationsfor medicine and disease. Physiol. Rev., 72(4):1063–1081.
Yoshihara, T., Naito, H., Kakigi, R., et al., 2013. Heat stress activates the Akt/mTOR signalling pathway in rat skeletalmuscle. Acta Physiol., 207(2):416–426. [doi:10.1111/apha.12040]
Zhang, M., Jiang, M., Bi, Y., et al., 2012. Autophagy and apoptosis act as partners to induce germ cell death afterheat stress in mice. PLoS ONE, 7(7):e41412. [doi:10.1371/journal.pone.0041412]
Author information
Authors and Affiliations
Corresponding authors
Additional information
The two authors contributed equally to this work
Project supported by the National 948 Program of China (No. 2011-G35), the National Basic Research Program (973) of China (No. 2012CB124704), and the Science and Technology Planning Project of Guangzhou, Guangdong Province, China (Nos. 201510010020 and 201300000035)
ORCID: Xiu-qi WANG, http://orcid.org/0000-0003-2033-9485
Rights and permissions
About this article
Cite this article
Gao, Cq., Zhao, Yl., Li, Hc. et al. Heat stress inhibits proliferation, promotes growth, and induces apoptosis in cultured Lantang swine skeletal muscle satellite cells. J. Zhejiang Univ. Sci. B 16, 549–559 (2015). https://doi.org/10.1631/jzus.B1400339
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1631/jzus.B1400339