Abstract
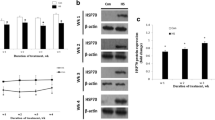
The present studies aimed to understand the interrelationships between stress, hormones and heat shock proteins (HSPs) in the ovary. We examined (1) whether HSP70.2, HSP72 and HSP105/110 can be produced and accumulated in porcine ovarian tissue, (2) whether these HSPs could be indicators of stress, i.e. whether two kinds of stress (high temperatures and malnutrition/serum deprivation) can affect them, and (3) whether some hormonal regulators of ovarian functions (insulin-like growth factor (IGF)-I, leptin and follicle-stimulating hormone (FSH)) can affect these HSPs and response of ovaries to HSP-related stress. We analysed the expression of HSP70.2, HSP72 and HSP105/110 mRNA (by using real-time reverse transcriptase polymerase chain reaction) in porcine ovarian granulosa cells, as well as the accumulation of HSP70 protein (by using sodium dodecyl sulphate polyacrylamide gel electrophoresis–Western) in either whole ovarian follicles and granulose cells cultured at normal (37.5°C) or high (41.5°C) temperature, with and without serum and with and without IGF-I, leptin and FSH. Expression of mRNA for HSP70.2, HSP72 and HSP105/110 in ovarian granulosa cells and accumulation of HSP70 protein in whole ovarian follicles and granulosa cells were demonstrated. In all the groups, addition of either IGF-I, leptin and FSH reduced the expression of HSP70.2, HSP72 and HSP105/110 mRNA. Both high temperature, serum deprivation and their combination resulted in increase in mRNAs for all three analysed HSPs. Additions of either IGF-I, leptin and FSH prevented the stimulatory effect of both high temperature and serum deprivation on the transcription of HSP70.2, HSP72 and HSP105/110. In contrast, high temperature reduced accumulation of peptide HSP70 in both ovarian follicles and granulosa cell. Serum deprivation promoted accumulation of HSP70 in granulosa cells, but not in ovarian follicles. Addition of IGF-I, leptin and FSH was able to alter accumulation of HSP70 in both follicles and granulosa cells. The present observations suggest (1) that HSPs can be synthesised in ovarian follicular granulosa cells; (2) that hormones (IGF-I, leptin and FSH) can inhibit, whilst stressors (both high temperature and malnutrition/serum deprivation) can stimulate transcription of HSP70.2, HSP72 and HSP105/110 genes, whilst heat stress, but not malnutrition, can promote depletion of HSP70 in ovarian cells, and (3) that hormones (IGF-I, leptin and FSH) can prevent stress-related changes in HSPs. The application of HSPs as indicators and mediators of stress and hormones on ovarian functions, as well as use of hormones and HSPs as anti-stressor molecules, are discussed.




Similar content being viewed by others
References
Akerfelt M, Trouillet D, Mezger V, Sistonen L (2007) Heat shock factors at a crossroad between stress and development. Ann NY Acad Sci 1391:1–13
Barb CR, Hausman GJ, Czaja K (2005) Leptin: a metabolic signal affecting central regulation of reproduction in the pig. Domest Anim Endocrinol 29:186–192
Beere HM (2004) ‘The stress of dying’: the role of heat shock proteins in the regulation of apoptosis. J Cell Sci 117:2641–2651
Berisha B, Schams D (2005) Ovarian function in ruminants. Domest Anim Endocrinol 29:305–317
Bombardier E, Vigna C, Iqbal S, Tiidus PM, Tupling AR (2009) Effects of ovarian sex hormones and downhill running on fiber-type-specific HSP70 expression in rat soleus. J Appl Physiol 106:2009–2015
Calderwood SK, Ciocca DR (2008) Heat shock proteins: stress proteins with Janus-like properties in cancer. Int J Hyperthermia 24:31–39
Calderwood SK, Mambula SS, Gray PJ Jr (2007) Extracellular heat shock proteins in cell signaling and immunity. Ann NY Acad Sci 1113:28–39
Driancourt MA, Guet P, Reynaud K, Chadli A, Catelli MG (1999) Presence of an aromatase inhibitor, possibly heat shock protein 90, in dominant follicles of cattle. J Reprod Fertil 115:45–58
Duvigneau JC, Hartl RT, Teinfalt M, Gemeiner M (2003) Delay in processing porcine whole blood affects cytokine expression. J Immunol Methods 272:11–21
Edwards JL, Hansen PJ (1997) Differential responses of bovine oocytes and preimplantation embryos to heat shock. Mol Reprod Dev 46:138–145
Ellis RJ (1997) Do molecular chaperones have to be proteins? Biochem Biophys Res Commun 238:687–692
Erickson GF, Danforth DR (1995) Ovarian control of follicle development. Am J Obstet Gynecol Ź 172:736–747
Guzeloglu A, Ambrose JD, Kassa T, Diaz T, Thatcher MJ, Thatcher WW (2001) Long-term follicular dynamics and biochemical characteristics of dominant follicles in dairy cows subjected to acute heat stress. Anim Reprod Sci 66:15–34
Hillier SG (1991) Cellular basis of follicular endocrine function. In: Hillier SG (ed) Ovarian endocrinology. Blackwell Science, Oxford, pp 73–105
Jayachandran M, Miller VM (2002) Ovariectomy upregulates expression of estrogen receptors, NOS, and HSPs in porcine platelets. Am J Physiol Heart Circ Physiol 283:H220–H226
Jego G, Hazoumé A, Seigneuric R, Garrido C (2010) Targeting heat shock proteins in cancer. Cancer Lett. PubMed PMID: 21078542.
Lanks KW (1986) Modulators of the eukaryotic heat shock response. Exp Cell Res 165:1–10
Lawrence JL, Payton RR, Godkin JD, Saxton AM, Schrick FN, Edwards JL (2004) Retinol improves development of bovine oocytes compromised by heat stress during maturation. J Dairy Sci 87:2449–2454
Makarevich A, Sirotkin A, Chrenek P, Bulla J, Hetenyi L (2000) The role of IGF-I, cAMP/protein kinase A and MAP kinase in the control of steroid secretion, cyclic nucleotide production, granulosa cell proliferation and preimplantation embryo development in rabbits. J Steroid Biochem Mol Biol 73:123–133
Maniwa J, Izumi S, Isobe N, Terada T (2005) Studies on substantially increased proteins in follicular fluid of bovine ovarian follicular cysts using 2-D PAGE and MALDI-TOF MS. Reprod Biol Endocrinol 3:23
Narayansingh RM, Senchyna M, Vijayan MM, Carlson JC (2004) Expression of prostaglandin G/H synthase (PGHS) and heat shock protein-70 (HSP-70) in the corpus luteum (CL) of prostaglandin F2 alpha-treated immature superovulated rats. Can J Physiol Pharmacol 82:363–371
Pfaffl MW (2001) A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 29(9):2002–2007
Putney DJ, Mullins S, Thatcher WW, Drost M, Gross TS (1989) Embryonic development in superovulated dairy cattle exposed to elevated ambient temperatures between the onset of estrus and insemination. Anim Reprod Sci 19:37–51
Queitsch C, Sangster TA, Lindquist S (2002) Hsp90 as a capacitor of phenotypic variation. Nature 417:618–624
Salvetti NR, Baravalle C, Mira GA, Gimeno EJ, Dallard BE, Rey F, Ortega HH (2008) Heat shock protein 70 and sex steroid receptors in the follicular structures of induced ovarian cysts. Reprod Domest Anim 44:805–814
Schams D, Berisha B, Kosmann M, Einspanier R, Amselgruber WM (1999) Possible role of growth hormone, IGFs, and IGF-binding proteins in the regulation of ovarian function in large farm animals. Domest Anim Endocrinol. 17:279–285
Sirotkin AV (2010) Effect of two types of stress (heat shock/high temperature and malnutrition/serum deprivation) on porcine ovarian cell functions and their response to hormones. J Exp Biol 213:2125–2130. doi:10.1242/jeb.040626
Sirotkin AV (1996) Inter-relationships between nonapeptide hormones and cyclic nucleotides within cultured porcine granulosa cells. J Endocrinol. 150:343–348
Sirotkin AV, Makarevich AV (1999) GH regulates secretory activity and apoptosis in cultured bovine granulosa cells through the activation of the cAMP/protein dinase A system. J Endocrinol. 163:317–327
Sirotkin AV, Makarevich AV, Corkins MR, Kotwica J, Bulla J (2001) The transfection-induced overexpression of IGF-binding protein-4 affects the secretory activity of porcine ovarian granulosa cells and their response to hormones and IGF-I. J Mol Endocrinol. 26:241–248
Sirotkin AV, Makarevich AV, Kotwica J, Marnet P-G, Kwon HB, Hetenyi L (1998) Isolated porcine ovarian follicles as a model for the study of hormone and growth factor action on ovarian secretory activity. J Endocrinol 159:313–321
Sirotkin AV, Mlyncek M, Kotwica J, Makarevich AV, Florkovicova I, Hetenyi L (2005) Leptin directly controls secretory activity of human ovarian granulosa cells: possible inter-relationship with the IGF/IGFBP system. Horm Res 64:198–202
Smith GD, Jackson LM, Foster DL (2001) Leptin regulation of reproductive function and fertility. Theriogenology 57:73–86
Sonna LA, Fujita J, Gaffin SL, Lilly CM (2002) Effects of heat and cold stress on mammalian gene expression. J Appl Physiol 92:1725–1742
Spicer LJ (2001) Leptin: a possible metabolic signal affecting reproduction. Domest Anim Endocrinol 21:251–270
Theodorakis NG, Morimoto RI (1987) Posttranscriptional regulation of hsp70 expression in human cells: effects of heat shock, inhibition of protein synthesis, and adenovirus infection on translation and mRNA stability. Mol Cell Biol 7:4357–4368
Trinklein ND, Murray JI, Hartman SJ, Botsein D, Myers RM (2004) The role of heat shock transcription factor 1 in the genome-wide regulation of the mammalian heat shock response. Mol Biol Cell 15:1254–1261
Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, De Paepe A, Speleman F (2002) Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol 3(7):1–11
Verfaillie T, Cox E, To LT, Vanrompay D, Bouchaut H, Buys N, Goddeeris BM (2001) Comparative analysis of porcine cytokine production by mRNA and protein detection. Vet Immunol Immunopathol 81:97–112
Zieba DA, Amstalden M, Williams GL (2005) Regulatory roles of leptin in reproduction and metabolism: a comparative review. Domest Anim Endocrinol 29:166–185
Acknowledgements
The authors thank Ing. Ž. Kuklová and K. Tothová for technical assistance. The present studies were supported by Ministry of Agriculture of Slovak Republic (RVVU 07-02).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sirotkin, A.V., Bauer, M. Heat shock proteins in porcine ovary: synthesis, accumulation and regulation by stress and hormones. Cell Stress and Chaperones 16, 379–387 (2011). https://doi.org/10.1007/s12192-010-0252-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12192-010-0252-4