Abstract
Background
Pervasive introductions of non-native taxa are behind processes of homogenization of various types affecting the global flora and fauna. Chile’s freshwater ecosystems encompass a diverse and highly endemic fish fauna that might be sensitive to the introduction of non-native species, an ongoing process that started two centuries ago, but has to date received little attention. Using historical (native) and present-day (native and non-native) presence-absence data sets of compositional similarity, our goal was twofold: (1) evaluate patterns of taxonomic homogenization at various spatial scales and (2) identify clusters of widely versus narrowly distributed species to assess their relative role in compositional changes. We expect that non-native species with wide distributions might have a larger influence in taxonomic homogenization than those with narrow distributions.
Results
Chile’s fish assemblages have become increasingly homogenized during the last two centuries when evaluating changes in compositional similarity among 201 watersheds (65.3 % of total comparisons showed homogenization) distributed among six defined biotic units. Taxonomic differentiation was significantly more prevalent than taxonomic homogenization within biotic units. Among biotic units, comparisons between historical and current compositional similarity were all significantly different. We identified one cluster of non-native fishes that were distributed across the entire five or six biotic units. This cluster included Brown Trout (Salmo trutta) and Rainbow Trout (Oncorhynchus mykiss) as the two most representative species. A second cluster we identified included fishes such that on average spanned only one or two biotic units.
Conclusions
We provide first evidence for an ongoing and large-scale process of taxonomic homogenization among Chile’s watersheds occurring at various scales. Our findings provide taxonomic and biogeographic baseline information for management plans and courses of action for conservation of native fishes, many of which are endemic. We also discuss management guidelines of non-native fishes in Chile. Baseline information of both native and non-native fish taxa might be applicable to other isolated regions elsewhere.
Similar content being viewed by others
Background
The biodiversity of freshwater systems is threatened and constitute an overriding conservation priority worldwide (Dudgeon et al. 2006; Johnson et al. 2008; Hopper et al. 2012). Human-mediated introductions of species have been a key factor leading to diversity losses in these systems (Clavero and García-Berthou 2006). Fishes are in particular among the most documented introduced species around the world (Rahel 2000) due to aquaculture, recreational fisheries, biological control, and ornamental uses (Ruiz and Marchant 2004; Vargas et al. 2010; Marr et al. 2010, 2013). Biogeographic and ecological approaches to quantify changes in fish communities over time have thus emerged as fundamental means to understanding global as well as local issues involving non-native fishes and their effects upon native aquatic biota, and to illuminate conservation implications as a result (Olden et al. 2008; Marr et al. 2010; Habit et al. 2010).
Long-term ecological consequences of freshwater fish introductions have focused on the loss of differentiation of unrelated communities across regions due to the current wide spread of some taxa (e.g., Olden et al. 2004, 2006, 2008). Biotic homogenization between communities represents a major biogeographic and ecological threat for biodiversity at a regional and global scale (Olden and Poff 2004; Rooney et al., 2004; Olden 2006; Olden and Rooney 2006; Marr et al. 2010, 2013). This phenomenon might include loss of taxonomic, functional, or genetic differentiation (Olden 2006). Our focus was the magnitude of taxonomic homogenization (TH), which depends on the resolution and extent of the spatial scale, in addition to species richness and turnover (Baiser et al. 2012). On the contrary, if communities become more different as a result of introductions of non-native species, taxonomic differentiation (TD) might occur (McKinney, 2004; Olden 2006). Ultimately, TH represents a form of biodiversity loss and can result from local species turnover.
Freshwater systems from isolated regions provide ideal conditions to study TH and TD as they have shaped the evolution of endemic species, and thus, unique species assemblages. Because they are also exposed to the establishment of non-native species, the assessment of the magnitude of TH between different aquatic systems is imperative in favor of conservation of native fishes. The case of Chile is especially insightful as fish communities span an extensive latitudinal gradient of watersheds that drain to the Pacific Ocean (18°S–55°S). Specifically, the Atacama Desert, the Andean range, and the Pacific Ocean have historically isolated freshwater fishes from Chile that have evolved within the confines of short rivers with high gradients and parallel basins and glacial lakes and meadows (Arratia et al. 1981; Arratia 2002, Vila and Pardo 2006). Forty four inland native fishes can be found among three so-called ichthyogeographic provinces composed of four areas of endemism (Dyer 2000a, b; Habit et al. 2006a). Eighty one percent of these native fishes are endemic species (Vila et al. 2006; Habit et al. 2006a). It has been hypothesized that native fishes from this region of South America are especially vulnerable to fish introductions (Arratia 1978), but this hypothesis has never been formally tested. Chile’s native fishes have ancestral traits such as small body sizes (Vila et al. 1999; Vila et al. 2006) and sparse development of locomotion attributes (Zunino et al. 1999); they also exhibit low variation in life histories and tolerances for habitat degradation as well as narrow geographical distributions (Habit et al. 2006a). These attributes are in stark contrast with those from several of the 27 non-native species documented in the literature (Iriarte et al. 2005; Habit et al. 2006b; Quezada-Romegialli et al. 2009). These introductions have affected native fishes due to predation (Ibarra et al. 2011; Arismendi et al. 2012), interactive segregation (Penaluna et al. 2009), overlap of microhabitat preferences (Vargas et al. 2010), and changes in local distributions (Arismendi et al. 2009; Habit et al. 2010). However, biogeographic changes in fish composition following introductions remain poorly documented.
In this study, we assembled a comprehensive presence-absence database of freshwater fishes in Chile documented during the last two centuries in order to accomplish two objectives. Firstly, we conducted an extensive analysis of changes in TH and TD to establish comparisons within and among biotic units. Given the high endemism of freshwater fishes in Chile (Dyer 2000a), we expected a very unique composition of species assemblages among biotic units, prior to the non-native species introductions. We hypothesize that human-mediated fish introductions have homogenized some natural biogeographic boundaries, promoting TH of fish diversity. Secondly, we employed a clustering method to explore groups of widely versus narrowly distributed species to assess their relative role in compositional changes. Our expectation was that introduced species with wide distributions might have a larger influence in TH than those with narrow distributions, which conversely are more likely to drive TD. These processes are likely to be driven by similar fish taxa because of convergent human interests around the globe (e.g., salmonids, cyprinids, and poeciliids: Ruesink 2005; Clavero and García-Berthou 2006; Marr et al. 2010; Marr et al. 2013). To our knowledge, this is the first attempt to broadly evaluate TH in Chile (but see Marr et al. 2010), thus permitting assessments of changes in the freshwater fish fauna in order to evaluate current implications for conservation and aid policy-making decisions.
Methods
Study area and biotic units
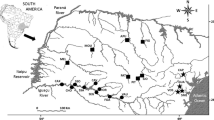
Chile’s freshwater fishes are distributed among three ichthyogeographic provinces and five areas of endemism between 18°S and 55°S in South America (Fig. 1; Dyer 2000a). Areas of endemism seem good candidates for our study, because (1) their boundaries are likely to become eroded as a result of taxonomic homogenization and (2) they represent different levels of habitat heterogeneity or ecoregions (see Olden et al. 2008 for an example). Our analyses have been restricted to Chile’s political limits, although provinces and areas of endemism spread beyond the country’s border. We designated each area of endemism as one biotic unit: Titicaca (U1, 18°S–23°S), Atacama (U2, 18°S–22°S), Central (U3, 28°S–33°S), South-Central (U4, 35°S–39°S), and South (U5, 39°S–42°S). Several studies have documented the presence of non-native taxa, especially salmonids, in both U5 and Patagonia province (e.g., Soto et al. 2006, Arismendi et al. 2009; Habit et al. 2010; Arismendi et al. 2012; Arismendi et al. 2014). Although Patagonia has not been described as an area of endemism, we included it as the sixth biotic unit of our study (U6, 42°S–55°S). However, when comparing biotic units, we limited comparisons of U6 with U5.
Historical and current presence-absence datasets
An exhaustive bibliographic search was undertaken to document the historical and current composition of Chile’s freshwater fishes. Our definition of “fishes” included both jawed and jawless taxa. The search yielded 227 articles about native and non-native (established or invasive) fishes published between 1782 and 2012 for 201 watersheds (Additional file 1: Appendix S1). Historical records represented the original composition of native fish assemblages before fish introductions (see Olden and Rooney 2006). We used a similar approach to Pool and Olden (2012) where if a native species was recorded within a watershed in any time period, then it was also present in all earlier time periods. Thus, native fishes during any time period represent the historical composition. We excluded marine fishes that occasionally occur in freshwater habitats. Current records represented the present-day composition of fish assemblages, including both native and non-native species since their first introduction was recorded (see Olden and Rooney 2006). We assumed that if a non-native species was recorded within a watershed it remained established in all subsequent years (Pool and Olden 2012). We built a latitudinal presence-absence data matrix represented in a binary format (1 = present, 0 = absent) from a total of 201 watersheds (available from the authors). We followed the descriptions of Mack et al. (2000) to distinguish between naturalized and invasive taxa and the synonymy of taxa.
Homogenization analyses
We assessed whether the taxonomic composition of fish assemblages is becoming similar (or dissimilar) over time. We employed pairwise estimates of compositional similarity (CS) between watersheds from historical (CShis) and current similarities (CScur) in fish composition using Sørensen’s index (Sørensen 1957), one of most commonly used indices to infer TH (see Olden et al. 2008; Leprieur et al. 2008; Marr et al. 2010, 2013). Prior to estimating Sørensen index, presence-absence data underwent a square root transformation and a double Wisconsin standardization (Oksanen et al. 2011). Changes in the similarity of fish composition were estimated according to ∆CS = CScur − CShis, where positive values were interpreted as TH and negative values as TD (Olden et al. 2008; Marr et al. 2010). We estimated the percentage of comparisons experiencing TH, TD, or ΔCS = 0 (i.e., CScur = CShis) by plotting CScur (y-axis) versus CShis (x-axis) values above or below a slope representing ΔCS = 0. Average changes in compositional similarity between historical and current fish assemblages (ΔCSav) were calculated for the entire country as well as within and between biotic units. We also estimated maximum taxonomic homogenization (Max TH) or differentiation (Max TD) to gauge whether ΔCSav was possibly influenced by extreme values of CS. All aforementioned statistics were calculated at various spatial scales including (1) a global estimation among all watersheds or across Chile, (2) among watersheds within each biotic unit, and (3) among watersheds found in different biotic units (i.e., between units).
We tested the hypothesis of no change in fish species composition between historical and current time periods within and among biotic units using an analysis of similarities (ANOSIM). ANOSIM provides a way to evaluate whether there is a significant difference between two or more groups of sampling units on a dissimilarity matrix, in our case, using the Bray-Curtis index of similarity (Clarke 1993). We also tested whether TH was more prevalent than TD (or vice versa) using a Pearson χ 2 (chi-square) test under the null hypothesis that TH and TD were equally likely to occur. Data transformation, estimation of ecological indices, and hypothesis testing were carried out using the package vegan (Oksanen et al. 2011) and core functions in R (R Development Core Team 2015).
Hierarchical clustering of presence-absence data from native and non-native fishes
Hierarchical clustering from presence-absence data was undertaken using the R package gplots. Our rationale was to gauge the impacts of widely (more likely to generate TH) versus narrowly distributed taxa (more likely to generate TD) on temporal changes in compositional similarity. We used gplots’ function “heatmap.2” and Ward’s minimum variance as clustering method from Euclidean distances to build a dendrogram without reordering watershed information. Keeping watershed information helped interpreting clusters in a geographic context (from low to high latitudes). We applied three internal measures—Dunn’s index, connectivity, and silhouette width—using the package clValid in R to validate the most probable number of clusters, which we varied between two and six. Merits and applications of these measures have been discussed elsewhere (Handl et al. 2005).
Results
Historical and current freshwater fish assemblages
The historical dataset of native fishes comprised 7 orders, 11 families, 17 genera, and 44 species of which 82 % were endemic and 72 % were classified within various categories of conservation (see Additional file 2: Table S1). Siluriforms (25 %), followed by Atheriniforms (20.4 %) and Osmeriforms (18.2 %), were the most represented orders of native fishes. The current dataset showed an increment in the number of orders (10), families (16), genera (33), and species (69) with a total of 27 non-native species, of which 19 can be considered invasive and 8 can be considered naturalized (Additional file 2: Table S1).
Homogenization analyses
Prevalent TH across Chile and among biotic units
We estimated a global CScur = 41.5 % and CShis = 40.3 % resulting in ∆CSav = 1.24 % and suggesting an overall TH for Chile’s freshwater fish assemblages. A total of 65.3 % of watersheds in the entire country showed TH, in contrast with 35.7 % that showed TD (Fig. 2). Most comparisons among biotic units showed a marked pattern of TH as suggested by Pearson χ 2 tests (Table 1), supporting the result for the entire country. The highest value of TH was found between Atacama (U2) and South (U5), whereas comparisons between Central (U4) and South (U5) were the sole exception as they showed TD to be more prevalent than TH. The highest proportion of watersheds with no change in CS over time was found in comparisons between South (U5) and Patagonia (U6). Significant differences between historical and current fish assemblages were found through ANOSIM across all comparisons (Table 1).
Scatterplot of historical (CShis: x-axis) versus current similarity composition (CScur: y-axis) of Chile’s freshwater fishes between 201 watersheds examined in this study. The diagonal line represents no temporal change in compositional similarity (ΔCS = 0). Empty circles above the diagonal line are positive values (ΔCS > 0) suggesting taxonomic homogenization, whereas filled circles below the diagonal line are negative (ΔCS < 0) values suggesting taxonomic differentiation
Prevalent TD within biotic units
Within all biotic units, a process of TD was evident (Table 2). Titicaca (U1) and Atacama (U2) showed the highest TD, whereas South (U5) showed the lowest. Patagonia (U6) showed the highest proportion of watersheds that did not change in CS over time. Results from the ANOSIM demonstrated significant differences between historical and current composition of fish assemblages within all biotic units, except for Central (U4). Pearson χ 2 tests indicated that TD was significantly more prevalent than TH for U1, U2, and U4. For the remaining biotic units, we found no evidence that TD was significantly more prevalent than TH.
Hierarchical clustering of presence-absence data from native and non-native fishes
The dendrogram suggested the presence of two clusters of fish assemblages (Fig. 3). Clusters were supported by the highest estimates of Dunn’s index (0.55) and silhouette width (0.39) and the lowest estimate of connectivity (11.02), thus, all three measures showed agreement. Cluster I comprised taxa with a narrow geographic distribution, spanning one or two biotic units. Native fishes included Pupfishes (genus Orestias), Silversides (genus Odontesthes), Pencil Catfishes (genus Trichomycterus), and Velvet Catfishes (genus Diplomystes); non-native fishes included Brook Charr (Salvelinus fontinalis), Lake Whitefish (Coregonus clupeaformis), Chum Salmon (Oncorhynchus keta), and the Uruguay Tetra (Cheirodon interruptus). Exceptions were the non-native Mosquitofishes Gambusia holbrooki and G. affinis, which spanned five units and native Silversides Odontesthes mauleanum and O. brevianalis that spanned four units. Cluster II comprised taxa with a wide geographic distribution that spanned four to six biotic units. Native fishes included Puyes (Galaxias maculatus and G. platei), Creole Perch (Percichtys trucha), and Pouched Lamprey (Geotria australis). Non-native fishes included several species of Pacific Salmon (genus Oncorhynchus) such as Coho (O. kisutch), Chinook (O. tshawytscha), and Rainbow Trout (O. mykiss). Rainbow Trout showed presence in all six biotic units as did the European Brown Trout (Salmo trutta).
Dendrogram of Chile’s current freshwater fish taxa (x-axis) presence (black area) and absence data (light-grey area) distributed among six biotic units ordered from north to south (y-axis: U1 Titicaca, U2 Atacama, U3 Central, U4 South-Central, U5 South, U6 Patagonia). Two clusters (I y II) can be identified that are separated by a white vertical line. Native and alien (non-native) taxa have been suffixed with “N” and “A,” respectively
Discussion
Our findings unraveled a large-scale process of TH that has significantly changed the structure of Chile’s historical fish assemblages over the past century. This process has been pervasive among biotic units, with the comparison between Central (U3) and South (U5) as the sole exception, and it might be invariably linked to widely distributed non-native species. Distributions for some of these span all six biotic units as well as many others that are distributed among four or five biotic units. Our results are consistent with many other studies that show the loss of uniqueness in regions worldwide after fish species introductions (Olden et al. 2008, Leprieur et al. 2008, Marr et al. 2010, 2013). Conversely, TD has been the dominant process within biotic units, and many non-native taxa with narrow geographic distributions might be the cause for this process. Parallel assessments in regions with similar climates have been crucial to improve our understanding of drivers of biological invasions (e.g., plants: Pauchard et al. 2004; fishes: Marr et al. 2010, 2013; but see Baiser et al. 2012). Marr et al. (2010) compared five Mediterranean climate regions of the world, including central Chile (32°S–40°S); they found that TH was prevalent with a ∆CSav = 8.4 %, and this is higher than our estimate for comparisons involving watersheds in Central (U3) and South-Central (U4) (∆CSav = 2.9 %). Clearly, scale (level of resolution or grain and geographic coverage) has an impact on assessments of TH as well as differential species richness and turnover (see McKinney 2004; Olden, 2006; Baiser et al. 2012). Because we document no local extinctions, species turnover seems less likely to have a significant impact, suggesting a difference in the level of resolution might be the best explanation for differences between Marr et al. (2010) and this study.
Which species and processes may influence TH in freshwaters of Chile?
Not all non-native fishes have contributed to TH of Chile’s fish fauna as evidenced by our hierarchical clustering analyses. So-called naturalized species with narrow geographic distributions are likely to contribute to TD within and between biotic units rather than TH, which is seemingly driven by invasive species widely distributed across watersheds. All three dimensions—the invader, biotic interactions, and human—have been recognized as crucial to understand the complexity of invasions (Arismendi et al. 2014), even though their importance are still the subject of much debate (Hayes and Barry 2008; Martin et al. 2009; Sol et al. 2012). Parallel patterns and processes of invasions among Chile’s watersheds and regions around the world might help evaluate the importance of such dimensions. Indeed, similarities between invasive taxa in Chile and many regions around the world are astounding; they include salmonids, cyprinids, and poeciliids (Ruesink, 2005; Clavero and García-Berthou 2006; Marr et al. 2010; Pool and Olden 2012). These are discussed below in separate paragraphs.
Salmonids are among the most widely introduced fishes worldwide (Crawford and Muir 2008); Rainbow Trout has been introduced in 87 countries and Brown Trout has been introduced in 40 countries (Kitano 2004). Introduction of salmonids in Chile has occurred since the late 1800s motivated by the establishment of recreational fisheries, with stocking efforts as common practice since 1960 until the present (Basulto 2003; Arismendi and Nahuelhual 2007; Arismendi et al. 2014). During the last three decades, both recreational fishing and salmon farming have experienced an explosive growth, especially in the southern portion of the country spanning South (U5) and Patagonia (U6) units (Basulto 2003; Gajardo and Laikre 2003; Arismendi et al. 2009, Buschmann et al. 2009; Arismendi et al. 2014). However, both Rainbow Trout and Brown Trout are also abundant at northern latitudes as far as Atacama (U2), which likely explains the highest estimates of TH found in our study when we compared U2 and U5. Collectively, propagule pressure, species traits (e.g., demography, migratory life history, phenotypic plasticity, and physiological tolerance), and low environmental resistance have concomitantly contributed to the current dominance of free-living salmonids and their invasion success in Chile (Soto et al. 2006; Arismendi et al. 2014). Yet, environmental and landscape attributes such as temperature, connectivity between lakes, and hydrological position have been documented to restrict the viability and current abundance of both native fishes and salmonids in Patagonia (U6: Soto et al. 2006; Habit et al. 2012; Correa and Hendry 2012).
Other human activities that have likely facilitated invasions and promoting TH are the use of non-native species for biological control and the artificial alteration of habitat components (see Marchetti et al. 2001; Alcaraz et al. 2005; Olden et al. 2006). First, species such as Gambusia holbrooki, Cyprinus carpio, Cnesterodon decenmaculatus, and Ctenopharyngodon idella were initially introduced for biological control (Ruiz and Marchant 2004; Iriarte et al. 2005; De los Ríos 2010). Mosquitofish (G. holbrooki) have spread throughout the country from Atacama (U2) to Patagonia (U6), while Carp (C. carpio) range from Central (U3) to South (U5). Second, invasive Carp dominate most tributaries of the Bio-Bío River and coastal eutrophic lakes in the South-Central (U4) unit, which have been intensively affected by cellulose waste discharges (see Parra et al. 2003; Habit et al. 2006b). Similarly, Olden et al. (2008) found that habitat alteration by human settlements, infrastructure, and land use are chief factors explaining the degree of TH of fish assemblages between Australian watersheds. In the particular case of Chile, further research is needed to gauge the relative importance of these factors driving different processes of TH between regions, so we can predict effects of existing invasive species and prevent future invasions (Clavero and García-Berthou 2006).
Implications for conservation of native fishes and strategies for management of non-native species
One of the chief implications of our results is the impact of TH in a large and isolated region of South America containing 44 native species, of which 81 % are endemic (Vila et al. 1999). The species responsible appear to be non-native and invasive fishes that have caused a loss of taxonomic regional distinctiveness and a reduction in the variability of fish communities (e.g., Olden et al. 2004, Olden 2006, Clavero and García-Berthou 2006). Although we have yet to document native extinctions, there is overwhelming evidence on the negative ecological impacts of salmonids on Chile’s native fishes (Vargas et al. 2010; Correa and Hendry 2012; Arismendi et al. 2012; Arismendi et al. 2009; Habit et al. 2012; Habit et al. 2010). During the last decades, there has been a shift from an enthusiastic promotion of species introductions to a more conscientious view of native fish fauna and its conservation value. The first attempt to protect freshwater fauna came from the regulation of recreational fishing, but this regulation only targeted introduced trout and salmon species (see Soto et al. 2006; Arismendi and Nahuelhual 2007). Later, the identification and protection of public lands has contributed to discouraging new introductions, particularly in those protected areas, as overseen by the National Plan for Biodiversity Conservation and the creation of the National System of Wild Areas Protected by the State (Sistema Nacional de Áreas Silvestres Protegidas por el Estado, SNASPE) in 1984. This system covers less than 20 % of watersheds contemplated in our study but includes most aquatic systems of Titicaca (U1) and Atacama (U2) units. This is encouraging because Atacama (U2) is an area with high endemism (Dyer, 2000a) that shows the highest estimates of TH and shares most invasive fishes with other units that stretch as far south as South (U5) and Patagonia (U6). SNASPE exerts special protection of native fishes indirectly by promoting the recreational fishery of salmonids. The potential for the use of these watersheds to establish hatcheries of salmonids in the future will increase the risk of fish escapements (Sepulveda et al. 2013) that in turn will promote the expansion of existing aquaculture-related fish introductions.
The introduction of salmonids has resulted in arguably the most complex management strategy of non-native freshwater fishes. Due to their social and economic importance as game species, eradication is unlikely to occur. However, an alternative such as co-existence (reviewed by Arismendi et al. 2014) may require a drastic reduction of propagule pressure and their negative impacts (Simberloff 2009). Mitigation of escapes from farms may be one of these measures (Sepulveda et al. 2013), and one of the pressing issues is that escape events are often under reported (Buschmann et al. 2009; Sepúlveda et al. 2009). While escapes of Atlantic Salmon are the highest in number, because it is the most farmed salmonid species in Chile, they may be quickly removed by fishers or fail to establish in the wild, possibly due to multiple generations of domestication selection (Soto et al. 2001). Escapes of Rainbow Trout may be more damaging, and have longer lasting consequences to the environment as they can establish self-sustaining populations in the wild more rapidly, than any other salmonid (Sepulveda et al. 2013). Some initiatives to prevent the escape of salmonids from aquaculture facilities in South (U5) and Patagonia (U6) have recently ocurred including the Environmental Regulation for Aquaculture (Regulación Ambiental para la Acuicultura, RAMA) during 2001 and the self-regulation from the salmon farming industry through the Integrated Management System of Salmon Chile (Sistema Integrado de Gestión de Salmon Chile, SIGES) in 2003. An incipient and yet not significant process of TH affecting South (U5) and Patagonia (U6) units therefore represents a unique opportunity to implement efficient management measures aimed at slowing down the rate of this process.
Caveats and limitations
Our study has limitations that commonly affect biogeography studies combining information from literature review and quantitative approaches from community ecology. These might include, but are not limited to, the occurrence of false negative detections, taxonomic discrepancies, the selection of specific ecological indices, and issues related to the poor knowledge about fish distributions prior to species introductions. Regarding the first limitation, we made our best effort to include both the primary and grey literature, which resulted in 227 documents (Additional file 1: Appendix S1), even though there is a possibility for some introduced species to be undetected. It also remains possible that individuals from many non-native species that were introduced to Chile’s watersheds fail in their establishment (reviewed by Arismendi et al. 2014). This could be the case for Sockeye (Oncorhynchus nerka), Chum (O. keta), and Pink salmon (O. gorbusha). Despite a well-documented history of introductions, their establishment remains contentious (Arismendi et al. 2014). Regarding taxonomic discrepancies, we included two species of mosquitofishes (Gambusia hoolbroki and G. affinis), but Ruiz and Marchant (2004) suggest that there is only one species, G. hoolbroki. We thus welcome further taxonomic and phylogenetic assessments to validate this issue. Indeed, recent phylogeographic studies of native Orestias, Basilichthys, Hatcheria, Trichomycterus, and Aplochiton have provided molecular evidence for increasing or decreasing the number of taxa, and such evidence is incorporated in our analyses to the extent of our knowledge (e.g., Vila 2006; Unmack et al. 2009; Quezada-Romegialli et al. 2010; Vila et al. 2011; Véliz et al. 2012; Alò et al. 2013). Regarding our choice of Sørensen’s index, this has been sometimes criticized as it may fail to distinguish the effects of species turnover and species richness on beta diversity (Baselga 2010, Carvalho et al. 2012, Baisier et al. 2012). However, with little or no evidence for local extinctions of native Chile’s fishes, the role of species turnover remains questionable to explain changes in beta diversity among watersheds. Lastly, the exact fish composition of most basins included here is unknown before fish introductions, but this issue is commonly found elsewhere (e.g., Olden and Rooney 2006; Marr et al. 2010). Thus, the decision about defining time periods (historical and current) is based on best judgement supported by an exhaustive review of 227 documents.
Conclusions
Following two centuries of human-mediated introduction of fishes to Chile, to our knowledge, this is the first attempt to provide a comprehensive and broad examination of TH, even though TD was also evident at smaller spatial scales, such as within biotic units. We show that species introductions and invasions have altered historical fish assemblages and affected the uniqueness of isolated and endemic freshwater fish diversity. Our results provide taxonomic and biogeography baseline information for conservation of native species and management of non-native taxa. Finally, we also have shown that not only have salmonids led TH in Chile but also other groups of species, such as mosquitofishes (genus Gambusia), and this is also well documented for other climatically similar regions of the world. Effective management recommendations have to include watershed-specific management alternatives given the isolated nature of the biogeography of native fishes. It is also imperative to acknowledge the diversity of biotic units and their differential composition of introduced species. A consideration of a full range of alternatives such as prevention, control, eradication, and restoration will allow a better understanding of the role of multiple interacting stressors during the invasion process (Arismendi et al. 2014). Management of species introductions should additionally include the tradeoff between valuable fisheries and aquaculture operations and the conservation and persistence of native species from freshwater ecosystems.
Abbreviations
- TH:
-
taxonomic homogenization
- TD:
-
taxonomic differentiation
- CS:
-
compositional similarity
- ANOSIM:
-
analysis of similarities
- SNASPE:
-
National System of Wild Areas Protected by the State (Sistema Nacional de Áreas Silvestres Protegidas por el Estado, in Spanish)
- RAMA:
-
Environmental Regulation for Aquaculture (Regulación Ambiental para la Acuicultura, in Spanish)
- SIGES:
-
Integrated Management System of Salmon Chile (Sistema Integrado de Gestión de SalmonChile, in Spanish)
References
Alcaraz C, Vila-Gispert A, García-Berthou E (2005) Profiling invasive fish species: the importance of phylogeny and human use. Divers Distrib 11:289–298
Alò D, Correa C, Arias C, Cárdenas L (2013) Diversity of Aplochiton fishes (Galaxiidea) and the Taxonomic Resurrection of A. marinus. PLoS One 8:e71577
Arismendi I, Nahuelhual L (2007) Non-native salmon and trout recreational fishing in Lake Llanquihue, Southern Chile: economic benefits and management implications. Rev Fish Sci 15:311–325
Arismendi I, Soto D, Penaluna B, Jara C, Leal C, León-Muñoz J (2009) Aquaculture, non-native salmonid invasions, and associated declines of native fishes in lakes of the northern Chilean Patagonia. Freshw Biol 54:1135–1147
Arismendi I, González J, Soto D, Penaluna B (2012) Piscivory and diet overlap between two non-native fishes in southern Chile. Austral Ecology 37:346–354
Arismendi I, Penaluna BE, Dunham JB, García De Leaniz C, Soto D et al (2014) Differential invasion success of introduced salmonids in Southern Chile: patterns and hypotheses. Rev Fish Biol Fish 24:919–941
Arratia G (1978) Comentarios sobre la introducción de peces exóticos en aguas continentales de Chile. Ciencias Forestales 1:21–30
Arratia G (1997) Brazilian and austral inland fish faunas of Southern America. In: Ulrich H (ed) A contrast. Tropical biodiversity and systematic. Museum Alexander Koenig, Bonn, pp 179–187
Arratia G (2002) La importancia de algunos peces Chilenos en el contexto evolutivo. Anales Academia de Ciencias 7:71–84
Arratia G, Rojas G, Chang A (1981) Géneros de peces de aguas continentales de Chile. Museo Nacional de Historia Natural Santiago de Chile. Publicación Ocasional 34:3–118
Baiser B, Record S, Olden JD, Lockwood JL, Mckinney ML (2012) Pattern and process of biotic homogenization in the New Pangaea. Proc R Soc B Biol Sci 279:4772–4777
Baselga A (2010) Partitioning the turnover and nestedness components on beta diversity. Glob Ecol Biogeogr 19:134–143
Basulto S (2003) El Largo Viaje de los Salmones: Una Crónica Olvidada, Propagación y Cultivo de especies Acuáticas en Chile. Editorial Maval Ltda, Santiago
Buschmann AH, Cabello F, Young K, Carvajal J, Varela DA, Henríquez L (2009) Salmon aquaculture and coastal ecosystem health in Chile: analysis of regulations, environmental impacts and bioremediation systems. Ocean Coast Manag 52:243–249
Carvalho JC, Cardoso P, Gomes P (2012) Determining the relative roles of species replacement and species richness differences in generating beta-diversity patterns. Glob Ecol Biogeogr 21:760–771
Clarke K (1993) Non-parametric multivariate analysis of changes in community structure. Aust J Ecol 18:117–143
Clavero M, García-Berthou E (2006) Homogenization dynamics and introduction routes of invasive freshwater fish in the Iberian Peninsula. Ecol Appl 16:2313–2324
Correa C, Hendry AP (2012) Invasive salmonids and lake order interact in the decline of puye grande Galaxias platei in western Patagonia lakes. Ecol Appl 22:828–842
Crawford SS, Muir AM (2008) Global introductions of salmon and trout in the genus Oncorhynchus: 1870–2007. Reviews on fish Biology and Fisheries 18:313–344
De Los Ríos PR (2010) Freshwater ecosystems in oceanic islands of Chile: Conservation of endemic microfauna and the role of introduced species in the biological control of tropical diseases. Rev Chil Hist Nat 83:549–460
Dudgeon D, Arthington AH, Gessner MO, Kawabata ZI, Knowler C et al (2006) Freshwater biodiversity: importance, threats, status and conservation challenges. Biol Rev 81:163–182
Dyer B (2000a) Systematic review and biogeography of the freshwater fishes of Chile. Estudios Oceanológicos 19:77–98
Dyer B (2000b) Revisión sistemática de los pejerreyes de Chile (Teleostei, Atheriniformes). Estudios Oceanológicos 19:99–127
Gajardo G, Laikre L (2003) Chilean aquaculture boom is based on introduced salmon resources: a conservation paradox. Conserv Biol 17:1173–1174
Habit E, Dyer B, Vila I (2006a) Estado de conocimiento de los peces dulceacuícolas de Chile. Gayana 70:100–113
Habit E, Belk MC, Tuckfield RC, Parra O (2006b) Response of fish community to human-induced changes in the Bio-bio river in Chile. Freshwater Biology 51:1–11
Habit E, Piedra P, Ruzzante DE, Walde SJ, Belk MC et al (2010) Changes in the distribution of native fishes in response to introduced species and other anthropogenic effects. Glob Ecol Biogeogr 19:697–710
Habit E, Gonzalez J, Ruzzante DE, Walde SJ (2012) Native and introduced fish species richness in Chilean Patagonian lakes: inferences on invasion mechanisms using salmonid-free lakes. Divers Distrib 18:1153–1165
Handl J, Knowles J, Kell DB (2005) Computational cluster validation in post-genomic data analysis. Bioinformatics 21:3201–3212
Hayes KR, Barry SC (2008) Are there any consistent predictors of invasion success? Biol Invasions 10:483–506
Hopper DV, Adair EC, Cardinale BJ, Byrnes JEK, Hungate BA et al (2012) A global synthesis reveals biodiversity loss as a major driver of ecosystem change. Nature 486:105–108
Ibarra J, Habit E, Barra B, Solís K (2011) Juveniles de salmón Chinook (Oncorhynchus tshawytscha Walbaum, 1792) en ríos y lagos de la Patagonia Chilena. Gayana 75:17–25
Iriarte JA, Lobos GA, Jaksic FM (2005) Invasive vertebrate species in Chile and their control and monitoring by government agencies. Rev Chil Hist Nat 78:143–154
Johnson PTJ, Olden J, Vander Zanden MJ (2008) Dam invaders: impoundments facilitate biological invasions into freshwaters. Front Ecol Environ 6:357–363
Kitano S (2004) Ecological impacts of rainbow, brown and brook trout in Japanese inland waters. Global Environ Res 8:41–50
Leprieur F, Beauchard O, Hugueny B, Grenouillet G, Brosse S (2008) Null model of-biotic homogenization: a test with the European freshwater fish fauna. Divers Distrib 14:291–300
Mack R, Simberloff D, Lonsdale WM, Evans H, Clout M et al (2000) Biotic invasions: causes, epidemiology, global consequences, and control. Ecol Appl 10:689–710
Marchetti M, Light T, Feliciano J, Armstrong T, Hogan Z et al (2001) Homogenization of California’s fish fauna through abiotic change. In: Lockwood JL, Mckinney ML (eds) Biotic homogenization. Kluwer Academic/ Plenum Publishers, New York, pp 33–56
Marr SM, Marchetti MP, Olden JD, García-Berthou E, Morgan DL et al (2010) Freshwater fish introductions in Mediterranean-climate regions: are there commonalities in the conservation problem? Divers Distrib 16:606–619
Marr SM, Olden JD, Leprieur F, Arismendi I, Caleta M et al (2013) A global assessment of freshwater fish introductions in Mediterranean-climate regions. Hydrobiologia. doi:10.1007/s10750-013-1486-9
Martin P, Canham C, Marks PL (2009) Why forests appear resistant to exotic plant invasions: intentional introductions, stand dynamics, and the role of shade tolerance. Front Ecol Environ 7:142–149
McKinney M (2004) Do exotics homogenize or differentiate communities? Roles of sampling and exotic species richness. Biol Invasions 6:495–504
Oksanen J, Blanchet FG, Kindt R, Legendre P, Minchin PL et al. (2011) vegan 2.0-2: Community Ecology Package. URL http://vegan.r-forge.r-project.org/. (accessed September 14, 2015 )
Olden JD (2006) Biotic homogenization: a new research agenda for conservation biogeography. J Biogeogr 33:2027–2039
Olden JD, Poff NL (2004) Ecological processes driving biotic homogenization: testing a mechanistic model using fish faunas. Ecology 85:1867–187
Olden JD, Rooney TP (2006) On defining and quantifying biotic homogenization. Glob Ecol Biogeogr 15:113–120
Olden JD, Poff NL, Douglas MR, Douglas ME, Fausch KD (2004) Ecological and evolutionary consequences of biotic homogenization. Trends Ecol Evol 19:18–24
Olden JD, Poff NL, Bestgen KR (2006) Life history strategies predict fish invasions and extirpation in the Colorado River Basin. Ecol Monogr 76:25–40
Olden JD, Kennard MJ, Pusey JB (2008) Species invasions and the changing biogeography of Australian freshwater fishes. Glob Ecol Biogeogr 17:25–37
Parra O, Valdovinos C, Urrutia R, Cisternas M, Habit E et al (2003) Caracterización y tendencias tróficas de cinco lagos costeros de Chile. Limnética 22:51–83
Pauchard A, Cavieres LA, Bustamante RO (2004) Comparing alien plant invasions among regions with similar climates. Where to from here? Divers Distrib 10:371–375
Penaluna B, Arismendi I, Soto D (2009) Evidence of interactive segregation between introduced trout and native fishes in northern Patagonian Rivers, Chile. Trans Am Fish Soc 138:839–884
Pool TK, Olden JD (2012) Taxonomic and functional homogenization of an endemic desert fish fauna. Divers Distrib 18:366–376
Quezada-Romegialli C, Vila I, Véliz D (2009) A new invasive freshwater fish species in Central Chile: Jenynsia Multidentata (Jenyns, 1842) (Cyprinodontiformes: Anablepidae). Gayana 73:233–236
Quezada-Romegialli C, Fuentes M, Véliz D (2010) Comparative population genetics of Basilichthys microlepidotus (Atheriniformes, Atherinopsidae) and Tichomycterus areolatus (Siluriformes, Trucomychteridae) in north central Chile. Environ Biol Fish 89:173–186
R Development Core Team (2015) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria, URL http://www-r-project.org/. (accessed December 14, 2015)
Rahel FJ (2000) Homogenization of fish faunas across the United States. Science 288:854–856
Rooney TP, Wiegmann SM, Rogers DA, Waller DM (2004) Biotic impoverishment and homogenization in unfragmented forest understory communities. Conserv Biol 18:787–798
Ruesink JL (2005) Global analysis of factors affecting the outcome of freshwater fish introductions. Conserv Biol 19:1883–1893
Ruiz VH and Marchant M (2004) Ictiofauna de aguas continentales chilenas. 1ra edición. Departamento de Zoología, Universidad de Concepción, Concepción.
Sepúlveda M, Farías F, Soto E (2009) Salmon escapes in Chile. WWF Chile, Valdivia
Sepulveda M, Arismendi I, Soto D, Jara F, Farias F (2013) Escaped farmed salmon and trout in Chile: incidence, impacts, and the need for an ecosystem view. Aquac Environ Interact 4:273–283
Simberloff D (2009) The role of propagule pressure in biological invasions. Annu Rev Ecol Evol Syst 40:81–102
Sol D, Maspons J, Vall-Llosera M, Bartomeus I, García-Peña G et al (2012) Unraveling the life history of successful invaders. Science 337:580–58
Sørensen T (1957) A method of establishing groups of equal amplitude in plant sociology based on similarity of species and its application to analyses of the vegetation on Danish commons. Biologiske Skrifter. Kongelige Danske Videnskabernes, Selskab 5:1–34
Soto D, Jara F, Moreno C (2001) Escaped salmon in the inner seas, southern Chile: facing ecological and social conflicts. Ecol Appl 11(6):1750–1762. doi:10.2307/3061093
Soto D, Arismendi I, González J, Sanzana J, Jara F et al (2006) Southern Chile, trout and salmon country: invasion patterns and threats for native species. Rev Chil Hist Nat 79:97–117
Unmack PJ, Habit EM, Johnson JH (2009) New records of Hatcheria macraei (Siluriformes, Trichomycteridae) from Chilean province. Gayana 73:102–110
Vargas P, Arismendi I, Lara G, Millar J, Peredo S (2010) Evidencia de solapamiento de micro-hábitat entre juveniles del salmón introducido Oncorhynchus tshawytscha y el pez nativo Trichomycterus areolatus en el río Allipén, Chile. Rev Biol Mar Oceanogr 45:285–292
Véliz D, Catalán L, Pardo R, Acuña P, díaz P et al (2012) The genus Basilichthys (Teleostei: Atherinopsidae) revisited along its Chilean distribution range (21° to 40° S) using variation in morphology and mtDNA. Rev Chil Hist Nat 85:49–59
Vila I (2006) A new species of killifish in the genus Orestias (Teleostei: Cyprinodontidae) from the southern high Andes, Chile. Copeia 3:473–477
Vila I, Pardo R (2006) Peces límnicos. In: CONAMA( ed) Biodiversidad de Chile: Patrimonio y desafíos. Editores Ocho Libros, Santiago, Chile, pp 306–311
Vila I, Fuentes L, Contreras M (1999) Peces límnicos de Chile. Boletín del Museo Nacional de Historia Natural 48:61–75
Vila I, Pardo R, Dyer B, Habit E (2006) Peces límnicos: diversidad origen y estado de conservación. In: Vila I, Veloso A, Schlatter R, Ramírez C (eds) Macrófitas y vertebrados de los ecosistemas límnicos de Chile. Editorial Universitaria, Santiago, pp 72–102
Vila I, Scott S, Mendez MA, Valenzuela F, Iturra P, Poulin E (2011) Orestias gloriae, a new species of cyprinodontid fish from saltpan spring of the southern high Andes (Teleostei: Cyprinodontidae). Inchthyological Exploration of Freshwaters 22:345–353
Zunino S, Baeza M, Quiroz S, Rivera R (1999) Ampliación distribucional de la carmelita Percilia gillissi Girard, 1854 (Pisces: Perciliidae). Anales del Museo de Historia Natural de Valparaíso 24:119–120
Acknowledgements
CONICYT N°22110331 scholarship supported PVV through a Master of Science program in Zoology at Universidad de Concepción. FONDAP 15110027, FONDECYT 1130807, and DIUC 212.113.082-1.0 provided funding to DG-U to conduct research on invasive salmonids. Cristian Correa provided recommendations and constructive criticisms that improved an earlier draft. We also appreciate valuable suggestions and discussions on these topics from Javier Millar, Brooke Penaluna, Ximena Sierra, Irma Vila, Victor Ruiz, and Enrique Rodriguez.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
PVV and IA conceived the study. PVV assembled the database and performed the majority of analyses and interpretation in collaboration with DG-U. PVV and DG-U drafted the manuscript. All authors read and approved the final manuscript.
Authors’ information
PVV is a Biologist and Master of Science (Zoology) student at the Department of Zoology, Universidad de Concepción. Her interests revolve around the ecological and biogeographic impacts of invasive fishes on native freshwater fish communities. She is also interested in developing management and conservation strategies for native fishes. IA is an Assistant Professor (Senior Research) at the Department of Fisheries and Wildlife, Oregon State University. His research focuses on the ecology of invasive species in temperate ecosystems and climate change influences on hydrological processes in forested streams. DG-U is an Assistant Professor in the Department of Zoology at Universidad de Concepción. He is interested in the application of genomic tools to resolve relevant questions in ecology, evolution, and conservation. Currently, he and his group study invasive salmonids in South America.
Additional files
Additional file 1: Appendix S1.
References used to build the presence-absence data set. (DOCX 69 kb)
Additional file 2: Table S1.
Native and non-native (alien) fishes present in inland waters in Chile. (DOCX 24 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Vargas, P.V., Arismendi, I. & Gomez-Uchida, D. Evaluating taxonomic homogenization of freshwater fish assemblages in Chile. Rev. Chil. de Hist. Nat. 88, 16 (2015). https://doi.org/10.1186/s40693-015-0046-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40693-015-0046-2