Abstract
Background
Oxidative stress has been considered as a contributory aspect for major complications of diabetes mellitus consisting of diabetic nephropathy. This study aimed to examine the therapeutic effect of formononetin in streptozotocin (STZ)-induced diabetic nephropathy through measuring biochemical parameters, oxidative indicators, and histopathological examination of renal tissues.
Results
Administration of a dose of STZ (55 mg/kg of body weight) intraperitoneal induced diabetic nephropathy in rats as indicated by an increase in serum glucose, creatinine, triglyceride, cholesterol, and BUN levels related to the depletion of serum albumin level. Besides, STZ treatment led to the depletion of antioxidant enzymes together with superoxide dismutase (SOD), glutathione (GSH), and catalase (CAT). Administration of formononetin at the dose of 10, 20, and 40 mg/kg extensively decreased biochemical parameters with a rise in serum albumin level. Formononetin was observed to improved antioxidant enzyme ranges and offered protection against lipid peroxidation (LPO). STZ administered rats show an elevated level of TNF-α and IL-6. Meanwhile, formononetin-treated rats inhibited the elevated level of cytokine.
Conclusion
This study concluded that formononetin may additionally modulate oxidative stress and protected renal tissues from STZ injury. It also showed improvement in renal histopathological architecture in STZ-induced diabetic nephropathy.
Similar content being viewed by others
Background
Diabetic nephropathy (DN), also known as diabetic kidney disorder, is a major complication of long-standing diabetes mellitus. Nearly 40% of patients of diabetes expand DN because of continual hyperglycemia. Chronic hyperglycemia leads to progressive structural changes inside the renal tissues such as extracellular accumulation in the mesangium, glomerular basement membrane, and tubulointerstitial tissue [1]. DN is characterized by specific renal morphological alterations consisting of glomerular sclerosis and tubular fibrosis leading to functional impairment inside the form of an upward push of urinary albumin excretion [2]. Persistently, high glucose level induces oxidative stress leading to the production of reactive oxygen species (ROS) which can be responsible for renal cellular injury. Current treatment modalities for diabetes primarily focus on glycemic and lipid control or lifestyle changes. These therapeutic approaches are insufficient to control oxidative stress and subsequent progression of DN. Hence, there is an urgent need to explore the underlying mechanism and novel treatment for DN.
Formononetin is a non-steroidal polyphenolic compound obtained from Astragalus membranaceus and Astragalus mongholicus root [3]. This phytochemical is known for its estrogenic activity due to its binding to the estrogen receptor (ER) and the induction of specific estrogen-responsive gene product. It is commonly found in legume plants including green peas, licorice roots, soybeans, flowering tops of red clover, astragalus roots [4], cauliflower, and iceberg lettuce [5]. Previous clinical study concluded the role of formononetin along with another isoflavone in the improvement of systemic arterial compliance [6]. Additionally, it was observed to be powerful in postmenopausal girls and obese men for a reduction in arterial stiffness and blood pressure [7]. Formononetin affords effective cardioprotection using inhibiting mitogen-activated protein (MAP) kinase activity [8]. It is called a potent activator of peroxisome proliferator-activated receptors (PPAR) alpha and gamma which modify glucose metabolism and dyslipidemi a[9]. In an experimental study, formononetin is known to enhance the antihyperglycemic effect of fangchinoline through growing insulin secretion from pancreatic beta cells in type 1 diabetic mice [10]. In vitro study demonstrated the defensive effect of formononetin by inhibiting pancreatic beta-cell apoptosis in diabetes mellitus. This effect was produced through the downregulation of nuclear factor-kappa B (NF-kB) and a decrease in the production of nitric oxide [11]. However, formononetin did not produce a hypoglycemic effect in streptozotocin-induced type 1 diabetes in C57BL/6 mice [12]. Therefore, the present study was undertaken to analyze the renoprotective effect of formononetin on STZ caused DN in rat version.
Methods
Animals
Thirty-six male Albino Wistar rats weighing 180–220 g were used for this experimental examination. Rats were procured from animal house facility of the R. C. Patel Institute of Pharmaceutical Education and Research, Shirpur, Dist-Dhule-Maharashtra). Animals were placed in nicely ventilated polypropylene cages and maintained under an ambient temperature of 25 ± 2 °C, 12-h light/dark cycle in the departmental animal house. The animals had been fed with trendy pelletized feed (Amrut Rat Feed, Pune) and water ad libitum. The experimental protocol was pre-accepted by the institutional animal ethical committee (IAEC), protocol approval no. RCPIPER/IAEC/2018-19/06.
Chemicals and drugs
STZ, formononetin, metformin, and carboxymethylcellulose were obtained from Sigma Aldrich (Mumbai, India). Glucose kit, creatinine kit, urea/BUN kit, albumin kit, and total protein kit were received from ERBA (Mumbai, India). Tumor necrosis factor-alpha (TNF-α) and interleukin-6 (IL-6) were purchased from Loba Chemicals Pvt Ltd (Mumbai, India). All other chemicals used in the study were of analytical grade.
Induction of diabetic nephropathy
Diabetes was induced in rats by a single dose of STZ (55 mg/kg) intraperitoneal route except for the normal group. STZ was prepared in cold phosphate buffer solution (pH-7.4) in dark conditions. After 72 h of STZ administration, DN was induced in animals [13].
Experimental design
Rats were randomly divided into six groups (n = 6).
Group 1: Normal - received 0.5% CMC
Group 2: Diabetic nephropathic group (DN) - injected with STZ (55 mg/kg)
Group 3: DN + Standard drug (Metformin) - injected with STZ (55 mg/kg) + metformin (70 mg/kg)
Group 4 to 6: Injected with STZ (55 mg/kg) and 10, 20, and 40 mg/kg of formononetin, respectively.
Metformin and formononetin suspension was prepared in 0.5% CMC and administered by oral route for 14 days. On the 15th day, animals were anesthetized by urethane (1.4 mg/kg) [14] and blood samples were collected. For separation of the serum, the blood was centrifuged at 5000 rpm for 10 min at 4 °C and the serum was separated and used for further biochemical estimation. Finally, animals were sacrificed and both kidneys were isolated. A 10% tissue homogenate of the kidney was prepared using ice-cold 50 mM phosphate buffer saline (pH 7.4). The homogenate was centrifuged at 10000 rpm for 10 min at 4 °C, and the supernatant was separated and used for further estimation.
Physiological parameters
The body weight of each animal was recorded weekly by using an electronic weighing balance. At the end of the experiment, the weight of both kidney and liver was taken.
Assessment of kidney function
On the 14th day of study, rats were kept individually in metabolic cages for 24 h urine collection for the measurement of urine output and renal function. Before urine collection, blood samples were collected by retro-orbital puncture for separation of the serum. Renal function was assessed by measuring serum levels of fasting glucose by the glucose oxidase-peroxidase method, creatinine, and BUN, and blood albumin was measured using the commercially available marketed kit (Erba kit) [15].
Biochemical estimations in kidney homogenates
Renal oxidative stress biomarkers like GSH, SOD, and MDA were estimated using commercially available marketed kits according to the manufacturer’s guidelines, and CAT was estimated using commercially available UV spectroscopic methods. Briefly, the kidney homogenate supernatant (10 μL) was added to 0.5 mL of 10 mM hydrogen peroxide (H2O2) solution. The reduction in optical density of this mixture was measured at 240 nm by using a UV spectrophotometer. The rate of decrease in the optical density within 3 min from the addition of kidney homogenate was taken as an indicator of the catalase activity present in the homogenate [16].
Cytokine parameters
Proinflammatory cytokines like TNF-α and IL-6 were measured in kidney homogenate by using respective kits according to the manufacturer’s protocol, and the final concentration was determined by using the standard curve [17].
Histopathological examinations
The kidney was removed from each rat by injecting a high dose of anesthesia (urethane 1.4 mg/kg i.p.) and buffered in formalin (10%) solution and embedded in paraffin wax. The sample was then cut into around 5-μm-thick sections with a microtome and deparaffined with xylene. Then, sections were subjected to standard staining of hematoxylin/eosin. Sections were observed under a light microscope at magnifications of ×40. Light microscopy was used to evaluate tubular necrosis and glomerular damage [18].
Statistical analysis
Data were presented as mean ± S.E.M. of each group. The result values were statistically analyzed by using one-way ANOVA and two-way ANOVA followed by Bonferroni’s test using graph pad prism version 7.0. A difference was regarded as significant when P < 0.05, P < 0.01, and P < 0.001.
Results
Effect of STZ and formononetin on change in body and organ weight
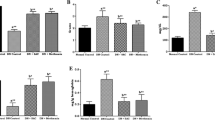
The bodyweight of the animals significantly decreased in STZ-treated group as compared to the normal group. Treatment with formononetin increased the body weight as compared to STZ-injected group (P < 0.001) shown in Fig. 1a, Whereas the metformin-treated group showed an increase in the body weight as compared to the STZ group (P < 0.001). Meanwhile, STZ-injected rats showed a decrease in kidney weight (Fig. 1b, c) and an increase in liver weight (Fig. 1d) as compared to normal rats. But the entire treatment group showed the reverse effect of STZ.
Effect of STZ and formononetin on urine output
Excretion of the urine was monitored at the end of the study for the evaluation of DN in the STZ-treated and drug-treated groups. Twenty-four hours of average urine volume was found to be 0.91 ml in the control group. The STZ-treated group exhibited a significant rise in the average urine volume up to 4.1 ml. Metformin and formononetin treatment significantly inhibited such increased urine output as compared to STZ-treated group (P < 0.01) as showed in Fig. 2.
Effect of formononetin on serum biochemical parameter
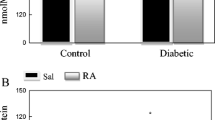
As shown in Fig. 3, STZ-treated rats showed marked impairment in renal functions as evident from the rise in the markers of glomerular and tubular damage like serum glucose, serum albumin, serum creatinine, BUN, serum cholesterol, and serum triglyceride activity as compared to the control group. Contrary, formononetin received group 3 showed a significant reduction in the serum level of BUN, glucose, albumin, and creatinine (P < 0.001).
Effect of STZ and formononetin on oxidative stress in kidney tissue homogenate
As shown in Fig. 4, lipid peroxidation was significantly high in STZ-treated rats as suggested by a marked increase in kidney MDA levels as compared to control rats (P < 0.001). Administration of different 3 doses of formononetin to the STZ-treated rats significantly restored the increased lipid peroxidation levels as compared to the STZ-treated rats (P < 0.001). STZ-treated groups showed a significant decrease in SOD and reduced GSH level and catalase activities (P < 0.001) in the kidney tissues. Metformin-treated rats show the similar effect like the normal rats. Administration of formononetin into the STZ-treated rats significantly increases the levels of SOD, reduced GSH, and kidney catalase activities as compared with STZ-treated rats (P < 0.001).
Effect of STZ and formononetin on cytokine release
The release of cytokine was measured by ELISA assay and showed a significant rise in TNF-α and IL-6 in STZ-treated rats. This was indicated by a marked increase in kidney cytokine levels as compared to control rats (P < 0.001). Formononetin treatment at different doses in the STZ-treated rats significantly restored the increased cytokine levels as compared to the STZ-treated rats (P < 0.001) (Fig. 5).
Effect of STZ and formononetin on histopathological examination
Histopathological examination of the kidney section of the normal group showed healthy kidney tissue architecture. The section of the STZ-treated group exhibited similar features of the control group in addition to tubular necrosis with inflammatory cells. Blood vessels were congested with interstitial edema. Cell damage was also observed in the STZ-treated group. STZ + metformin group showed comparatively least damage in the glomerulus. It can be observed that necrosis was also least compared to the STZ-treated group. However, formononetin treatment significantly reduced inflammatory changes and necrosis in renal cells. STZ-induced renal tissue damage attenuated by formononetin with an increase in its doses shown in Fig. 6.
Discussion
Oxidative stress and inflammation play a crucial role in the initiation and development of DN. Polyphenolic phytochemicals such as resveratrol, catechin, and genistein were proved for antidiabetic activities [19, 20]. Isoflavone was a polyphenolic compound with a potent antioxidant effect. It was also recognized to enhance insulin secretion and improves glycemic management in diabetics [15, 21]. Hyperlipidemia, oxidative pressure, and anti-inflammatory approaches were proposed inside the pathogenesis of type 2 diabetes [22]. Phytochemicals were known to act as a powerful antioxidant and halt the process of inflammation by controlling oxidative stress. Therefore, phytochemicals play a significant role in the control and management of diabetes mellitus and its complications. Previous research had stated the function of formononetin as a hypolipidemic, anti-inflammatory, and antioxidant agent. It is far recognized to set off PPAR-α and PPAR-γ receptors and inhibit the activation of the NF-kB pathway [23]. Additionally, formononetin exhibited an inhibitory effect on pancreatic β-cellular apoptosis and precipitated β-cellular regeneration [24].
In the present study, it was observed, Wistar rats were selected to induce pathological changes just like type 2 diabetes mellitus in humans. Streptozotocin was injected into rats to induce type 2 diabetes in the experimental model. As noted previously, the severity of β-cell destruction depends on the dose of STZ treatment [10]. In order to produce type 2 diabetes, various authors recommended the STZ dose in the range of 25–55 mg/kg in rodent models. Hence, in the current study, STZ in a dose of 55 mg/kg was used to induce insulin resistance and partial β-cellular disorder [13].
The administration of STZ significantly reduces the weight of both kidneys in diabetic rats compared to normal rats. However, treatment with metformin and formononetin improved the weight of both kidneys. Similarly, the weight of the liver was increased in STZ-administered groups and got reduced with the administration of metformin and formononetin. Results of the current study suggested that STZ in a dose of 55 mg/kg induced hyperglycemia and hyperinsulinemia in experimental animals. Treatment with formononetin produced better glycemic control which was equivalent to standard drug metformin. Results of the glucose test supported these findings. Formononetin at a dose of 40 mg/kg exhibited significant improvement in fasting glucose levels as compared to the diabetic control group. These data suggested that formononetin could produce an antidiabetic effect in diabetic rats.
Urinary albumin level was a selective marker of glomerular damage. Enhanced albumin excretion in experimental rats suggests modern nephropathy resulting in a loss of auto-regulation and inflammation. Several studies have mentioned that the reduction of microalbuminuria indicates a better prognosis in diabetic nephropathy [25]. Formononetin treatment efficiently reversed microalbuminuria equivalent to metformin remedy. This shows the role of formononetin in attenuating hyperfiltration and halting the progression of early diabetic nephropathy [13].
STZ treatment in diabetic rats leads to an increase in oxidative stress responsible for the development of diabetic complications [26]. The resemblance of pathological status in human diabetes with STZ caused hyperglycemia makes it a good model for the preliminary screening active agent against diabetes [27]. Continual hyperglycemias suppress the activity of antioxidant enzymes resulting in growth in the production of reactive oxygen species (ROS). ROS causes cellular damage leading to functional abnormalities in the organ. Cellular membranes and lipids were highly susceptible to the oxidative stress and peroxide reaction induced on by ROS [28]. Lipid peroxidation was traditionally an unfastened radical chain response initiated by the absorption of a hydrogen atom from a polyunsaturated fatty acid side chain. This process was initiated via ROS main to cellular damage by using the inactivation of membrane enzymes and receptors, depolymerization of polysaccharides, protein cross-linking, and fragmentation. Additionally, lipid peroxidation leads to the production of an extensive variety of cytotoxic merchandize which includes aldehydes. MDA is a main marker of endogenous lipid peroxidation [29]. In the current study, the activity of MDA was significantly increased in diabetic animals as compared to the normal group. However, treatment with formononetin significantly decreased MDA activity in a dose-dependent manner comparable to standard drug metformin. TNF-α and IL-6 were known as pro-inflammatory cytokines and are predictors of type 2 diabetes [30]. Both are thought to consider to mediate adverse metabolic effects resulting in insulin resistance and deteriorating glucose homeostasis [31]. The level of TNF-α and IL-6 was significantly increased in STZ-induced diabetic animals as compared to the normal group. Treatment with formononetin significantly decreased both and effect was equivalent to metformin.
On histopathological examination, the renal tissue of diabetic rats exhibited the presence of inflammatory, degenerative, necrotic, and hyperplastic lesions. Atrophy of renal tissue has also been observed in diabetic rats. Treatment with formononetin at the dose of 10 mg/kg showed little improvement in histological damage induced by diabetes. At the higher dose of 20 and 40 mg/kg dose of formononetin, there were no signs of necrosis and atrophy of renal tissue. Taken together, the above results indicated that formononetin ameliorated diabetes-induced renal cell injury in DN rats.
Conclusion
Our study provides evidence for the renoprotective effect of formononetin in diabetes-induced renal injury. Antidiabetic activity elicited by formononetin might be due to its ability to activate antioxidant enzymes. The study results suggest that formononetin can be used in diabetic nephropathy and management of blood glucose, BUN, and serum creatinine. Further studies are needed to evaluate the therapeutic potential of formononetin in diabetic nephropathy in the human cell line model to find the mechanism.
Availability of data and materials
All data and material are available upon request
References
Gilbert RE et al (1998) Expression of transforming growth factor-beta1 and type IV collagen in the renal tubulointerstitium in experimental diabetes: effects of ACE inhibition. Diabetes 47(3):414–422. https://doi.org/10.2337/diabetes.47.3.414
Ha H and Lee HB (2000) Reactive oxygen species as glucose signaling molecules in mesangial cells cultured under high glucose. Kidney Interna 2000. 58: S19-S25. DOI: https://doi.org/10.1046/j.1523-1755.2000.07704.x
Mu H et al (2009) Research on antioxidant effects and estrogenic effect of formononetin from Trifolium pratense (red clover). Phytomedi 16(4):314–319. https://doi.org/10.1016/j.phymed.2008.07.005
Kaczmarczyk-Sedlak I et al (2013) Effect of formononetin on mechanical properties and chemical composition of bones in rats with ovariectomy-induced osteoporosis. Evid Based Complement Alternat Med:2013. https://doi.org/10.1155/2013/457052
Kühnle A (2009) Self-assembly of organic molecules at metal surfaces. Curr Opin Colloid Interface Sci 14(2):157–168. https://doi.org/10.1016/j.cocis.2008.01.001
Nestel PJ et al (1999) Isoflavones from red clover improve systemic arterial compliance but not plasma lipids in menopausal women. J Clinl Endocrinol Metabol 84(3):895–898. https://doi.org/10.1210/jcem.84.3.5561
Nestel P, Fujii A and L. Zhang (2007) An isoflavone metabolite reduces arterial stiffness and blood pressure in overweight men and postmenopausal women. Atherosclerosis, 192(1): 184-189. https://doi.org/10.1016/j.atherosclerosis.2006.04.033
Dubey RK et al (1999) Phytoestrogens inhibit growth and MAP kinase activity in human aortic smooth muscle cells. Hypertension, 1999 33(1):177–182. https://doi.org/10.1161/01.hyp.33.1.177
Alicic RZ, Rooney MT, Tuttle KR (2017) Diabetic kidney disease: challenges, progress, and possibilities. Clin J Ameri Socie Nephrol 12(12):2032–2045. https://doi.org/10.2215/CJN.11491116
Ma W et al (2007) Combined effects of fangchinoline from Stephania tetrandra Radix and formononetin and calycosin from Astragalus membranaceus Radix on hyperglycemia and hypoinsulinemia in streptozotocin-diabetic mice. Biol Pharmaceu Bulletin 30(11):2079–2083. https://doi.org/10.1248/bpb.30.2079
Wang Y et al (2012) Formononetin attenuates IL-1β-induced apoptosis and NF-κB activation in INS-1 cells. Mol 2012. 17(9):10052–10064. https://doi.org/10.3390/molecules170910052
Qiu L et al (2012) Red clover extract ameliorates dyslipidemia in streptozotocin-induced diabetic C57BL/6 mice by activating hepatic PPARα. Phytother Res 26(6):860–864. https://doi.org/10.1002/ptr.3641
Somani R et al (2012) Asparagus racemosus Willd (Liliaceae) ameliorates early diabetic nephropathy in STZ induced diabetic rats. Indian J Exp Biol 50(7):469–475 http://nopr.niscair.res.in/handle/123456789/14328
Golder FJ, Robertson SA, Valverde A, Bolser DC (2003) Urethane anesthesia in adult female rats: preliminary observations. Vet Anaesth Analg 30(2):115–115. https://doi.org/10.1046/j.1467-2995.2003.13335.x
Rufer CE, Kulling SE (2006) Antioxidant activity of isoflavones and their major metabolites using different in vitro assays. J Agric Food Chem 54(8):2926–2931. https://doi.org/10.1021/jf053112o
Soon Y, Tan B (2002) Evaluation of the hypoglycemic and anti-oxidant activities of Morinda officinalis in streptozotocin-induced diabetic rats. Singapore Med J. 43(2):077–085
Jain PG et al (2018) Cardioprotective role of FA against isoproterenol induced cardiac toxicity. Mole Biol Reports 45(5):1357–1365. https://doi.org/10.1007/s11033-018-4297-2
Khairnar SI et al (2020) Disulfiram and its copper chelate attenuate cisplatin-induced acute nephrotoxicity in rats via reduction of oxidative stress and inflammation. Biol Trace Elem Res 193(1):174–184. https://doi.org/10.1007/s12011-019-01683-w
Tsuneki H et al (2004) Effect of green tea on blood glucose levels and serum proteomic patterns in diabetic (db/db) mice and on glucose metabolism in healthy humans. BMC Pharmacol 4(1):18–26. https://doi.org/10.1186/1471-2210-4-18
Cao Z et al., (2007) Learning to rank: from pairwise approach to listwise approach. in Proceedings of the 24th international conference on Machine learning. 2007; 129-136. https://doi.org/10.1145/1273496.1273513
Lu MP et al (2008) Dietary soy isoflavones increase insulin secretion and prevent the development of diabetic cataracts in streptozotocin-induced diabetic rats. Nutri Res 28(7):464–471. https://doi.org/10.1016/j.nutres.2008.03.009
Rains JL, Jain SK (2011) Oxidative stress, insulin signaling, and diabetes. Free Radic Biol Med 50(5):567–575. https://doi.org/10.1016/j.freeradbiomed.2010.12.006
Medjakovic S, Mueller M, Jungbauer A (2010) Potential health-modulating effects of isoflavones and metabolites via activation of PPAR and AhR. Nutri 2(3):241–279. https://doi.org/10.3390/nu2030241
Qiu G et al (2017) Formononetin exhibits anti-hyperglycemic activity in alloxan-induced type 1 diabetic mice. Experi Biol Med 242(2):223–230. https://doi.org/10.1177/1535370216657445
Mogensen C (2003) Microalbuminuria and hypertension with focus on type 1 and type 2 diabetes. J Inter Med 254(1):45–66. https://doi.org/10.1046/j.1365-2796.2003.01157.x
Johansen JS et al (2005) Oxidative stress and the use of antioxidants in diabetes: linking basic science to clinical practice. Cardiovascul Diabetol 4(1):5–12. https://doi.org/10.1186/1475-2840-4-5
Srinivasan K, Ramarao P (2007) Animal model in type 2 diabetes research: an overview. Ind J Med Res 125(3):451–458
Blake D, Allen R, Lunec J (1987) Free radicals in biological systems—a review orientated to inflammatory processes. Br Med Bull 43(2):371–385. https://doi.org/10.1093/oxfordjournals.bmb.a072188
Oktem F, Ozguner F, Sulak O, Olgar S, Akturk O, Yilmaz HR, Altuntas I (2005) Lithium-induced renal toxicity in rats: protection by a novel antioxidant caffeic acid phenethyl ester. Mole Cellu Biochem 277(1-2):109–115. https://doi.org/10.1007/s11010-005-5426-5
Mirza S, Hossain M, Mathews C, Martinez P, Pino P, Gay JL, Rentfro A, McCormick JB, Fisher-Hoch SP (2012) Type 2-diabetes is associated with elevated levels of TNF-alpha, IL-6 and adiponectin and low levels of leptin in a population of Mexican Americans: a cross-sectional study. Cyto 57(1):136–142. https://doi.org/10.1016/j.cyto.2011.09.029
Moller DE (2000) Potential role of TNF-α in the pathogenesis of insulin resistance and type 2 diabetes. Trends Endrocrinol Metab 11(6):212–217. https://doi.org/10.1016/s1043-2760(00)00272-1
Acknowledgements
Not applicable
Funding
Not applicable
Author information
Authors and Affiliations
Contributions
PGJ designed all the experiments, analyzed data, and wrote the manuscript.
PGN performed the experiments.
DJP-performed the experiments.
SDS analyzed data and wrote the manuscript.
SJS provided reagents and information.
The authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The experimental protocol was approved by the institutional animal ethical committee of R.C. Patel Institute of Pharmaceutical Education and Research, Shirpur with protocol no. RCPIPER/IAEC/2018-19/06.
Consent for publication
Not applicable
Competing interests
The authors do not have conflicts of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Jain, P.G., Nayse, P.G., Patil, D.J. et al. The possible antioxidant capabilities of formononetin in guarding against streptozotocin-induced diabetic nephropathy in rats. Futur J Pharm Sci 6, 53 (2020). https://doi.org/10.1186/s43094-020-00071-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43094-020-00071-9