Abstract
Three new phenylethanol glycosides (1-3) and one known analogue (4) were isolated from the seeds of Aesculus chinensis Bge. var. chekiangensis. To the best of our knowledge, this represents the first isolation of phenylethanol glycosides from the genus of Aesculus, which enriched its chemical composition. Structure elucidations were performed via extensive NMR and HRESIMS data together with comparison with literature data. Thereafter, the isolated compounds were assayed for their neuroprotective activities against CoCl2-induced cytotoxicity in PC12 cells and compound 3 exhibited moderate activity. 
Similar content being viewed by others
Introduction
The genus Aesculus, which belongs to the family Hippocastanaceae contains about 30 species found worldwide. The dried seeds of Aesculus chinensis Bge. var. chekiangensis (Hu et Fang) Fang, Aesculus chinensis Bge and Aesculus wilsonii Rehd are commonly used to treat chest and abdomen pain, dysentery and ague [1, 2] in traditional Chinese medicine. Previous studies on the genus of Aesculus revealed the presences of diverse secondary metabolites such as triterpenoids [3,4,5,6,7], flavonoids [8, 9], coumarins [10] and steroids [11]. And a number of pharmacological studies have suggested that A. chinensis exhibited beneficial effects on antitumor [12], neuroprotective [13], anti-inflammatory [14] and cardio-protective activities [15]. Nevertheless, compared to other species of Aesculus genus, the chemical investigation of Aesculus chinensis Bge. var. chekiangensis (Hu et Fang) Fang is limited. Our interests in cytotoxic and neuroprotective components from A. chinensis Bge. var. chekiangensis (Hu et Fang) Fang have led to the isolation of numerous new ones [16, 17]. As a continuous search for structurally novel compounds with diverse bioactivities, three new phenylethanol glycosides (1-3) and one known analog (4) were obtained (Fig. 1), which represent the first examples of phenylethanol glycosides obtained from the genus of Aesculus. Herein, the isolation, structure identification and biological evaluation of 1-4 are described.
Methods
General experimental procedures
The chemicals and material were similar to our previous researches [16, 17].
Plant material
The plant was the same batch of medicinal material as our previous reports [16, 17].
Extraction and isolation
The extracted method was the same to our previous studies [16, 17]. Chopped, dried seeds of A. chinensis Bge. (8.8 kg) were extracted with 70% ethanol, then partitioned via D101 resin column eluting with a stepwise gradient of H2O-EtOH.
The 60% EtOH-H2O part was loaded onto a silica gel column using CH2Cl2/CH3OH (100:1 → 1:1) to yield 4 fractions (A–D). Fraction A was further separated by RP C18 CC (MeOH–H2O, from 0:100 to 100:0) to give four subfractions (A1–A4). Subfraction A2 was chromatographed over a Sephadex LH-20 column (MeOH) then RP-HPLC (MeOH–H2O, 35:65, 3.0 mL/min) to give compounds 1 (11.0 mg) and 4 (20.0 mg). Subfraction A3 was further subdivided with an ODS RP-C18 column (MeOH/H2O, 10:90 to 100: 0) to give seven subfractions (A3A–A3G). The subfraction A3G was applied to a Sephadex LH-20 column (MeOH), and then purified by recycling preparative HPLC with 40% MeOH/H2O to yield compounds 2 (3.7 mg) and 3 (9.0 mg).
4-methoxy-phenylethanol-8-O-α-l-rhamnopyranosyl-(1 → 6)-β-d-glucopyranoside (1)
Brown amorphous powder; [α]25 D–7.3 (c 0.10, MeOH); Proton nuclear magnetic resonance (1H-NMR) and carbon-13 nuclear magnetic resonance (13C-NMR): Table 1; HR-ESI–MS: m/z 505.1918 [M + COOH]− (calculated for C22H33O13, 505.1921).
4-methoxy-phenylethanol-8-O-β-d-glucopyranosyl-(1 → 2)-β-d-glucopyranoside (2)
Brown amorphous powder; [α]25 D–11.2 (c 0.11, MeOH); 1H-NMR and 13C-NMR: Table 1; HR-ESI–MS: m/z 521.1870 [M + COOH]− (calculated for C22H33O14, 521.1870).
4-methoxy-phenylethanol-8-O-β-d-glucopyranosyl-(1 → 2)-[β-d-glucopyranosyl-(1 → 3)]-β-d-glucopyranoside (3)
Brown amorphous powder; [α]25 D–14.6 (c 0.10, MeOH); 1H-NMR and 13C-NMR: Table 1; HR-ESI–MS: m/z 683.2398 [M + COOH]− (calculated for C28H43O19, 683.2399).
Hydrolysis and determination of absolute configuration of sugars
Compounds 1–3 (1.0 mg, respectively) was hydrolyzed with 2 M HCl (4.0 mL) at 90 °C for 2 h. Then the hydrolysed materials were disposed and tested by means of the procedure described in our previous work [16, 17].
Neuroprotective effect assay
Compounds 1-4 were assayed for their neuroprotective effects against CoCl2-induced PC12 cell injury [18] by 3-(4,5-dimethylthiazol)-2,5-diphenyltetrazolium bromide (MTT) method with trolox as the positive control according to our previously reported procedure [16, 17]. PC12 cells were cultured in RPMI-1640 medium containing 10% fetal bovine serum as well as 100 U/mL penicillin/streptomycin and were incubated at 37 °C with 5% CO2. PC12 cells were placed into a 96-well plate at a density of 2 × 104 cells/well and kept there for 24 h. Cells were incubated with test compounds and trolox (10 μM) for 2 h. To induce an oxidative stress, 1 mM CoCl2 was added to the cells and incubated for 24 h. Then, the supernatant was changed with 100 μL MTT solution (5 mg/mL) for 2.5 h, the plate was vibrated, and the absorbance at 490 nm was measured using a microplate reader.
Cytotoxicity assay
Cell viability was determined with the MTT method [19, 20]. The human hepatocellular carcinomas cells (HepG2), the human colorectal carcinoma cells (HCT-116) and the human gastric carcinoma cells (MGC-803) were purchased from ATCC. HepG2, MGC-803 and HCT-116 were respectively cultured in DMEM and RPMI-1640 mediums, which were supplemented with 10% fetal bovine serum at 37 °C in a humidified atmosphere containing 5% CO2. HepG2, HCT-116, and MGC-803 cells (1 × 104) were seeded in 96-well tissue culture plates. Cells were treated in triplicate with five concentrations (50, 25, 12.5, 6.25 and 3.125 μM) of the tested compounds for 24 h, with 5-fluorouracil (5-FU) as positive control. Subsequently, 100 μL of MTT (5 mg/mL) was added and the cells were incubated for additional 2.5 h. Thereafter, the supernatant was discarded and 0.15 ml of DMSO was added to each well, then the plate was mixed on a microshaker for 10 min and read on a microplate reader at 490 nm.
Results and discussion
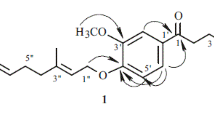
Compound 1 was obtained as brown amorphous powder with a molecular formula of C21H32O11 deduced from its HR-ESI-MS spectrum (m/z 505.1918 [M + COOH]−, calcd. for C22H33O13, 505.1921). The 1H-NMR spectrum of compound 1 exhibited signals characteristic for a 1, 4-disubstituted benzene ring [δH 7.17 (2H, d, J = 8.5 Hz, H-2, 6), 6.83 (2H, d, J = 8.5 Hz, H-3, 5)], an ethoxy moiety [δH 2.78 (2H, dt, J = 8.2, 6.3 Hz), 3.83 (1H, dt, J = 8.2, 6.3 Hz)] as well as a O-methyl at δH 3.71 (3H, s) (Table 1). The heteronuclear multiple bond correlations (HMBC) (Fig. 2) of H-2 (δH 7.17) to C-4 (δC 157.6), C-6 (δC 129.9), C-7 (δC 34.8); H-3 (δH 6.83) to C-1 (δC 130.5), C-5 (δC 113.7); H-8 (δH 3.83) to C-1 (δC 130.5) and OCH3 (δH 3.71) to C-4 (δC 157.6) indicated 1 contains a 4-methoxy-phenylethanol moiety.
The two anomeric protons at δ 4.17 (1H, d, J = 7.7 Hz), 4.59 (1H, d, J = 1.2 Hz) correlated with carbons at δ 103.0 and 100.8 in heteronuclear single quantum coherence (HSQC) spectrum, respectively, indicated a disaccharide residue. Acid hydrolysis of 1 liberated d-glucose and l-rhamnose, which were identified by HPLC analysis after derivatization [21, 22]. The β-orientation of the glucopyranosyl unit was deduced from the coupling constant (J = 7.7 Hz, H-1′). The α- anomeric configuration of rhamnose was determined from the absence of nuclear overhauser effect spectroscopy (NOESY) correlations between protons H‑1 and H‑3/H‑5. The β-d-glucose was attached to the 4-methoxy-phenylethanol nucleus at C-8, evidenced by the HMBC correlation between H-1′ (δH 4.17) to C-8 (δC 69.9). In addition, the downfield chemical shift of C-6′ (δC 67.0) of the glucose coupled with the cross peak of H-1′′ (δH 4.59) to C-6′ (δC 67.0) in HMBC spectrum suggesting the α-l-rhamnose was linked to C-6′. Based on these data, compound 1 was concluded to be 4-methoxy-phenylethanol‐8-O-α-l-rhamnopyranosyl-(1 → 6)-β-d-glucopyranoside.
The elemental formula of compound 2 was confirmed to be C21H32O12 with one oxygen more than that of 1 according to the [M + COOH]− ion peak at m/z 521.1870 in its HRESIMS spectrum. The 1H and 13C NMR data of 2 revealed a close resemblance to 1 except for the corresponding signals to the two sugar units. Careful analysis of the NMR data and the acid hydrolysis results affirmed the existence of two β-d-glucose groups in 2 instead of one β-d-glucose and one α-l-rhamnose in 1. HMBC correlations from H-1′ (δH 4.33) to C-8 (δC 69.8) and H-1′′ (δH 4.41) to C-2′ (δC 82.8) revealed the position and sequences of the sugar moiety in 2 as shown in Fig. 2. Hence, compound 2 was assigned as 4-methoxy-phenylethanol-8-O-β-d-glucopyranosyl-(1 → 2)-β-d-glucopyranoside.
Compound 3 was also acquired as a brown solid with the molecular formula of C27H42O17 (m/z 683.2398 [M + COOH]−; calcd. for C28H43O19, 683.2399), which is 162 mass units more than that of 2. The NMR data of 3 were closely resemble to those of 2, indicating the same aglycone with the difference of an additional hexose moiety. d-glucose was afforded from 3 via the same procedure as before and the β configuration was inferred from the large coupling constants: [δH 4.39 (1H, d, J = 7.5 Hz, H-1′), 4.55 (1H, d, J = 8.0 Hz, H-1′′), 4.38 (1H, d, J = 7.9 Hz, H-1′′′)]. HMBC correlations from H-1′ (δH 4.39) to C-8 (δC 69.9) supported the attachment of the sugar units to C-8. The sequence of the sugar chain was further established by the long correlations of H-1′′ (δH 4.55) and C-2′ (δC 79.6), H-1′′′ (δH 4.38) and C-3′ (δC 86.2) (Fig. 2). Consequently, compound 3 was assigned as 4-methoxy-phenylethanol-8-O-β-d-glucopyranosyl-(1 → 2)-[β-d-glucopyranosyl-(1 → 3)]-β-d-glucopyranoside.
The other known one, phenylethanol-8-O-α-l-rhamnopyranosyl-(1 → 6)-β-d-glucopyranoside (4) were also obtained and identified by NMR analysis and comparison with literature data [23].
All compounds (1–4) were tested in three human cancer cell lines, HepG2, HCT-116 and MGC-803, using 5-FU as the positive control. However, they did not show obvious cytotoxicity (IC50 > 50 μM).
The neuroprotective effects of 1–4 were also evaluated in CoCl2-induced PC12 cell damage [24] by MTT assay. According to the references [25, 26] and our study, the positive control, trolox, exhibited statistically significant neuroprotective effect at 10 μM (Fig. 3). Therefore, the concentration of 10 μM was selected for the cytotoxic and neuroprotective evaluation of these compounds. First, the cytotoxic activity of compounds 1–4 against PC12 cell line was tested and none of them showed cytotoxicity at 10 μM (Additional file 1: Fig. S16). Subsequently, 10 µM compounds were bioassayed for their neuroprotective properties. And according to Fig. 3, compound 3 exhibited moderate activities against CoCl2-induced PC12 cell injury.
Neuroprotective activities of compounds 1-4 (10 μM) against CoCl2-induced cell death in PC12 cells. The data (cell viability, measured by MTT assay) are expressed as mean ± SD. Three independent experiments were performed. Trolox was used as the positive control at 10 μM. Compared with CoCl2 treated group, *P < 0.05, **P < 0.01
Conclusion
In this paper, three new phenylethanol glycosides (1-3) and one known compound (4) were obtained from the seeds of A. chinensis Bge. var. chekiangensis., which represents the first isolation of phenylethanol glycosides from the genus of Aesculus. The findings also provided more insights into the chemotaxonomy of the Aesculus genus. Besides, the neuroprotective activities of the phenylethanol glycosides were also evaluated and compound 3 exhibited statistically significant neuroprotective activity.
Availability of data and materials
All other datasets generated for this study are included in the article and Additional file 1.
Abbreviations
- DMSO-d6 :
-
Deuterated dimethyl sulfoxide
- 1H-NMR:
-
Proton nuclear magnetic resonance
- 13C-NMR:
-
Carbon-13 nuclear magnetic resonance
- HMBC:
-
Heteronuclear multiple bond correlation
- HSQC:
-
Heteronuclear single quantum coherence
- NOESY:
-
Nuclear overhauser effect spectroscopy
- HepG2:
-
The human hepatocellular carcinomas cells
- HCT-116:
-
The human colorectal carcinoma cells
- MGC-803:
-
The human gastric carcinoma cells
- MTT:
-
3-(4,5-dimethylthiazol)-2,5-diphenyltetrazolium bromide
- 5-FU:
-
5-Fluorouracil
References
Yang X, Zhao J, Cui Y, Liu X, Ma C, Hattori M, Zhang L (1999) Anti-HIV-1 protease triterpenoid saponins from the seeds of Aesculus chinensis. J Nat Pro. 62(11):1510–1513
Zhang Z, Li S, Zhang S, Gorenstein D (2006) Triterpenoid saponins from the fruits of Aesculus pavia. Phytochemistry 67(8):784–794
Zhao J, Yang XW, Cul YX, Liu XH, Ouyangz SH (1999) A new triterpenoid oligoglycoside escin IVe from the seeds of Aesculus Chinensis. Chin Chem Lett 10(6):473–476
Jie G, Xiu WY (2004) Studies on Triterpenoid Saponins of seeds of Aesculus chinensis Bunge var. chekiangensis (Hu et Fang) Fang. J Chin Pharm Sci. 13(2):87–91
Zhang Z, Kazuo K, Jia Z, Nikaido T, Guo D, Zheng J (1999) New saponins from the seeds of Aesculus chinensis. Chem Pharm Bull 47(11):1515–1520
Yang X, Zhao J, Cui Y, Zhang L (1999) A pair of new geometrically isomeric Triterpenoid Saponins from the seeds of Aesculus chinensis. Chin Chem Lett 11:925–928
Cheng JT, Chen ST, Guo C, Jiao MJ, Cui WJ, Wang SH (2018) Triterpenoid Saponins from the seeds of Aesculus chinensis and their cytotoxicities. Nat Prod Bioprospect 8(1):47–56
Ireneusz K, Bogdan J, Barbara S, Anna S, Sonia P, Cosimo P, Federico F, Chlodwig F, Wieslaw O (2007) Flavonoids in horse chestnut (Aesculus hippocastanum) seeds and powdered waste water byproducts. J Agric Food Chem 55(21):8485–8490
Wei F, Ma S, Ly M, But PP, Lin RC, Khan IA (2004) Antiviral flavonoids from the seeds of Aesculus chinensis. J Nat Prod 67(4):650–653
Niu X, Wang Y, Li W, Zhang H, Wang X, Mu Q, He Z, Yao H (2015) Esculin exhibited anti-inflammatory activities in vivo and regulated TNF-α and IL-6 production in LPS-stimulated mouse peritoneal macrophages in vitro through MAPK pathway. Int Immunopharmacol 29(2):779–786
Zhang CL, Wu SQ, Li XS (2009) Analysis on fatty acids composition in Aesculus chinensis seeds. Seed 28(8):53–55
Patlolla JM, Raju J, Swamy MV, Rao CV (2006) Beta-escin inhibits colonic aberrant crypt foci formation in rats and regulates the cell cycle growth by inducing p21(waf1/cip1) in colon cancer cells. Mol Cancer Ther 5:1459–1466
Peng C, Fang K, Gong J (2016) Aescin reduces oxidative stress and provides neuroprotection in experimental traumatic spinal cord injury. Free Radical Bio Med 99:405–417
Matsuda H, Li Y, Murakami T, Ninomiya K, Araki N, Yoshikawa M (1997) Antiinflammatory effects of escins Ia Ib IIa and IIb from horse chestnut the seeds of Aesculus hippocastanum L. Bioorg Med Chem Lett 7:1611–1616
Piller NB (1976) Drug-induced proteolysis: a correlation with oedema-reducing ability. Br J Exp Path 57:266–273
Zhang N, Huang WX, Cao SJ, Zhang Q, Kang N, Ding LQ, Qiu F (2020) Bioactive Triterpenoid Saponins from the seeds of Aesculus chinensis Bge. var. chekiangensis. Front Chem 7:908–922
Zhang N, Cao SJ, Huang WX, Li P, Kang N, Ding LQ, Qiu F (2019) New indole glycosides from Aesculus chinensis var. chekiangensis and their neuroprotective activities. Molecules 24:4063–4071
Tan YZ, Yong Y, Dong YH, Wang RJ, Li HX, Zhang H, Guo DL, Zhang SJ, Dong XP, Xie XF (2016) A new secoiridoid glycoside and a new sesquiterpenoid glycoside from Valeriana jatamansi with neuroprotective activity. Phytochem Lett 17:177–180
Elreadi MZ, Eid S, Ashour ML, Tahrani A, Wink M (2013) Modulation of multidrug resistance in cancer cells by chelidonine and Chelidonium majus alkaloids. Phytomedicine 20:282–294
Xia YZ, Yang L, Wang ZD, Guo C, Zhang C, Geng YD, Kong LY (2015) Schisandrin a enhances the cytotoxicity of doxorubicin by the inhibition of nuclear factor-kappa B signaling in a doxorubicin-resistant human osteosarcoma cell line. RSC Adv 5:13972–13984
Tanaka T, Nakashima T, Ueda T, Kenji T, Isao K (2007) Facile discrimination of aldose enantiomers by reversed-phase HPLC. Chem Pharm Bull 55:899–901
Zhang N, Huang WX, Xia GY, Oppong MB, Ding LQ, Li P (2018) Methods for determination of absolute configuration of monosaccharides. Chin Herb Med 10:14–22
Nakamura S, Zhang Y, Nakashima S, Oda Y, Matsuda H (2016) Structures of aromatic glycosides from the seeds of cassia auriculata. Chem Pharm Bull 64(7):970–974
Zou W, Zeng J, Zhuo M, Xu W, Sun L, Wang J (2002) Involvement of caspase-3 and p38 mitogen-activated protein kinase in cobalt chloride-induced apoptosis in PC12 cells. J Neurosci Res 67:837–843
Li GL, Hong G, Li XY, Zhang Y, Xu ZP, Mao LN, Feng XZ, Liu TJ (2018) Synthesis and activity towards Alzheimer’s disease in vitro: tacrine, phenolic acid and ligustrazine hybrids. Eur J Med Chem 148:238–254
Lubica H, Licht A, Sandig G, Manuela J, Zdena D, Tilman G (2003) Standardized extracts of flavonoids increase the viability of PC12 cells treated with hydrogen peroxide: effects on oxidative injury. Arch Toxicol 77:22–29
Funding
This work was financially supported by the State Key Program of National Natural Science of China (Grant No. 81430095).
Author information
Authors and Affiliations
Contributions
NZ conceived and designed the experiments. NZ and DL were responsible for the isolation and elucidation the structures. NZ tested cytotoxicity and neuroprotective effects of the compounds. NZ interpreted the data and wrote the paper. DL, SW, SC, XF and KW revised the manuscript. LD and FQ were the project leaders organizing and guiding the experiment. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
No potential conflict of interest was reported by authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Additional file 1:
HR-ESI-MS, 1D- and 2D-NMR spectra of compounds 1–3 (Figures S1–S15), cytotoxic activities of compounds 1–4 on PC12 cells at 10 µM (Figure S16).
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Zhang, N., Liu, D., Wei, S. et al. Phenylethanol glycosides from the seeds of Aesculus chinensis var. chekiangensis. BMC Chemistry 14, 31 (2020). https://doi.org/10.1186/s13065-020-00685-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13065-020-00685-3