Abstract
Begonia semperflorens Link et Otto has been broadly raised up for ornamental purpose as well comestible blossom. As the reproductive structures of phanerogams, flowers contain various secondary metabolites and have many biological activities. Accordingly, we began the contrivance for isolation and analysis of flavonoids contained in B. semperflorens flowers. MeOH extraction of B. semperflorens followed solvent fractionation was prosecuted. Column chromatography of non-polar fraction gave four flavonoids using several resins. Identification of the flavonols were established as quercetin (1), kaempferol (2), astragalin (3), and isoquercetin (4) by interpreting a variety of spectral information. Quercetin (1) and kaempferol (2) inhibited NO production and protected against t-BHP-induced oxidative stress. Kaempferol (2) also protected cell death of glutamate-treated HT22. Quantitative analysis of flavonoid content in B. semperflorens flowers was also performed using HPLC experiment.
Similar content being viewed by others
Introduction
A flower, as the reproductive organ of a plant, is pollinated by insects, water, and wind and produces various secondary metabolites, including volatiles, pigments, and flavonoids, for alluring pollinating insects as well definite pollination. Pollinators, especially insects, are attracted by floral colors and scents. Volatile compounds have been suggested as the main drivers of visitation decisions by pollinators [1,2,3]. Many flowers have UV patterns that are specifically visible to insects, and UV-absorbing pigments concentrated in the center of the flower increase its attractiveness [4].
Flowers have been used as ornamental plants for thousands of years because of their flavors, colors, and pleasing shapes. However, many flowers are also used as food ingredients. KFDA acknowledges approximately twenty edible flowers including pansies (Viola tricolor), jasmine (Jasminum polyanthum), camellia (Camellia japonica), peaches (Prunus persica), geranium (Pelargonium inquinans), and begonias (Begonia semperflorens). These flowers include a variety of active components showing anti-inflammatory [5], antioxidant [6], antibacterial [7], and NO-inhibition effects [8]. In addition, the Rural Development Administration (RDA) reported that edible flowers contain a 10-fold higher concentration of antioxidant constituents compared to vegetables and fruits. Among the edible flowers, B. semperflorens has a high content of total polyphenols and flavonoids, and NMR and MS analyses have shown it to contain anthocyanins [9, 10]. Therefore, the flowers of B. semperflorens were also expected to contain polyphenols and flavonoids.
B. semperflorens (Begoniaceae), native to Brazil, is broadly raised in tropical wetlands areas. This plant is in height by 15–45 cm with broad oval-shaped leaves, and its flowers bloom throughout the growing season until frost. As mentioned above, NMR and MS analyses of B. semperflorens flowers have shown the presence of acylated anthocyanins [11]. The anthocyanin cyanidin 3-(2G-xylosylrutinoside) was also reported from the leaves of this plant [12]. Anthocyanins provide photoprotection under stressful conditions [9].
In this study, four flavonoids were isolated from B. semperflorens flowers using extraction, fractionation as well repeated chromatography. The flavonoids were identified using spectroscopic methods, NMR, IR, MS. The flavonoids were quantitatively analyzed through HPLC experiment. And antioxidant, hepatoprotective, and neuroprotective effects of the flavonoids were then assessed.
Materials and methods
Plant materials
Begonia semperflorens flowers were acquired in Busan flower plantation, Korea, 2017, and Dr. D.G. Kim of Woosuk University, Jeonju, Korea identified. A voucher specimen (NPCL-20170716) was deposited at the Natural Products Chemistry Laboratory of Kyung Hee University, Yongin, Korea.
Reagents and instrumentation
The reagents and instruments used in this study were same as those used in the previous study [13].
Extraction of Begonia semperflorens flowers and isolation of flavonoids
Extraction of the fresh flowers of B. semperflorens (3.0 kg) was executed using 100% methanol (MeOH, 18 L) and 80% aqueous MeOH (27 L × 2) at r.t. for 24 h. The filtrates were evaporated under reduced pressure to yield an alcohol extract (Ext, 58 g). Ext was added to water (H2O, 2 L) and successively fractionated with ethyl acetate (EtOAc, 2 L × 2) and n-butanol (n-BuOH, 2 L × 2). The evaporated EtOAc Ext (BSE, 11.2 g) was put in application for SiO2 column chromatography (CC) (7 × 15 cm) and eluated by n-hexane:EtOAc (15:1 → 10:1 → 7:1 → 3:1 → 1:1, 7 L of each). Fraction (Fr) BSE-12 (459.0 mg, elution volume/total volume (VET) 0.772–0.797) was subjected to ODS CC (3.5 × 5 cm) and eluated by acetone:H2O (1:2, 8 L), resulting in 6 Frs (BSE-12-1 to BSE-12-6) with isolation of 1 in BSE-12-3 (4.6 mg, VET 0.070–0.172, TLC using ODS Rf 0.51 in 4:2 acetone:H2O). Fr BSE-12-5 (126.5 mg, VET 0.175–0.787) was subjected to SiO2 CC (2.5 × 13 cm) and eluated by CHCl3:MeOH:H2O (36:3:1 → 25:3:1 → 18:3:1 → 65:35:10, 470 mL of each), resulting in 6 Frs (BSE-12-5-1 to BSE-12-5-6) with isolation of 2 in Fr BSE-12-5-2 (3.5 mg, VET 0.118–0.101, TLC using ODS Rf 0.42 in 4:2 acetone:H2O). Fr BSE-18 (5.85 g, VET 0.956–1.000) was subjected to SiO2 CC (5.0 × 13 cm) and eluated by CHCl3:MeOH:H2O (20:3:1, 15.7 L), resulting in 20 Frs (BSE-18-1 to BSE-18-20). Fr BSE-18-15 (137.3 mg, VET 0.573–0.725) was subjected to SiO2 CC (3.0 × 14 cm) and eluted by CHCl3:MeOH:H2O (25:3:1 → 20:3:1, 4.1 L of both), resulting in 7 Frs (BSE-18-15-1 to BSE-18-15-7) with isolation of 3 in Fr BSE-18-15-2 (6.7 mg, VET 0.257–0.324, TLC using ODS Rf 0.43 in 2:2 acetone:H2O) and 4 in Fr BSE-18-15-4 (22.5 mg, VET 0.545–0.665, TLC using ODS Rf 0.50 in 2:2 acetone:H2O).
-
quercetin (1) yellow crystals; m.p. 277 °C; IRν (KBr) 3425, 1660, 1610, and 1505 cm−1; positive FAB/MS (pFABMS) m/z 303 [M + H]+.
-
kaempferol (2) light yellow crystals; m.p. 178–180 °C; IRν (KBr) 3396, 3021, 2867, 1642, and 1609 cm−1; EI/MS m/z 286 [M]+, 258, 229, 213, 184, 153, and 121.
-
astragalin (3) yellow crystals; m.p. 230–232 °C; \(\left[ \alpha \right]_{\text{D}}^{25}\) +16.0°; IRν (KBr) 3420, 1680, and 1628 cm−1; pFABMS m/z 449 [M + H]+ and 287.
-
isoquercetin (4) yellow crystals; m.p. 230–232 °C; \(\left[ \alpha \right]_{\text{D}}^{25}\) 230–231°; IRν (KBr) 3400, 2919, 1656, 1606, and 1508 cm−1; pFABMS m/z 465 [M + H]+, 447, 423, 389, 297, and 204.
1H-NMR (400 MHz, CD3OD, δH) and 13C-NMR (100 MHz, CD3OD, δC) see Table 1.
Inhibitory effects on NO production in LPS-induced RAW 264.7
Cell culture of murine macrophage RAW 264.7 cells and measurement of nitrite (NO) production can be referred to literature [14]. Butein was used as a positive control.
Protective effect on cell death of glutamate-treated HT22
Cytoprotective effect was assayed according to the same methods reported in literature [15]. Trolox was used as a positive control.
Protective effect on oxidative stress in treated HepG2 cells by t-BHP
Human hepatoma HepG2 cell culture and Hepatoprotective effect assay was accomplished using the same method reported in the previous study [14]. Curcumin was used as a positive control.
Quantitative analysis of the flavonoids isolated from Begonia semperflorens flowers
The MeOH Ext of B. semperflorens flowers was fractionated using EtOAc and H2O. The organic phase Fr was utilized to analyze the isolated flavonoids. The flavonoids were diluted to various concentrations to establish calibration curves (1: 1.890625, 3.78125, 7.5625, 15.125, and 31.25 μg/mL; 2: 3.78125, 7.5625, 15.125, 31.25, and 62.5 μg/mL; 3 and 4: 15.125, 31.25, 62.5, 125, 250 μg/mL).
The equipment and materials for HPLC analysis were as the followings. An Waters 600S (Milford, MA), a reverse phase column (Waters C18, 5 μm, 250 × 4.6 mm). The eluting solvents, aqueous 0.05% trifluoroacetic acid (A) and 100% acetonitrile (B). 0.6 mL/min with gradient of B: 0–5 min, 10–30%; 5–20 min, 30%; 20–23 min, 30–40%; 23–38 min, 40%; 38–43 min, 40–100%. Injection volume, 10 μL. Detection was carried out using a photodiode spectrophotometer at 280 nm. The analysis was repeated three times.
Results and discussion
TLC for alcohol Ext of B. semperflorens flowers revealed yellow spots after spraying with a 10% H2SO4 solution and heating, indicating the presence of flavonoids in the Ext. The Ext was fractionated into EtOAc, n-BuOH, and H2O Frs through solvent fractionation. And repeated SiO2 and ODS CC of EtOAc Fr afforded four flavonoid compounds. All compounds were isolated as yellow crystals and exhibited yellow spots on TLC plate after by same treatment, which led to deduction that they were flavonoids. The UV absorption pattern of the compounds confirmed the above-mentioned ratiocination.
The molecular weight (MW) of 1 was determined to be 302 amu based on the molecular ion peak (MIP) [M + H]+ at m/z 303 in the pFABMS. IR spectrum showed absorption peaks at 3425 (OH), 1660 (conjugated ketone), and 1610 cm−1 (aromatic double bond). The 1H-NMR (PMR) spectrum (400 MHz, CD3OD) showed two olefin methine proton signals at δH 6.68 (br. s, H-8) and 6.73 (br. s, H-6) due to a 1,2,3,5-tetrasubstituted benzene ring and three olefin methine proton signals at 7.35 (d, J = 8.4 Hz, H-5′, coupling pattern, coupling constant in J in Hz), δH 8.08 (br. d, 8.4, H-6′) and 8.55 (br. s, H-2′) due to a 1,2,4-trisubstituted benzene ring. The 13C-NMR (CMR) (100 MHz, CD3OD) spectrum included 15 carbon signals, suggesting 1 was a flavonoid. The five olefin methine carbon signals at δC 94.16 (C-8), 99.09 (C-6), 116.48 (C-5′), 116.48 (C-2′), and 120.93 (C-6′); two olefin quaternary carbon signals at 104.31 (C-10) and δC 123.43 (C-1′); seven oxygenated olefin quaternary carbon signals at δC 137.7 (C-3), 146.91 (C-4′), 147.59 (C-2), 149.66 (C-3′), 157.33 (C-9), 162.28 (C-5), and 165.37 (C-7); one conjugated ketone carbon signal at δC 177.12 (C-4) suggested that 1 was a flavonol. 1 was identified to be quercetin through intensive analysis of 2D-NMR (i.e., gHSQC and gHMBC) data as well comparison of the spectroscopic data with reported literature [16].
2 showed very similar NMR signals to those of 1 with the exception of the B-ring structure. The PMR signals of a para-substituted benzene ring at δH 6.89 (2H, d, 9.2, H-3′,5′) and 8.07 (2H, d, 9.2, H-2′,6′), as well the CMR signals of four olefin methines at δC 116.30 (C-3′,5′) and 130.66 (C-2′,6′), one olefin quaternary at δC 123.76 (C-1′), and one oxygenated olefin quaternary at δC 160.54 (C-4′) indicated that 2 was 5,7,4′-trihydroxyflavonol, kaempferol. The identification of 2 was confirmed through the molecular weight (MW) of 286 amu, which was 16 amu less than that of 1.
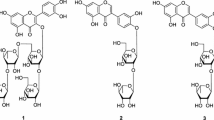
3 showed similar NMR signals as those of 2 with the exception of additional signals due to a β-glucopyranose. The hemiacetal PMR signal at δH 5.24 (d, 7.2, H-1″) and the chemical shifts of CMR signals confirmed the presence of a β-glucopyranosyl moiety. MW of 3 was determined to be 448 amu from a MIP [M + H]+ at m/z 449 in FABMS spectra, which was 162 amu more than that of 2. The β-glucopyranose was revealed to be linked to the 3-OH in the C-ring from the cross-peak between the anomeric PMR signal at δH 5.24 (d, 7.2, H-1″) and an oxygenated olefin quaternary CMR signal at δC 135.47 (C-3) in the gHMBC spectrum. 3 was identified to be kaempferol 3-O-β-d-glucopyranoside, astragalin.
4 showed similar NMR signals as those of 1 with the exception of additional β-glucopyranose signals. The hemiacetal PMR signal at δH 5.22 (d, 7.2, H-1″) and the chemical shifts of CMR signals confirmed the presence of a β-glucopyranosyl moiety. MW was determined to be 464 amu from MIP [M + H]+ at m/z 465 in pFABMS spectrum, which was 162 amu more than that of 1. The 3-OH linkage of the β-glucopyranose was determined from the cross-peak between the anomeric PMR signal at δH 5.22 (d, 7.2, H-1″) and an oxygenated olefin quaternary CMR signal at δC 135.63 (C-3) in the gHMBC spectrum. 4 was identified to be quercetin 3-O-β-d-glucopyranoside, isoquercetin. This study is the first report for isolation of the flavonoids from B. semperflorens flowers.
Inhibitory effects on NO production in RAW 264.7 treated by LPS
1 and 2 were estimated for inhibition effect against NO generation in LPS-treated RAW 264.7. LPS-stimulated macrophages were treated with each compound (1: 1, 5, 10, or 20 µM; 2: 5, 10, 20, or 40 μM). As can be seen in Fig. 1, 1 and 2 dose-dependently suppressed NO production in RAW 264.7. 1 and 2 showed slightly lower effect than butein. IC50 value of 1 and 2 was respectively estimated as 84.79 and 80.87 μM. Previous studies have also reported the suppressive activity of 1 and 2 on NO generation. Naturally occurring flavonoids are known to modulate various inflammatory and immune processes. Genistein inhibits NO synthase expression and NO generation with IC50 value, 26.8 μM [17].
Inhibitory activity of 1 and 2 on NO generation in RAW 264.7 induced by LPS. The cells were pretreated for 12 h with the indicated concentrations of compounds and stimulated for 18 h with LPS (1 µg/mL). The error bars represent the mean ± SD of three independent experiments. *p < 0.05 compared to the LPS-treated control group. Positive control, butein
Neuroprotective effects against glutamate-induced cell death in HT22
1-4 were investigated for their protective effects against glutamate-induced cell death in HT22 cells. Glutamate-stimulated HT-22 cells were treated with the compounds and trolox (100 μM). As shown in Fig. 2, 2 showed considerable protection (99.1%) against glutamate-induced toxicity at the low concentration at 80 μM, which was a higher protective effect than trolox (82.0%, 100 μM). The EC50 value of 2 was 19.95 μM. A previous study reported that 2 (100 μM) protected HT22 cells against glutamate-induced cytotoxicity by 62.4 ± 2.8% [18].
Hepatoprotective activity against oxidative stress in t-BHP-induced HepG2
To examine the protective effect against t-BHP-induced oxidative stress in HepG2, the cells were treated with 1 and 2 (1: 5, 10, 20, or 40 µM; 2: 10, 20, 40, or 80 μM) or curcumin (20 μM). As shown in Fig. 3, 1 and 2 showed high protective effects (86.5 and 78.7%, respectively) against t-BHP-induced cytotoxicity at a concentration of 20 μM, which was almost the same as that of the positive control, curcumin. EC50 value of 1 and 2 was calculated to be 1.019 and 5.321 μM, respectively. A previous study also reported 1 to show protective effect against t-BHP-induced oxidative stress [19].
Many flavonoids have been shown to have various pharmacological activities. Quercetin (1) is effective against inflammation, arteriosclerosis, bleeding, allergies, and swelling [10, 20]. Kaempferol (2) has antidiabetic [21] and antioxidant as well as anticancer [22] activities. Astragalin (3) exhibits antioxidant [23], anti-HIV [24], and anti-allergen [25] activities, and isoquercetin (4) shows antioxidant [26], anti-inflammatory [27], and antitumor [28] activities. Compounds 1 and 2 were proved to have anti-inflammatory, neuroprotective, and hepatoprotective effects through our experiments and previous studies as well. The compounds are sure to have potential to be developed as new drugs.
Quantitative HPLC analysis of the flavonoids in Begonia semperflorens flowers
Using HPLC, each flavonoid peak was clearly separated and identified through comparison of the retention time with those of the standards (Fig. 4). The calibration curves were built using various concentrations of each compound (1: 1.890625, 3.78125, 7.5625, 15.125, and 31.25 μg/mL; 2: 3.78125, 7.5625, 15.125, 31.25, and 62.5 μg/mL; 3 and 4: 15.125, 31.25, 62.5, 125, and 250 μg/mL). The regression equations and correlation coefficient (r2 0.9996–1.000) for 1–4 are listed in Table 2. The high value of each r2 confirmed this analysis to be reliable. The concentrations of 1–4 were determined using the peak areas in the chromatogram and the regression equations (Table 2). The contents of 1–4 in the EtOAc Fr were calculated to be 0.3 ± 0.02, 0.8 ± 0.09, 7.1 ± 0.16, and 11.9 ± 0.03%, respectively.
References
Dötterl S, Vereecken NJ (2010) The chemical ecology and evolution of bee-flower interactions: a review and perspectives. Can J Zool 88:668–697
Raguso RA (2008) Wake up and smell the roses: the ecology and evolution of floral scent. Annu Rev Ecol Evol Systemat 39:549–569
Wright GA, Schiestl FP (2009) The evolution of floral scent: the influence of olfactory learning by insect pollinators on the honest signalling of floral rewards. Funct Ecol 23:841–851
Gronquist M, Bezzerides A, Attygalle A, Meinwald J, Eisner M, Eisner T (2001) Attractive and defensive functions of the ultraviolet pitments of a flower (Hypericum calycinum). Proc Natl Acad Sci USA 98:13745–13750
Lee HH, Cho JY, Moon JH, Park KH (2011) Isolation and identification of antioxidative phenolic acid and flavonoid glycosides from Camellia japonica flowers. Hort Environ Biotechnol 52:270–277
Chung TY, Kim MA, Jones AD (1996) Antioxidative activity of flavonoids isolated from Jindalrae flowers (Rhododendron mucronulatum Turcz.). Agric Chem Biotechnol 39:320–326
Rashid F, Ahmed R, Mahmood A, Bibi N, Kazmi SU (2007) Flavonoid glycosides from Prunus armeniaca and the antibacterial activity of a crude extract. Arch Pharm Res 30:932–937
Lee DG, Lee SM, Bang MH, Park HJ, Lee TH, Kim YH, Kim JY, Baek NI (2011) Lignans from the flower of Osmanthus fragrans var. aurantiacus and their inhibition effect on NO production. Arch Pharm Res 34:2029–2035
Zhang KM, Yu HJ, Shi K, Zhou YH, Yu JQ, Xia XJ (2010) Photoprotective roles of anthocyanins in Begonia semperflorens. Plant Sci 179:202–208
Formica JV, Regelson W (1995) Review of the biology of quercetin and related bioflavonoids. Food Chem Toxicol 33:1061–1080
Chirol N, Jay M (1995) Acylated anthocyanins from flowers of Begonia. Phytochemistry 40:275–277
Ji S, Yokoi M, Saito N, Ueda Y, Shigihara A, Honda T (1995) Cyanidin 3-(2G-xylosylrutinoside) from brown-red spring leaves of Acer macrophyllum and Begonia semperflorens cultivars. Tech Bull Fac Hort Chiba Univ 49:13–17
Seo KH, Nam YH, Lee DY, Ahn EM, Kang TH, Baek NI (2015) Recovery effect of phenylpropanoid glycosides from Magnolia obovata fruit on alloxan-induced pancreatic islet damage in zebrafish (Danio rerio). Carbohydr Res 416:70–74
Jung YJ, Park JH, Seo KH, Shrestha S, Lee DS, Kim YC, Kang HC, Kim J, Baek NI (2014) Phenolic compounds from the stems of Zea mays and their pharmacological activity. J Korean Soc Appl Biol Chem 57:379–385
Jung YJ, Park JH, Cho JG, Seo KH, Lee DS, Kim YC, Kang HC, Song MC, Baek NI (2015) Lignan and flavonoids from the stems of Zea mays and their anti-inflammatory and neuroprotective activities. Arch Pharmacal Res 38:178–185
Aisyah LS, Yun YF, Herlina T, Julaeha E, Zainuddin A, Nurfarida I, Hidayat AT, Supratman U, Shiono Y (2017) Flavonoid compounds from the leaves of Kalanchoe prolifera and their cytotoxic activity against P-388 murine leukimia cells. Nat Prod Sci 23:139–145
Kim AR, Cho JY, Zou Y, Choi JS, Chung HY (2005) Flavonoids differentially modulate nitric oxide production pathways in lipopolysaccharide-activated RAW 264.7 cells. Arch Pharm Res 28:297–304
Taechowisan T, Chuaychot N, Chanaphat S, Wanbanjob A, Shen Y (2009) Cytoprotective activity of chemical constituents isolated from Streptomyces sp. Int J Biol Chem 3:11–17
Saw CL, Guo Y, Yang AY, Paredes-Gonzalez X, Ramirez C, Pung D, Kong AT (2014) The berry constituents quercetin, kaempferol, and pterostilbene synergistically attenuate reactive oxygen species: involvement of the Nrf2-ARE signaling pathway. Food Chem Toxicol 72:303–311
Havsten B (1983) Flavonoids, A class of natural products of high pharmacological potency. Biochem Pharmacol 32:1141–1148
Yoo NH, Jang DS, Yoo JL, Lee YM, Kim YS, Cho JH, Kim JS (2008) Erigeroflavanone, a flavanone derivative from the flowers of Erigeron annuus with protein glycation and aldose reductase inhibitory activity. J Nat Prod 71:713–715
Li S, Yan T, Deng R, Jiang X, Xiong H, Wang Y, Yu Q, Wang X, Chen C, Zhu Y (2017) Low dose of kaempferol suppresses the migration and invasion of triple-negative breast cancer cells by downregulating the activities of RhoA and Rac1. Onco Targets Ther 10:1–11
Choi JW, Kang HJ, Kim SZ, Kwon TO, Jeong SI, Jang SI (2013) Antioxidant effect of astragalin isolated from the leaves of Morus alba L. against free radical-induced oxidative hemolysis of human red blood cells. Arch Pharm Res 36:912–917
Lin YM, Anderson H, Flavin MT, Pai YH, Mata-Greenwood E, Pengsuparp T, Pezzuto JM, Schinazi RF, Hughes SH, Chen FC (1997) In vitro anti-HIV activity of biflavonoids isolated from Rhus succedanea and Garcinia multiflora. J Nat Prod 60:884–888
Matsumoto M, Kotani A, Fujita S, Higa T, Kishimoto M, Suemura T, Tanaka T (2002) Oral administration of persimmon leaf extract ameliorates skin symptoms and trasepidermal water loss in atopic dermatitis model mice, NC/Nga. Br J Dermatol 146:221
Jo SH, Ka EH, Lee HS, Apostolidis E, Jang HD, Kwon YI (2010) Comparison of antioxidant potential and rat intestinal α-glucosidases inhibitory activities of quercetin, rutin, and isoquercetin. Int J Appl Res Nat Prod 2:52–60
Vila-Real H, Alfaia AJ, Bronze MR, Calado AR, Ribeiro MH (2011) Enzymatic synthesis of the flavone glucosides, prunin and isoquercetin, and the aglycones, naringenin and quercetin, with selective α-l-rhamnosidase and β-d-glucosidase activities of naringinase. Enzyme Res 2011:11
Ramya S, Neethirajan K, Jayakumararaj R (2012) Profile of bioactive compounds in Syzygium cumini: a review. J Pharm Res 5:4548–4553
Authors’ contributions
J-HK and H-JO isolated the compounds and elucidated the structures; J-WJ contributed to the plant materials preparation; D-SL carried out the biological assay and helped with the preparation of the manuscript; S-JI and K-HS performed the NMRs, and HPLC of the samples; DYL assisted the revision of the manuscript; J-WK wrote the paper; N-IB designed and managed the research project. All authors read and approved the final manuscript.
Acknowledgements
This work was supported by the Korean Institute of Planning and Evaluation for Technology in Food, Agriculture, Forestry, and Fisheries (317071-03-2-SB020).
Competing interests
The authors declare that they have no competing interests.
Funding
The funding sponsors had no role in the design of the study; collection, analyses, or interpretation of the data; writing of the manuscript; or the decision to publish the results.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Kwon, JH., Oh, HJ., Lee, DS. et al. Pharmacological activity and quantitative analysis of flavonoids isolated from the flowers of Begonia semperflorens Link et Otto. Appl Biol Chem 62, 11 (2019). https://doi.org/10.1186/s13765-019-0416-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13765-019-0416-6