Abstract
Background
A rational and high-performance high-throughput screening system effectively improves the efficiency of strain screening.
Results
A rapid and efficient method was developed to detect carbon sources. And then by using glucose analog as screening pressure and two indexes for sophorolipids (SLs) production and glycerol consumption, a mutant of Candida bombicola L1.3 was successfully selected among 3000 mutants. It produces high SLs titer and co-utilizes glucose and glycerol simultaneously.
Conclusions
Compared to the wild-type strain, L1.3 exhibited 15.06% higher SLs titer and 35.69% higher glycerol consumption capacity in a 5-L bioreactor. We believe that L1.3 can potentially be used for the efficient industrial production of SLs with simple downstream processing for separating SLs and glycerol.

Similar content being viewed by others
Introduction
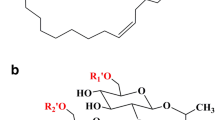
Sophorolipids (SLs) are biologically derived surfactants harboring a high biodegradability and low toxicity, thus being widely applied in food, cosmetic, pharmaceutical, and oil industries (Jezierska et al. 2018). SLs are mainly synthesized in yeast strains including Wickerhaniella (Chen et al. 2005), Pichia (Thaniyavarn et al. 2008), Rhodotorula (Sen et al. 2017), and Candida (Konishi et al. 2018). SLs are composed of a hydrophilic sophorose and a hydrophobic fatty acid, and harbor a wide structural diversity (Fig. 1) (Saerens et al. 2011; Hu and Ju 2001).
Glucose and oil are used as important hydrophilic and hydrophobic substrates, respectively, that are used in the fermentation of SLs (Shah et al. 2017). However, the use of fatty acids as substrates for synthesizing SLs implies that glycerol remains in the broth and that only a small part of it can further be metabolized. Increased concentrations of residual glycerol significantly affect the rheological properties of the broth influencing mass transfer and mixing. This in turn affects cell growth and metabolism. Moreover, residual glycerol complicates the processes of SLs separation and purification. Therefore, it is of great importance to obtain strains producing a high SLs content by co-utilizing glycerol and glucose capacity. This will not only improve the production yields of SLs, but also simplify the downstream processing.
Carbon catabolite repression is considered to be present inmost microorganisms. Indeed, microbes can favorably use carbon sources such as glucose that are quickly metabolized and repress the expression of enzymes involved in the metabolism of other slowly metabolized carbohydrates (Gancedo 1998). Thus, during SLs fermentation, residual glycerol in the broth can hardly be consumed since the concentration of available glucose is always maintained in the range of 40–80 g/L. In general, glucose analogs such as 2-deoxy-d-glucose (Afanas’Eva and Burd 1980) were used for breeding strains with eliminated or alleviated carbon catabolic repression (Zhu et al. 2018). On the other hand, a high-performance producing strain can increase the competitiveness of the companies. High-throughput screening technologies playa key role in reaching for improved strains, which is large scale, low cost, and high specificity (Ottenheim et al. 2018; Zhang et al. 2018). High-throughput screening technologies consist in generating a mutant library in high-throughput miniaturized cultivation platforms, such as microplates (Tan et al. 2013), shake flasks, micro-bioreactors (Tian et al. 2018), and using a high-throughput detection platform (Tan et al. 2014; Lv et al. 2016). Unlike previous reported studies, which only focused on single target of product titer or productivity, the present study characterized two indexes for the production of high SLs contents by co-utilizing glucose and glycerol capacity. Generally, SLs concentration is determined by high-performance liquid chromatography (HPLC), low-field nuclear magnetic resonance, or weighing method (Chen et al. 2019). The concentration of glycerol is usually determined by the periodate oxidation method, gas chromatography (GC), or high-performance liquid chromatography (HPLC) (Wu et al. 2011). However, these methods are not compatible with the high-throughput detection required in preliminary screening because they are time consuming and labor intensive. In a previous study, we developed a method for measuring SLs concentration with I2 since this molecule reacts with the double bond of the fatty acid chain in SLs (Zhou et al. 2019).
In this study, the iterative mutagenesis by atmospheric and room-temperature plasma (ARTP) technology was applied to Candida bombicola for constructing a mutant library. The I2 and Cu(OH)2 methods were used for detecting SLs and carbon sources concentrations, respectively. Furthermore, 2-deoxy-d-glucose was used as the rational screening pressure. This allowed us to identify a high SLs-producing mutant with co-utilizing glucose and glycerol capacity. The fermentation performance of the mutant was verified in shake flask and a 5-L bioreactor by the double indicators of SLs yield and glycerol consumption capacity.
Materials and methods
Microorganism, media, and culture conditions
Candida bombicola ATCC 22214 purchased from Guangdong Culture Collection Center (China) was stored at − 80 °C in 20% glycerol solution for SLs production.
The solid medium contained (g/L), glycerol 20, 2-deoxy-d-glucose 0.04% (w/w), yeast extract 10, peptone 20, and agar 20. The solid plate was cultured at 25 °C for 96 h.
The seed medium contained (g/L), glucose 50, KH2PO4 1, (NH4)2SO4 4, MgSO4·7H2O 0.5, corn steep liquor (CSL) 10. The seed cultured in a 1-L baffled shake flask with 200 mL working volume was carried out at 200 rpm and 25 °C for 96 h.
The fermentation medium (24-well microplates and shake flask) contained (g/L), glucose 100, glycerol 6, oleic acid 57.4, KH2PO4 1, (NH4)2SO4 4, MgSO4·7H2O 0.5, corn steep liquor 10, CaCO3 6. The strain was cultured in a 500-mL shake flask with 50 mL working volume for 96 h. The cultivation was conducted at 200 rpm and 25 °C.
The initial fermentation medium contained (g/L), glucose 100, KH2PO4 1, (NH4)2SO4 4, MgSO4·7H2O 0.5, corn steep liquor 10. All the media were sterilized at 115 °C for 30 min. The fermentation medium of 2.5 L in a 5-L bioreactor (Shanghai Guoqiang Bioengineering Equipment Co., Ltd., China) with an inoculum quantity of 3% was carried out at 25 °C for 168 h. Aeration was maintained at 0.5 vvm, and dissolved oxygen (DO) above 25% of saturation concentration by adjusting the agitation stepwisely and pH at 3.5 by adding 4 M NaOH solution were kept during the whole process. The concentrations of glucose and oil in the broth were maintained at 30–80 g/L and 2–10 g/kg, respectively.
High-throughput analytical methods for preliminary screening process
Determination of SLs concentration by I2 method
The hydrophobic hydroxyl fatty acid of SLs always contains one or two unsaturated double bonds, which can react with I2 by addition reaction. Thus, basing on this principle, a rapid and efficient determination of SLs can be realized by I2 method as described in our previous work (Zhou et al. 2019).
One milliliter I2 solution (containing 5 g/L KI), 1 mL SLs solution, 2 mL pH buffer (5.0), and 6 mL deionized water were sequentially added into a 10-mL colorimetric tube. The optical density at the wavelength of 350 nm of the mixture solution was measured after incubating at room temperature for 20 min.
Determination of carbon source concentrations by Cu(OH)2 method
Under alkaline condition, the freshly prepared Cu(OH)2 solution can react with polyhydroxyl compounds to form complex and navy blue solution, which has an absorption wavelength at 630 nm. Thus, both glucose and glycerol in the broth will cause this complexation reaction with Cu(OH)2 solution and this method could semi-quantitatively and qualitatively characterize the consumption of carbon sources (Norkus et al. 1995).
Freshly prepared Cu(OH)2 solution was composed of CuSO4 and NaOH solution, so the amount of Cu2+ should be slightly higher than the detection demand. The concentrations of CuSO4 solution were 2.5%, 5%, 7.5%, 10%, 12.5%, 15%, 17.5%, 20% (w/w). Then 150 μL CuSO4 and 200 μL of 50 g/L glycerol solution were sequentially added into 1.75 mL of 5% NaOH solution (w/v) and mixed vigorously for about 20 s. After standing for 5 min, the sample was centrifuged at 4000 rpm for 5 min. Finally, the optical density (OD) at 630 nm was recorded.
Since the complexation reaction should be carried out under alkaline conditions, the amount of NaOH should be much higher than Cu2+. The concentrations of NaOH were 1%, 3%, 5%, 7%, and 9% (w/w). And then 150 μL of 15% CuSO4 and 200 μL of 50 g/L glycerol solution were sequentially added into 1.75 mL NaOH solution (w/v) and mixed vigorously for about 20 s. After standing for 5 min, the sample was centrifuged at 4000 rpm for 5 min. Finally, OD at 630 nm was recorded.
Glucose analogs’ conditions
Effect of different carbon sources on cell growth
The strain was cultured in a 500-mL shake flask with 50 mL working volume for 72 h, in which the only carbon source was corn steep liquor (10 g/L) or glycerol (20 g/L), respectively. Glucose (20 g/L) as the only carbon source was used as the control group. The cell biomass was measured with OD600 every 12 h.
Determination of the concentration of 2-deoxy-d-glucose
2-Deoxy-d-glucose with the concentrations of 0, 0.01%, 0.05%, 0.1%, 0.5%, and 1% (w/w) were added into the media for solid plate and shake flask cultivations. The strain was cultured for 72 h with glycerol as the only carbon source.
High-throughput screening model for high SLs-producing strain with co-utilizing glucose and glycerol capacity
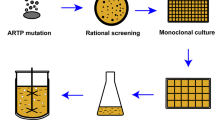
The flowchart of high-throughput screening model for high SLs-yielding C. bombicola with co-utilizing glucose and glycerol capacity is illustrated in Fig. 2. Firstly, 10 μL of the cell suspension (107–108 cells/mL) was treated by ARTP for 50 s with a lethality of approximately 90%. Subsequently, the cell suspension was mixed and cultured in the 96-well plates with 2-deoxy-d-glucose (0.05% w/w) and 10 g/L of glycerol (the only carbon source) for 72 h. Relatively, a clear zone with higher concentration of cell biomass was selected by OD600 detection, and then the cells were diluted to appropriate concentration and coated on the solid plate for 96 h cultivation. Subsequently, larger single colonies were selected and inoculated into 96-well plates for 48 h to obtain the seed culture. The seeds were transferred to 24-well plates for 96 h fermentation culture with an inoculation of 6%. After centrifugation at 4000 rpm for 5 min, the fermentation broth was diluted by 5 times and the supernatant was withdrawn for the determination of the concentrations of SLs and carbon source by I2 and Cu(OH)2 methods, respectively, which was miniaturized from the colorimetric tube of 10 mL volume to 96-well plates of 200 μL volume. Strains with better performances were selected for the rescreening procedure in the shake flask, in which the SLs production was more than 15% higher than that in the wild-type strain and higher consumption of glycerol was obtained at the same time. After sixteen rounds of iterative ARTP mutagenesis, a total of 150 potential mutant strains were screened out from 3000 mutants in the preliminary screening process, and then three top mutant strains were screened out through the rescreening process, in which glycerol concentration was analyzed by a glycerol detecting kit (Beijing Applygen Technologies Inc., China) and SLs was detected by DNS method. Finally, the fermentation performances of these three candidates were verified in a 5-L bioreactor and then the genetic stability was also tested afterward.
Analytical methods
Glucose concentration was analyzed by an enzymatic bio-analyzer (SBA-40C, Shandong Academy of Sciences, China). Concentration of oil in the broth was determined by weighing method in the verification process. Briefly, three parallel broth samples were extracted twice using the same volume of n-hexane for oil content determination. The upper layer was then transferred to another tube and dried for 24 h to constant weight by an oven. The bottom layer was then washed twice by alcohol and dried at 80 °C for 24 h to measure dry cell weight (DCW). Concentrations of SLs in the broth were determined by HPLC method. Two milliliter of fermentation broth was withdrawn and 2 mL of KOH/MeOH (4 M) solution was added, and then the mixture was heated at 80 °C for 15 min. After cooling to room temperature, methanol was added to a total volume of 10 mL, and NaH2PO4 pH buffer (0.2 M) was used to neutralize the solution. Finally, the sample was diluted to an appropriate concentration. For HPLC conditions, mobile phase (ammonium acetate, 0.02 mol/L; formic acid, 1% v/v; methanol, 75% v/v) C18 column (4.6 mm × 250 mm, Acchrom) refractive index detector (RID) and the flow rate of 0.9 mL/min were adopted. The injection volume was 20 μL, and the column and detector temperatures were controlled at 50 °C and 35 °C, respectively.
Results
Development of high-throughput analytical method for carbon sources detection
Evaluation of the Cu(OH)2 method for carbon sources detection
After fermentation in a 24-well microplate, 1 M NaOH was added to the broth to obtain an alkaline pH so that the lactone-form of SLs would be hydrolyzed into the acidic-form displaying a relatively high-water solubility.
As shown in Additional file 1: Fig. S1, in a 50-g/L glycerol solution, the absorbance increased with CuSO4 and NaOH concentrations. However, when the later were greater than 12.5% and 3%, respectively, the absorbance values were stable, indicating that glycerol had completely reacted. Therefore, the concentrations of the CuSO4 and NaOH solutions were set at 15% and 5%, respectively.
Standard curves of glycerol and glucose determination by the Cu(OH)2 method
As shown in Additional file 1: Fig. S2, the detection range of the Cu(OH)2 method for glycerol and glucose could be extended to at least 50 and 70 g/L, respectively. Overall, the glucose and glycerol concentrations were much lower than the maximum detection threshold. Thus, the standard curves were conducted between the ranges from 0 to 10 g/L and 40 g/L for glycerol and glucose determination, respectively (Additional file 1: Fig. S2).
The evaluating parameters of the spectrophotometric Cu(OH)2 method are shown in Additional file 1: Table S1. Seven replicates of glycerol (3 g/L) and glucose (20 g/L) standard solutions were assayed by the Cu(OH)2 method with the above optimized parameters. The average measured concentrations were 2.99 g/L and 20.01 g/L for glycerol and glucose, respectively. Standard deviation values were 0.045 g/L and 0.082 g/L for glycerol and glucose, respectively. These data showed that the Cu(OH)2 method is precise and accurate. Moreover, although Cu(OH)2 can simultaneously react with glycerol and glucose, the respective reactions do not interact with each other.
Rational high-through screening model
The carbon sources in the medium are glucose and glycerol while CSL is regarded as a nutrient-providing nitrogen. As shown in Additional file 1: Fig. S3, glucose was a favorable carbon source. Glycerol could also be used for cultivating C. bombicola with a longer lag phase. In contrast, CSL was obviously not suitable to support cell growth.
The molecular structure of 2-deoxy-d-glucose is similar to that of glucose. However, it cannot be utilized by the cells and has an inhibitory effect on cell growth. As illustrated in Fig. 3a and b, different concentrations of 2-deoxy-d-glucose would effectively inhibit cell growth on solid plates and shake flasks. The inhibitory concentrations were 0.05% and 0.04%, respectively. Therefore, these two concentrations of 2-deoxy-d-glucose were used in the following experiments.
High-throughput screening for high SLs-producing strain by co-utilizing glucose and glycerol capacity
After 16 rounds of iterative ARTP mutagenesis, a total of 3000 single colonies were selected on agar plates containing 20 g/L glycerol and 0.04% 2-deoxy-d-glucose and cultured in 24-well plates. As shown in Fig. 4a, the strains producing high SLs contents and showing high carbon source consumption were in the second quadrant. Among them, 150 mutants displayed 15% higher SLs production and a higher glycerol consumption compared to the parent strain. These strains were subsequently cultivated in shake flasks for rescreening (Fig. 4b). Finally, seven mutants with good performances were screened out. Among them, C. bombicola L1.1, L1.3, and L1.5 showed increased SLs yields and a glycerol consumption increased by more than 20% (Fig. 4c). Therefore, L1.1, L1.3, and L1.5 were selected for further analysis in a 5-L fermenter.
The results of preliminary screening with 24-well plates (a), rescreening in shake flasks (b, c). a The difference between yield of SLs of mutants and wild-type strain were taken as y-axis, and the carbon source residual (define as absorbance) of mutants and wild-type strain were taken as x-axis. b The differences between the yield of SLs and glycerol consumption of mutants and wild-type strain were taken as x-axis and y-axis, respectively
Validation of the fermentation performances of L1.1, L1.3, and L1.5
Although little changes were observed between the mutants and wild strain regarding cell growth, SLs production and glycerol consumption were significantly increased (Fig. 5). The final SLs titers of L1.1, L1.3, and L1.5 were increased by 8.13, 15.06, and 6.36%, respectively. However, glucose consumption by L1.1, L1.3, and L1.5 was not significantly different from the wild-type strain, leading to the increase of the ratio between SLs production and total substrate consumption (YSLs/S). These ratios were increased by 3.53, 15.79, and 7.10% for L1.1, L1.3, and L1.5, respectively (Table 1). The residual glycerol/oil consumption was lower in the three mutants compared to the wild-type strain. This was especially the case for L1.3, with a 35.69% higher glycerol consumption capacity than the wild-type strain. These data show that the mutant strain L1.3 can effectively alleviate the carbon catabolic repression. Moreover, after five continuous generations, L1.3 still presented a good fermentation performance regarding SLs titer and glycerol consumption in a 5-L bioreactor (Additional file 1: Figs. S4 and S5).
Furthermore, exogenous glycerol was added into the shake flasks to further verify the utilization of glycerol by L1.3. A fermentation medium containing 60 g/L rapeseed oil was used as a control and an additional 5 g/L glycerol was then added to the medium. After cultivation of 96 h, the SLs titer and glycerol consumption were determined for both the L1.3 mutant and the wild-type strain.
As shown in Fig. 6, the yield of SLs was relatively close for L1.3 and the wild-type strain in normal medium. However, SLs yield was much higher in L1.3 compared to the wild-type strain when cultivated in the medium containing additional glycerol. The difference between residual glycerol in normal medium and in the medium with added glycerol was 5.37 g/L for the wild type and 2.79 g/L for L1.3. In addition, the oil consumptions of L1.3 and the wild-type strain were 2.88 g/L and 2.51 g/L, respectively, indicating that L1.3 also consumed more oil than the control, other than the additional glycerol.
Discussion
The development of high-throughput screening technologies leads to wide applications in strain screening. Tan et al. (2014) used a novel and effective mixture culture method, and performed a high-throughput screening of realized the whole mutant library of Monascus purpureus. An integrated strategy for high-throughput screening of high l-lactic acid-productivity strains in deep-well microtiter plates (MTPs) was developed by Lv et al. (2016). And they confirmed that 24-well U-bottom MTPs could well alternate shake flasks for cell cultivation as a scale-down tool. Zhou et al. (2019) established a simple and rapid high-throughput SLs-detecting method (I2 method). A high SLs-producing mutant was obtained by high-throughput screening technology. In this paper, mixed culture and microtiter plates (96-, 24-well) were used. In addition, a high-throughput detection for rapid quantification of carbon source by the Cu(OH)2 method was established to improve the efficiency of strain screening.
Rational screening can effectively improve the positive mutant rate from mutagenized population. Zhou et al. (2019) introduced the chlorpromazine as a rational selecting pressure. Wang et al. (2018) found that the inhibition of New Delhi metallo-β-lactamase 1 (NDM-1) was enhanced by the use of inhibitor combinations as a rational screening pressure, where the IC50 was reduced, comparing to the alone. In this paper, 2-deoxy-d-glucose as a rational screening pressure was introduced, and a mutant with alleviated carbon catabolic repression was successfully obtained.
Compared with single index, two indexes can improve efficiency of screening for high-performance strains. Zhu et al. (2018) developed a rapid, dual-parameter, plate-based screening process to simultaneously improve the production and secretion rate of glucose oxidase in Aspergillus niger. Using this dual-parameter screening method, a strain with glucose oxidase activity 164% higher than the original strain was obtained. In this study, two indexes of sophorolipids (SLs) production and glycerol consumption were introduced. In addition, we adopted the ratio of the residual glycerol to oil consumption to characterize glycerol consumption ability of the strain, thus eliminating the influence of oil consumption on the final glycerol utilization. The lower residual glycerol/oil consumption demonstrated that, compared to the wild-type strain, the mutant strain could utilize more glycerol with the same level of rapeseed oil consumption.
Conclusion
Through the implementation of a rapid, efficient, and simple method for detecting carbon sources as well as the introduction of 2-deoxy-d-glucose as a rational screening pressure, C. bombicola L1.3 was successfully screened out among 3000 mutants for high SLs production and the capacity of co-utilizing glycerol and glucose. The SLs yield in L1.3 was 15.06% higher than that in the wild-type strain and it exhibited a 35.69% higher glycerol consumption ability. We believe the high-throughput screening system reported here will be readily extended to the identification of other bio-surfactant producers.
Availability of data and materials
The data presented in the manuscript were reviewed and concluded from the earlier reported studies and mostly presented in the form of text. All the figures in the manuscript have been drawn by the authors themselves.
Abbreviations
- SLs:
-
sophorolipids
- HPLC:
-
high-performance liquid chromatography
- GC:
-
gas chromatography
- ARTP:
-
atmospheric and room-temperature plasma
- RID:
-
refractive index detector
- DO:
-
dissolved oxygen
- DCW:
-
dry cell weight
- CSL:
-
corn steep liquor
References
Afanas’Eva VP, Burd GI (1980) Glucose transport and catabolite repression in Endomycopsis fibuligera yeasts. Mikrobiologiia 49(3):433–439
Chen J, Song X, Zhang H, Qu Y (2005) Production, structure elucidation and anticancer properties of sophorolipid from Wickerhamiella domercqiae. Enzyme Microb Technol 39(3):501–506
Chen Y, Lin YM, Tian XW, Li QH, Chu J (2019) Real-time dynamic analysis with low-field nuclear magnetic resonance of residual oil and sophorolipids concentrations in the fermentation process of Starmerella bombicola. J Microbiol Methods 157:9–15. https://doi.org/10.1016/j.mimet.2018.12.007
Gancedo JM (1998) Yeast carbon catabolite repression. Microbiol Mol Biol Rev 62(2):334–361
Hu Y, Ju LK (2001) Purification of lactonic sophorolipids by crystallization. J Biotechnol 87(3):263–272
Jezierska S, Claus S, Van Bogaert I (2018) Yeast glycolipid biosurfactants. FEBS Lett 592(8):1312–1329
Konishi M, Morita T, Fukuoka T, Masaaki K, Imura T, Uemura S, Iwabuchi H, Kitamoto D (2018) Efficient production of acid-form sophorolipids from waste glycerol and fatty acid methyl esters by Candida floricola. J Oleo Sci 67(4):489–496
Lv XY, Song JL, Yu B, Liu HL, Li C, Zhuang YP, Wang YH (2016) High-throughput system for screening of high l-lactic acid-productivity strains in deep-well microtiter plates. Bioprocess Biosyst Eng 39(11):1737–1747
Norkus E, Vaškelis A, Vaitkus R, Reklaitis J (1995) On Cu(II) complex formation with saccharose and glycerol in alkaline solutions. J Inorg Biochem 60(4):299–302
Ottenheim C, Nawrath M, Wu JC (2018) Microbial mutagenesis by atmospheric and room-temperature plasma (ARTP): the latest development. Bioresour Bioprocess 5(1):12
Saerens KMJ, Saey L, Soetaert W (2011) One-step production of unacetylated sophorolipids by an acetyltransferase negative Candida bombicola. Biotechnol Bioeng 108(12):2923–2931
Sen S, Borah SN, Bora A, Deka S (2017) Production, characterization, and antifungal activity of a biosurfactant produced by Rhodotorula babjevae YS3. Microb Cell Fact 16(1):95–108
Shah MUH, Sivapragasam M, Moniruzzaman M, Talukder MMR, Yusup SB, Goto M (2017) Production of sophorolipids by Starmerella bombicola yeast using new hydrophobic substrates. Biochem Eng J 127:60–67
Tan J, Chu J, Hao YY, Guo YX, Zhuang YP, Zhang SL (2013) High-throughput system for screening of cephalosporin C high-yield strain by 48-deep-well microtiter plates. Appl Biochem Biotechnol 169(5):1683–1695
Tan J, Chu J, WangYH Zhuang YP, Zhang SL (2014) High-throughput system for screening of Monascus purpureus, high-yield strain in pigment production. Bioresour Bioprocess 1(1):16
Thaniyavarn J, Chianguthai T, Sangvanich P, Roongsawang N, Washio K (2008) Production of sophorolipid biosurfactant by Pichia anomala. Biosci Biotechnol Biochem 72(8):2061–2068
Tian XW, Zhou G, Wang WF, Zhang M, Hang HF, Mohsin A, Zhuang YP, Zhang SL (2018) Application of 8-parallel micro-bioreactor system with non-invasive optical pH and DO biosensor in high-throughput screening of l-lactic acid producing strain. Bioresour Bioprocess 5(1):20
Wang J, Li Y, Yan HZ, Duan J, Luo XH, Feng XQ, Lu LF, Wang WJ (2018) Semi-rational screening of the inhibitors and β-lactam antibiotics against the New Delhi metallo-β-lactamase 1 (NDM-1) producing E. coli. RSC Adv 8(11):5936–5944
Wu J, Li MH, Lin JP, Wen DZ (2011) Determination of dihydroxyacetone and glycerol in fermentation process by GC after n-methylimidazole catalyzed acetylation. J Chromatogr Sci 49(5):375–378
Zhang X, Zhang XM, Xu GQ, Zhang XJ, Shi JS, Xu ZH (2018) Integration of ARTP mutagenesis with biosensor-mediated high-throughput screening to improve l-serine yield in Corynebacterium glutamicum. Appl Microbiol Biotechnol 102(4):1–13
Zhou G, Tian XW, Lin YM, Zhang SL, Chu J (2019) Rational high-throughput system for screening of high sophorolipids-producing strains of Candida bombicola. Bioproc Biosyst Eng. https://doi.org/10.1007/s00449-018-02062-w
Zhu XD, Sun JC, Chu J (2018) High-content screening of Aspergillus niger with both increased production and high secretion rate of glucose oxidase. Biotechnol Lett 40(1):103–110
Acknowledgements
This work was financially supported by the National Key Research and Development Program (2017YFB0309302), the National Basic Research Program of China (973 Program, No. 2012CB721006), and the Fundamental Research Funds for the Central Universities (WF1814032, 22221817014, 22221818014). The authors thank all the participants.
Funding
This work was financially supported by the National Key Research and Development Program (2017YFB0309302), the National Basic Research Program of China (973 Program, No. 2012CB721006), and the Fundamental Research Funds for the Central Universities (WF1814032, 22221817014, 22221818014).
Author information
Authors and Affiliations
Contributions
YML, YC, and QHL were in charge of the experiments and manuscript writing. XWT and JC directed the study as tutors. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
This article does not contain any studies with human participants or animals performed by any of the authors.
Consent for publication
All of the authors have read and approved to submit it to bioresources and bioprocessing.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Additional file
Additional file 1: Table S1.
The evaluating parameters of the spectrophotometric Cu(OH)2 method. Fig. S1. A, determination of CuSO4 solution concentration, B, determination of NaOH solution concentration. Fig. S2. A, relationship between glycerol content and absorbance, B, relationship between glucose content and absorbance, C, glycerol standard curve, D, glucose standard curve. Fig. S3. Cell growth curves with glucose, glycerol and CSL respectively as the sole carbon source. Fig. S4. Yield of SLs and glycerol consumption in subculture by L1.3. Fig. S5. Comparison of DCW (A), residual glycerol/oil consumption (B), SLs concentration (C), among the parent strain, L1.3 and L1.3.5 (sub-cultured by continuous five generations).
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Lin, Y., Chen, Y., Li, Q. et al. Rational high-throughput screening system for high sophorolipids production in Candida bombicola by co-utilizing glycerol and glucose capacity. Bioresour. Bioprocess. 6, 17 (2019). https://doi.org/10.1186/s40643-019-0252-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40643-019-0252-x