Abstract
Background
A new series of benzoxazole analogues was synthesized and checked for their in vitro antibacterial, antifungal and anticancer activities.
Results and discussion
The synthesized benzoxazole compounds were confirmed by IR, 1H/13C-NMR, mass and screened for their in vitro antimicrobial activity against Gram-positive bacterium: Bacillus subtilis, four Gram-negative bacteria: Escherichia coli, Pseudomonas aeruginosa, Klebsiella pneumoniae, Salmonella typhi and two fungal strains: Candida albicans and Aspergillus niger using tube dilution technique and minimum inhibitory concentration (MIC) was noted in µM and compared to ofloxacin and fluconazole. Human colorectal carcinoma (HCT116) cancer cell line was used for the determination of in vitro anticancer activity (IC50 value) by Sulforhodamine B assay using 5-fluorouracil as standard drug.
Conclusion
The performed study indicated that the compounds 1, 10, 13, 16, 19, 20 and 24 had highest antimicrobial activity with MIC values comparable to ofloxacin and fluconazole and compounds 4, 6, 25 and 26 had best anticancer activity in comparison to 5-fluorouracil.

Similar content being viewed by others
Background
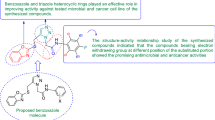
A great number of deaths are occurring throughout the world because of infectious diseases [1]. It has been observed that there is a rapid increase in multi drug resistant infections these days which are causing a rise in various public health problems. There are number of diseases which are now hard to treat with traditional antibiotics drugs and clinicians have to depend on limited drugs such as vancomycin [2]. Because of this there is an increased demand to develop newer antimicrobial agents [3]. One of the most dangerous diseases in the world is cancer and irrespective of so much medical advancement, cancer remains the second leading cause of death in developing as well as developed countries. Although chemotherapy is mostly used for treating cancer, the failure of available chemotherapeutics to treat cancer underscores the need of developing new chemical entities [4]. Human colorectal cancer (CRC) has poor prognosis and is the third most commonly diagnosed malignancies. Therapy is very much required with better efficacy, less adverse effects and improved survival rates [5]. Benzoxazole derivatives have gained a lot of importance in the past few years because of their use in intermediates for the preparation of new biological materials. Benzoxazoles are prominent in medicinal chemistry due to their wide spectrum of pharmacological activities such as antibacterial [2], antifungal [6], anticancer [7], anti-inflammatory [8], antimycobacterial [9], antihistamine [10], antiparkinson [11], inhibition of hepatitis C virus [12], 5-HT3 antagonistic effect [13], melatonin receptor antagonism [14], amyloidogenesis inhibition [15] and Rho-kinase inhibition [16]. A number of marketed drugs (Fig. 1) are available having benzoxazole as core active moiety like, nonsteroidal anti-inflammatory drug (NSAID)—flunoxaprofen, benoxaprofen, antibiotic—calcimycin, antibacterial—boxazomycin B, muscle relaxant—chloroxazone. Prompted by the above findings (Fig. 2) in the present study, we hereby report the synthesis, antimicrobial and anticancer activities of a series of benzoxazole derivatives.
Results and discussion
Chemistry
The method to synthesize the designed benzoxazole derivatives is given in Scheme 1. Initially, 2-(chloromethyl)-1H-benzo[d]imidazole (I) was synthesized by the reaction of ortho phenylenediamine, chloroacetic acid and hydrochloric acid. Benzo[d]oxazole-2-thiol (II) was synthesized by the reaction of methanolic solution of 2-aminophenol with potassium hydroxide, followed by the addition of carbon-di-sulfide. A mixture of I and II was stirred in the presence of triethylamine so as to obtain 2-(((1H-benzimidazol-2-yl) methyl)thio)benzoxazole (III). To a mixture of III and anhydrous potassium carbonate in dry acetone, ethyl chloroacetate was added so as to get ethyl 2-(2-((benzoxazol-2-ylthio)methyl)-1H-benzimidazol-1-yl)acetate (IV). Further reaction of IV with hydrazine hydrate yielded 2-(2-((benzoxazol-2-ylthio) methyl)-1H-benzimidazol-1-yl) acetohydrazide (V). Finally reaction of V with various substituted aldehydes gave the title compounds (1–26). The physicochemical properties of newly synthesized compounds are given in Table 1. The molecular structures of the synthesized compounds (1–26) were determined by IR (ATR, cm−1), 1H/13C-NMR (DMSO-d6, 400 MHz, ppm) and mass spectral studies.
The presence of IR absorption band at 3214 cm−1 in the spectral data of synthesized derivatives (26) corresponds to the group Ar–OH. The C–Br stretching of aromatic bromo compounds shows band around 705 cm−1 (19 and 20). The presence of Ar–NO2 group in compounds (11, 12 and 13) was indicated by the appearance of asymmetric Ar–NO2 stretches in the scale of 1347–1339 cm−1. Arylalkyl ether category (Ar-OCH3) present in the compounds 2, 3, 4, 5 and 6 shows IR absorption stretching at 3053–2835 cm−1. In case of halogen group Ar–Cl vibration appears at 747–740 cm−1 whereas existence of Ar–F group in compounds 8, 17 and 18 was indicated by appearance of Ar–F stretches at 1383–1119 cm−1. The presence of IR stretching at 759–660 cm−1 reflected the presence of C–S group. The presence of CO–NH group is reflected by the presence of absorption bands at 1629–1605 cm−1 whereas the absorption bands at 3213–2919 cm−1, 1496–1452 cm−1 and 1688–1654 cm−1 corresponds to the presence of C–H, C=C and C=N group respectively. In case of 1H-NMR spectra the presence of multiplet signals between 6.85 and 8.83 ppm reflected the presence of aromatic protons in synthesized derivatives. The compound 26 showed singlet at 4.6 ppm because of the presence of OH of Ar–OH. The appearance of singlet at 7.01–8.24 ppm, 7.49–8.26 ppm, 4.61–4.63 ppm and 4.57–4.59 ppm is due to the existence of –CONH, N=CH, N–CH2 and CH2–S groups respectively. Compound 7 showed doublet around 1.22 ppm due to existence of isopropyl group at para position. Compounds 2, 3, 4, 5 and 6 showed singlet at range of 3.72–3.81 ppm due to presence of OCH3 of Ar–OCH3. Finally, DMSO-d6 was used for recording the 13C-NMR spectra of benzoxazole derivatives and it was observed that the spectral signals and proposed molecular structure of the prepared compounds showed good agreement.
Antimicrobial activity
The screening of antibacterial and antifungal activity of the synthesized derivatives was done by tube dilution method [21] and the results are shown in Table 2 as well as Figs. 3 and 4. The study revealed that the prepared derivatives showed moderate to good antimicrobial activity against various microbial strains used. Particularly, compounds 1, 10, 13, 16, 19, 20 and 24 have shown better antimicrobial activity than the standards ofloxacin and fluconazole. Compound 10 (MICbs = 1.14 × 10−3 µM) was found to be most effective against B. subtilis. Compound 24 (MICec = 1.40 × 10−3 µM) was found to be active against E. coli, compound 13 (MICpa = 2.57 × 10−3 µM) against P. aeruginosa, compounds 19 and 20 (MICst = 2.40 × 10−3 µM) against S. typhi, compound 16 (MICkp = 1.22 × 10−3 µM) against K. pneumonia. The results of antifungal activity indicated that compound 19 (MICan = 2.40 × 10−3 µM) was most potent against A. niger and compound 1 (MICca = 0.34 × 10−3 µM) was most effective against C. albicans. The other derivatives showed average to poor antimicrobial activity against all seven species.
Anticancer activity
Human colorectal carcinoma [HCT-116 (ATCC CCL-247)] cancer cell line was used for evaluating the anticancer activity of the prepared benzoxazole compounds using Sulforhodamine B (SRB) assay [22]. 5-Fluorouracil was used as standard drug and the results are shown in Table 2. The results indicated that the compound 6 (IC50 = 24.5 µM) exhibited the best anticancer activity in comparison with the standard drug (IC50 = 29.2 µM) whereas the compounds 4 and 26 displayed IC50 values closer to the reference drug (39.9 µM and 35.6 µM, respectively).
SAR (structure activity relationship) studies
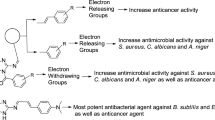
The structure–activity relationship of the synthesized benzoxazole derivatives with their antibacterial and anticancer activity results is summarized in Fig. 5.
-
The substitution of aromatic aldehydes with di-methoxy (compound 4) and tri-methoxy groups (compound 6) improved the anticancer activity of prepared derivatives.
-
Presence of ortho hydroxy group (compound 26) improved the anticancer activity.
-
Presence of unsubstituted benzylidene hydrazide (compound 1) in synthesized oxazole derivatives improved the antifungal activity against C. albicans.
-
Using (methoxymethyl)benzene (compound 10) enhanced the antibacterial activity against B. subtilis.
-
Presence of electron withdrawing groups (compounds 13, 16, 19 and 20) improved the antimicrobial activity against P. aeruginosa, K. pneumonia, S. typhi and A. niger.
-
Substitution of five member cyclic aldehyde i.e., thiophene (compound 24) improved the antibacterial activity of benzoxazole derivatives against E. coli.
Experimental part
The analytical grade chemicals procured from commercial sources were used as such without further purification. Thin-layer chromatography on 0.25 mm silica gel (Merck) plates was performed for monitoring the progress of reaction, using chloroform and methanol as mobile phase in ratio of 9:1 and exposure to iodine vapours helped in observing the spots. Open capillary tube was used for determining the melting points of synthesized compounds. Bruker 12060280, software: OPUS 7.2.139.1294 spectrometer was used for recording infrared spectrum (ATR). Bruker Avance III 600 NMR spectrometer was used for recording 1H and 13C NMR spectra in appropriate deuterated solvents and are expressed in parts per million (ppm) downfield from tetramethylsilane (internal standard). NMR data are given as multiplicity (s, singlet; d, doublet; t, triplet; m, multiplet) and number of protons. Perkin-Elmer 2400 C, H and N analyzer was utilized for the elemental analysis of the new synthesized compounds. All the compounds gave C, H and N analysis within ± 0.4% of the theoretical results. Mass spectra were obtained on Waters Micromass Q-ToF Micro instrument. The physicochemical and spectral data of the prepared compounds helped in their characterization.
Procedure for synthesis of benzoxazole derivatives (2-(2-((benzoxazol-2-ylthio) methyl)-1H-benzimidazol-1-yl) acetohydrazide)
Step 1: Synthesis of 2-(chloromethyl)-1H-benzo[d]imidazole (I)
Phenylenediamine (5.4 g), chloroacetic acid (7.1 g) and 4 N hydrochloric acid were refluxed for 16 h, the mixture was then allowed to stand overnight, filtered and diluted with 100 ml of water, cooled and carefully neutralized with solid sodium bicarbonate. The yellow solid was filtered, washed well with water, recrystallized with ethanol and dried to give the title compound (Yield: 80%). MP: 157–159 °C.
Step 2: Synthesis of benzo[d]oxazole-2-thiol (II)
A mixture of 2-aminophenol (1.1 g) in methanol (15 ml) was prepared to which potassium hydroxide (0.7 g) in water (3 ml) was added, followed by the addition of carbon-di-sulfide (0.9 ml). Resulting solution was refluxed at 65 °C for 5 h. After the completion of reaction, the mixture was poured in water, which was neutralized with concentrated hydrochloric acid. Solid separated was filtered and washed with hexane, recrystallized with ethanol and dried to afford the pure compound (Yield: 90%). MP: 168–170 °C.
Step 3: Synthesis of 2-(((1H-benzimidazol-2-yl) methyl)thio)benzoxazole (III)
A mixture of 2-(chloromethyl)-1H-benzimidazole (1) (1.66 g) and benzoxazole-2-thiol (II) (1.51 g) in dry THF (30 ml) was stirred in the presence of triethylamine (2 ml) for 6 h at room temperature. The reaction was monitored by TLC (chloroform: methanol/9:1, Rf: 0.82). After the completion of reaction, THF was removed and ice cold water (30 ml) was added to the residue with stirring. The solid precipitated was filtered, washed with water followed by hexane, recrystallized with ethanol and dried to afford crude product III (2.5 g, 88%). MP: 181–183 °C.
Step 4: Synthesis of ethyl 2-(2-((benzoxazol-2-ylthio)methyl)-1H-benzimidazol-1-yl)acetate (IV)
A mixture of 2-(((1H-benzimidazol-2-yl)methyl)thio)benzoxazole (III) (2.8 g) and anhydrous potassium carbonate (1 g) in dry acetone (15 ml) was prepared to which ethyl chloroacetate (1.2 ml) was added and the mixture was stirred for 8 h at room temperature. The reaction was monitored by TLC (TLC System: chloroform: methanol/9:1, Rf: 0.65). The resulting solution was then evaporated and solid obtained was suspended in cold water with stirring, which was then filtered, washed with water, recrystallized with ethanol and dried to give desired product IV (Yield: 3.1 g, 85%). MP: 163–165 °C.
Step 5: Synthesis of 2-(2-((benzoxazol-2-ylthio) methyl)-1H-benzimidazol-1-yl) acetohydrazide (V)
A suspension of ethyl 2-(2-((benzoxazol-2-ylthio)methyl)-1H-benzimidazol-1-yl)acetate (IV) (3.57 g) in isopropyl alcohol (30 ml) was added with hydrazine hydrate (98%, 5 ml) and was stirred at room temperature for 1 h. After the completion of reaction as indicated by TLC (chloroform: methanol/9:1, Rf: 0.4), the reaction mixture was poured into ice cold water and the precipitated solid was filtered, washed with cold isopropyl alcohol and recrystallized with ethanol to give compound V as white solid (2.9 g, 82%). MP: 236–238 °C.
Step 6: Synthesis of final derivatives (1–26)
A solution of 2-(2-((benzoxazol-2-ylthio)methyl)-1H-benzimidazol-1-yl) acetohydrazide (V) (0.71 g) in acetic acid (5 ml) was added with corresponding substituted aldehydes The reaction mixture was stirred at room temperature for 30 min. After completion of the reaction as monitored by TLC (chloroform: methanol/9:1), the solution was poured in ice cold water and stirred for 30 min at room temperature. Solid separated out was then filtered, washed with water followed by isopropyl alcohol and recrystallized with ethanol to give pure product.
Spectral data of intermediates and final compounds (1–26)
Intermediate I
IR: 3048 (C–H str., aromatic), 1456 (C=C str., aromatic), 1662 (C=N, N=CH str.), 1189 (C–H str., –CH2), 745 (C–Cl str., Cl); 1H-NMR: 7.35–7.76 (m, 4H, ArH), 4.67 (s, 1H, –NH of imidazole), 4.52 (s, 2H, –CH2); 13C-NMR: 140.8, 137.9, 122.5, 114.6, 40.7; MS ES + (ToF): m/z 167 [M++1]; CHN: Calc. C8H7ClN2: C, 57.67; H, 4.23; N, 16.81; Found: C, 57.72; H, 4.35; N, 16.97.
Intermediate II
IR: 3072 (C–H str., aromatic), 1462 (C=C str., aromatic), 1658 (C=N, N=CH str.), 1183 (C–O–C str. of oxazole), 2498 (–SH str.); 1H-NMR: 7.32 (m, 4H, ArH), 3.61 (s, 1H, –SH); 13C-NMR: 178.3, 151.2, 142.7, 124.4, 118.2, 111.7; MS ES + (ToF): m/z 152 [M++1]; CHN: Calc. C7H5NOS: C, 64.04; H, 3.94; N, 14.94; Found: C, 64.09; H, 3.98; N, 14.97.
Intermediate III
IR: 3046 (C–H str., aromatic), 1485 (C=C str., aromatic), 1670 (C=N, N=CH str.), 1243 (C–N str.), 687 (CH2S, C–S str.), 1189 (C–O–C str. of oxazole); 1H-NMR: 7.31–7.70 (m, 8H, ArH), 3.61 (s, 2H, –CH2S), 4.88 (s, 1H, –NH of imidazole); 13C-NMR: 163.3, 151.3, 141.1, 124.6, 124.4, 118.3, 110.2, 38.8; MS ES + (ToF): m/z 282 [M++1]; CHN: Calc. C15H11N3OS: C, 64.04; H, 3.94; N, 14.94; Found: C, 64.09; H, 3.98; N, 14.97.
Intermediate IV
IR: 3078 (C–H str., aromatic), 1475 (C=C str., aromatic), 1668 (C=N, N=CH str.), 1249 (C–N str.), 689 (CH2S, C–S str.), 1197 (C–O–C str. of oxazole), 3945 (C–H str., –CH3), 1782 (C=O str.), 2745 (C–H str., –OC2H5); 1H-NMR: 7.46–7.72 (m, 8H, ArH), 4.59 (s, 2H, –CH2S), 4.62 (s, 2H, –NCH2), 3.97 (s, 2H, –CH2), 1.92 (s, 3H, –CH3); 13C-NMR:164.7, 151.1, 141.8, 139.8, 132.9, 124.9, 124.4, 119.3, 114.4, 110.9, 55.2, 29.5; MS ES + (ToF): m/z 368 [M++1]; CHN: Calc. C19H17N3O3S: C, 62.11; H, 4.66; N, 11.44; Found: C, 62.16; H, 4.72; N, 11.49.
Intermediate V
IR: 3031 (C–H str., aromatic), 1472 (C=C str., aromatic), 1674 (C=N, N=CH str.), 1240 (C–N str.), 694 (CH2S, C–S str.), 1194 (C–O–C str. of oxazole), 1624 (CONH str., amide), 1778 (C=O str.), 3392 (C–NH2 str.); 1H-NMR: 7.41–7.78 (m, 8H, ArH), 4.57 (s, 2H, –NCH2), 7.89 (s, 1H, –NH), 4.24 (s, 2H, –CH2S), 2.51 (s, 2H, –NH2); 13C-NMR: 167.9, 151.1, 141.7, 139.8, 132.8, 124.8, 124.4, 119.3, 113.7, 110.9, 32.3, 29.7; MS ES + (ToF): m/z 354 [M++1]; CHN: Calc. C17H15N5O2S: C, 57.78; H, 4.28; N, 19.82; Found: C, 57.84; H, 4.34; N, 19.92.
Compound 1
IR: 3062 (C–H str., aromatic), 1490 (C=C str., aromatic), 1669 (C=N, N=CH str.), 1245 (C–N str.), 697 (CH2S, C–S str.), 1196 (C–O–C str. of oxazole), 1621 (CONH str., amide); 1H-NMR: 7.34–7.69 (m, 13H, ArH), 8.15 (s, 1H, N=CH–Ar), 4.63 (s, 2H, –NCH2), 7.95 (s, 1H, –NH), 4.59 (s, 2H, –CH2S); 13C-NMR: 170.4, 165, 151.1, 143.1, 141.7, 139.8, 134.1, 133.9, 130.1,129.7,128.7, 124.9, 124.4, 119.7, 113.7, 110.9, 33.3, 29.5; MS ES + (ToF): m/z 442 [M++1]; CHN: Calc. C24H19N5O2S: C, 65.29; H, 4.34; N, 15.86; Found: C, 65.49; H, 4.40; N, 15.92.
Compound 2
IR: 3211 (C–H str., aromatic), 1455 (C=C str., aromatic), 1666 (C=N, N=CH str.), 1252 (C–N str.), 705 (CH2S, C–S str.), 1196 (C–O–C str. of oxazole), 1624 (CONH str., amide), 3053 (C–H str., –OCH3); 1H-NMR: 6.88–7.79 (m, 12H, ArH), 8.25 (s, 1H, N=CH–Ar), 4.62 (s, 2H, –NCH2), 8.08 (s, 1H, –NH), 4.59 (s, 2H, –CH2S), 3.77 (s, 3H, –OCH3); 13C-NMR: 170.2, 164.7, 151.1, 143.1, 141.8, 139.8, 132.9, 131.7, 126.5, 124.9, 124.4, 119.3, 114.4, 114.2, 110.9, 55.2, 33.3, 29.5; MS ES + (ToF): m/z 472 [M++1]; CHN: Calc. C25H21N5O3S: C, 63.68; H, 4.49; N, 14.85; Found: C, 63.75; H, 4.54; N, 14.92.
Compound 3
IR: 3053 (C–H str., aromatic), 1456 (C=C str., aromatic), 1671 (C=N, N=CH str.), 1248 (C–N str.), 675 (CH2S, C–S str.), 1167 (C–O–C str. of oxazole), 1625 (CONH str., amide), 2941 (C–H str., –OCH3); 1H-NMR: 6.85–7.69 (m, 12H, ArH), 8.24 (s, 1H, N=CH–Ar), 4.62 (s, 2H, –NCH2), 7.89 (s, 1H, –NH), 4.58 (s, 2H, –CH2S), 3.81 (s, 3H, –OCH3); 13C-NMR: 170.3, 164.8, 151.1, 142.1, 141.8, 138.7, 132.8, 131.5, 131.2, 124.9, 124.4, 119.3, 113.7, 110.9, 55.6, 33.3, 29.5; MS ES + (ToF): m/z 472 [M++1]; CHN: Calc. C25H21N5O3S: C, 63.68; H, 4.49; N, 14.85; Found: C, 63.78; H, 4.52; N, 14.88.
Compound 4
IR: 3011 (C–H str., aromatic), 1456 (C=C str., aromatic), 1661 (C=N, N=CH str.), 1244 (C–N str.), 703 (C–S str., CH2S), 1176 (C–O–C str. of oxazole), 1629 (CONH str., amide), 2880 (C–H str., –OCH3); 1H-NMR: 6.90–7.70 (m, 11H, ArH), 4.60 (s, 2H, –CH2S), 4.62 (s, 2H, –NCH2), 8.24 (s, 1H, N=CH–Ar), 8.05 (s, 1H, –NH), 3.77 (s, 6H, (–OCH3)2); 13C-NMR: 170.3, 164.7, 152.2, 150.3, 148.9, 143.2, 141.7, 139.8, 132.9, 126.7, 124.8, 124.4, 121.7, 119.3, 113.7, 110.9, 55.4, 33.34, 29.6; MS ES + (ToF): m/z 502 [M++1]; CHN: Calc. C26H23N5O4S: C, 62.26; H, 4.62; N, 13.96; Found: C, 62.31; H, 4.72; N, 13.99.
Compound 5
IR: 3072 (C–H str., aromatic), 1456 (C=C str., aromatic), 1665 (C=N, N=CH str.), 1247 (C–N str.), 683 (C–S str., CH2S), 1168 (C–O–C str. of oxazole), 1626 (CONH str., amide), 2835 (C–H str., –OCH3); 1H-NMR: 6.93–7.76 (m, 11H, ArH), 4.59 (s, 2H, –CH2S), 4.62 (s, 2H, –NCH2), 8.26 (s, 1H, N=CH–Ar), 8.24 (s, 1H, –NH), 3.74 (s, 6H, (–OCH3)2); 13C-NMR: 170.5, 164.8, 153.1, 151.1, 142.1, 141.7, 138.6, 132.8, 124.9, 124.4, 122.5, 119.2, 116.8, 110.9, 110.8, 108.9, 55.3, 33.3, 29.6; MS ES + (ToF): m/z 502 [M++1]; CHN: Calc. C26H23N5O4S: C, 62.26; H, 4.62; N, 13.96; Found: C, 62.33; H, 4.68; N, 13.98.
Compound 6
IR: 3208 (C–H str., aromatic), 1454 (C=C str., aromatic), 1657 (C=N, N=CH str.), 1238 (C–N str.), 704 (C–S str., CH2S), 1158 (C–O–C str. of oxazole), 1625 (CONH str., amide), 3002 (C–H str., –OCH3); 1H-NMR: 6.9–7.74 (m, 10H, ArH), 3.82 (s, 2H, –CH2S), 4.61 (s, 2H, –NCH2), 8.24 (s, 1H, N=CH–Ar), 8.07 (s, 1H, –NH), 3.72 (s, 9H, (–OCH3)3); 13C-NMR: 170.6, 164.9, 151.1, 148.2, 141.7, 138.8, 132.8, 129.4, 124.9, 124.4, 119.2, 113.7, 110.8, 104.2, 55.8, 33.3, 29.7; MS ES + (ToF): m/z 532 [M++1]; CHN: Calc. C27H25N5O5S: C, 61.00; H, 4.74; N, 13.17; Found: C, 61.05; H, 4.78; N, 13.24.
Compound 7
IR: 3055 (C–H str., aromatic), 1455 (C=C str., aromatic), 1669 (C=N, N=CH str.), 1245 (C–N str.), 706 (C–S str., CH2S), 1165 (C–O–C str. of oxazole), 1622 (CONH str., amide), 3002 (C–H str., –OCH3); 1H-NMR: 7.2–7.79 (m, 12H, ArH), 4.59 (s, 2H, –CH2S), 4.62 (s, 2H, –NCH2), 8.25 (s, 1H, N=CH–Ar), 8.11 (s, 1H, –NH), 3.37 (s, 1H, –CH), 1.22 (d, 6H, (–CH3)2); 13C-NMR: 170.3, 164.9, 150.6, 150.3, 143.1, 141.8, 139.8, 132.9, 131.6, 127.1, 126.6, 124.9, 124.4, 119.3, 113.7, 110.9, 38.8, 33.2, 29.6, 23.6; MS ES + (ToF): m/z 484 [M++1]; CHN: Calc. C27H25N5O2S: C, 67.06; H, 5.21; N, 14.48; Found: C, 67.09; H, 5.28; N, 14.52.
Compound 8
IR: 3213 (C–H str., aromatic), 1456 (C=C str., aromatic), 1670 (C=N, N=CH str.), 1242 (C–N str.), 682 (C–S str., CH2S), 1160 (C–O–C str. of oxazole), 1622 (CONH str., amide), 1119 (C–F); 1H-NMR: 7.39–7.68 (m, 12H, ArH), 4.59 (s, 2H, –CH2S), 4.63 (s, 2H, –NCH2), 8.21 (s, 1H, N=CH–Ar), 8.01 (s, 1H, –NH); 13C-NMR: 170.7, 165.3, 151.1, 144.9, 141.4, 138, 137.9, 132.8, 129.5, 125.3, 124.8, 124.4, 122.6, 119.2, 113.7, 110.9, 33.3, 29.6; MS ES + (ToF): m/z 510 [M++1]; CHN: Calc. C25H18F3N5O2S: C, 58.93; H, 3.56; N, 13.75; Found: C, 58.99; H, 3.62; N, 13.78.
Compound 9
IR: 3085 (C–H str., aromatic), 1460 (C=C str., aromatic), 1673 (C=N, N=CH str.), 1239 (C–N str.), 709 (C–S str., CH2S), 1186 (C–O–C str. of oxazole), 1620 (CONH str., amide), 2227 (C≡N str., cyanide); 1H-NMR: 7.40–7.8 (m, 12H, ArH), 4.58 (s, 2H, –CH2S), 4.63 (s, 2H, –NCH2), 8.19 (s, 1H, N=CH–Ar), 7.98 (s, 1H, –NH); 13C-NMR: 170.8, 165.3, 151.1, 144.7, 141.7, 138.5, 138.3, 132.8, 127.5, 124.9, 124.4, 119.2, 113.7, 110.9, 33.3, 29.5; MS ES + (ToF): m/z 467 [M++1]; CHN: Calc. C25H18N6O2S: C, 64.36; H, 3.89; N, 18.01; Found: C, 64.38; H, 3.93; N, 18.07.
Compound 10
IR: 3053 (C–H str., aromatic), 1456 (C=C str., aromatic), 1671 (C=N, N=CH str.), 1248 (C–N str.), 675 (C–S str., CH2S), 1167 (C–O–C str. of oxazole), 1625 (CONH str., amide); 1H-NMR: 6.96–7.78 (m, 17H, ArH), 4.58 (s, 2H, –CH2S), 4.62 (s, 2H, –NCH2), 8.24 (s, 1H, N=CH–Ar), 8.08 (s, 1H, –NH), 5.15 (s, 2H, –OCH2–Ar); 13C-NMR: 170.2, 164.7, 151.1, 142.9, 141.8, 139.8, 132.9, 128.6, 127.7, 126.8, 124.8, 124.4, 119.3, 115.1, 114.9, 110.9, 69.2, 31.6, 29.5; MS ES + (ToF): m/z 548 [M++1]; CHN: Calc. C31H25N5O3S: C, 67.99; H, 4.60; N, 12.79; Found: C, 68.03; H, 4.64; N, 12.84.
Compound 11
IR: 2955 (C–H str., aromatic), 1456 (C=C str., aromatic), 1673 (C=N, N=CH str.), 1242 (C–N str.), 692 (C–S str., CH2S), 1164 (C–O–C str. of oxazole), 1624 (CONH str., amide), 1339 (N=O, Nitro); 1H-NMR: 7.40–8.23 (m, 12H, ArH), 4.59 (s, 2H, –CH2S), 4.63 (s, 2H, –NCH2), 8.13 (s, 1H, N=CH–Ar), 8.02 (s, 1H, –NH); 13C-NMR: 170.8, 165.4, 151.2, 150.9, 144.2, 141.7, 139.7, 132.8, 124.9, 123.9, 123.8, 119.2, 113.7, 110.8, 33.3, 29.6; MS ES + (ToF): m/z 487 [M++1]; CHN: Calc. C24H18N6O4S: C, 59.25; H, 3.73; N, 17.27; Found: C, 59.16; H, 3.78; N, 17.33.
Compound 12
IR: 3064 (C–H str., aromatic), 1456 (C=C str., aromatic), 1672 (C=N, N=CH str.), 1244 (C–N str.), 701 (C–S str., CH2S), 1166 (C–O–C str. of oxazole), 1625 (CONH str., amide), 1342 (N=O, Nitro); 1H-NMR: 7.41–8.23 (m, 12H, ArH), 4.58 (s, 2H, –CH2S), 4.63 (s, 2H, –NCH2), 8.09 (s, 1H, N=CH–Ar), 8.00 (s, 1H, –NH); 13C-NMR: 172.1, 165.3, 151.1, 148.2, 142.1, 141.7, 138.5, 133.6, 132.8, 130.3, 125.3, 124.9, 124.4, 119.2, 113.7, 110.8, 33.3, 29.5; MS ES + (ToF): m/z 487 [M++1]; CHN: Calc. C24H18N6O4S: C, 59.25; H, 3.73; N, 17.27; Found: C, 59.29; H, 3.65; N, 17.34.
Compound 13
IR: 3075 (C–H str., aromatic), 1453 (C=C str., aromatic), 1666 (C=N, N=CH), 1241 (C–N str.), 677 (C–S str., CH2S), 1167 (C–O–C str. of oxazole), 1624 (CONH str., amide), 1347 (N=O, Nitro); 1H-NMR: 7.38–8.48 (m, 12H, ArH), 4.59 (s, 2H, –CH2S), 4.63 (s, 2H, –NCH2), 8.11 (s, 1H, N=CH–Ar), 8.03 (s, 1H, –NH); 13C-NMR: 170.7, 165.3, 151.1, 148.2, 144.3, 141.7, 139.8, 135.8, 133.1, 130.2, 124.9, 124.2, 123.9, 119.2, 113.7, 110.9, 33.3, 29.5; MS ES + (ToF): m/z 487 [M++1]; CHN: Calc. C24H18N6O4S: C, 59.25; H, 3.73; N, 17.27; Found: C, 59.30; H, 3.75; N, 17.30.
Compound 14
IR: 3058 (C–H str., aromatic), 1456 (C=C str., aromatic), 1670 (C=N, N=CH str.), 1246 (C–N str.), 660 (C–S str., CH2S), 1167 (C–O–C str.. of oxazole), 1625 (CONH str., amide), 747 (C–Cl str., Ar–Cl); 1H-NMR: 7.25–7.77 (m, 12H, ArH), 4.59 (s, 2H, –CH2S), 4.63 (s, 2H, –NCH2), 8.23 (s, 1H, N=CH–Ar), 7.94 (s, 1H, –NH); 13C-NMR: 170.6, 165.1, 151.1, 142.5, 141.7, 139.1, 133, 132.8, 129.8, 127.3, 124.9, 124.4, 119.2, 113.7, 110.8, 33.4, 29.5 MS ES + (ToF): m/z 476 [M++1]; CHN: Calc. C24H18ClN5O2S: C, 60.56; H, 3.81; N, 14.71; Found: C, 60.58; H, 3.85; N, 14.73.
Compound 15
IR: 3082 (C–H str., aromatic), 1460 (C=C str., aromatic), 1670 (C=N str., N=CH str.), 1223 (C–N str.), 708 (C–S str., CH2S), 1186 (C–O–C str. of oxazole), 1619 (CONH str., amide), 745 (C–Cl str., Ar–Cl); 1H-NMR: 7.38–7.70 (m, 12H, ArH), 4.58 (s, 2H, –CH2S), 4.62 (s, 2H, –NCH2), 8.13 (s, 1H, N=CH–Ar), 7.93 (s, 1H, –NH); 13C-NMR: 170.53, 165, 151, 141.8, 141.7, 139.8, 134.4, 132.9, 128.8, 124.8, 124.4, 119.3, 113.7, 110.9, 33.3, 29.5; MS ES + (ToF): m/z 476 [M++1]; CHN: Calc. C24H18ClN5O2S: C, 60.56; H, 3.81; N, 14.71; Found: C, 60.50; H, 3.87; N, 14.65.
Compound 16
IR: 2946 (C–H str., aromatic), 1454 (C=C str., aromatic), 1667 (C=N, N=CH str.), 1242 (C–N str.), 672 (C–S str., CH2S), 1167 (C–O–C str. of oxazole), 1623 (CONH str., amide), 740 (C–Cl str., Ar–Cl); 1H-NMR: 7.28–7.69 (m, 11H, ArH), 4.57 (s, 2H, –CH2S), 4.62 (s, 2H, –NCH2), 8.22 (s, 1H, N=CH–Ar), 7.90 (s, 1H, –NH); 13C-NMR: 170.6, 165.2, 151.1, 141.7, 141.5, 139.8, 138.1, 135, 134.7, 132.8, 130.3, 130.2, 127.7, 124.9, 124.4, 119.2, 113.7, 110.9, 33.4, 29.5; MS ES + (ToF): m/z 511 [M++1]; CHN: Calc. C24H17Cl2N5O2S: C, 56.48; H, 3.36; N, 13.72; Found: C, 56.52; H, 3.44; N, 13.75.
Compound 17
IR: 3060 (C–H str., aromatic), 1452 (C=C str., aromatic), 1663 (C=N, N=CH str.), 1242 (C–N str.), 738 (C–S str., CH2S), 1169 (C–O–C str. of oxazole), 1625 (CONH str., amide), 1383 (C–F str., Ar–F); 1H-NMR: 7.39–7.75 (m, 10H, ArH), 4.58 (s, 2H, –CH2S), 4.62 (s, 2H, –NCH2), 8.23 (s, 1H, N=CH–Ar), 8.11 (s, 1H, –NH); 13C-NMR: 170.9, 165.3, 151.3, 151.1, 143.4, 141.7, 138.1, 132.8, 124.9, 124.4, 119.2, 113.7, 111.2, 110.8, 33.3, 29.5; MS ES + (ToF): m/z 496 [M++1]; CHN: Calc. C24H16F3N5O2S: C, 58.18; H, 3.25; N, 11.50; Found: C, 58.10; H, 3.27; N, 11.53.
Compound 18
IR: 3130 (C–H str., aromatic), 1496 (C=C str., aromatic), 1671 (C=N, N=CH str.), 1267 (C–N str.), 706 (C–S str., CH2S), 1152 (C–O–C str. of oxazole), 1605 (CONH str., amide), 1351 (C–F str., Ar–F); 1H-NMR: 7.16–7.71 (m, 12H, ArH), 4.58 (s, 2H, –CH2S), 4.62 (s, 2H, –NCH2), 8.24 (s, 1H, N=CH–Ar), 8.14 (s, 1H, –NH); 13C-NMR: 170.4, 165.1, 151.1, 145.6, 141.7, 139.8, 132.8, 130.7, 129.2, 124.8, 124.4, 119.3, 113.7, 33.3, 29.5; MS ES + (ToF): m/z 460 [M++1]; CHN: Calc. C24H18FN5O2S: C, 62.73; H, 3.95; N, 15.24; Found: C, 62.76; H, 3.98; N, 15.16.
Compound 19
IR: 3055 (C–H str., aromatic), 1485 (C=C str., aromatic), 1688 (C=N, N=CH str.), 1245 (C–N str.), 705 (C–S str., CH2S), 1188 (C–O–C str. of oxazole), 1626 (CONH str., amide), 705 (C–Br str., Ar–Br); 1H-NMR: 7.40–7.77 (m, 12H, ArH), 4.58 (s, 2H, –CH2S), 4.62 (s, 2H, –NCH2), 8.24 (s, 1H, N=CH–Ar), 8.11 (s, 1H, –NH); 13C-NMR: 170.5, 165.1, 151.1, 141.9, 141.7, 139.8, 133.3, 132.8, 131.7, 131.6, 125.3, 124.9, 124.4, 123.2, 119.2, 113.7, 110.9, 33.3, 29.5; MS ES + (ToF): m/z 521 [M++1]; CHN: Calc. C24H18BrN5O2S: C, 55.39; H, 3.49; N, 13.46; Found: C, 55.42; H, 3.51; N, 13.50.
Compound 20
IR: IR: 3057 (C–H str., aromatic), 1458 (C=C str., aromatic), 1669 (C=N, N=CH str.), 1287 (C–N str.), 707 (C–S str., CH2S), 1247 (C–O–C str. of oxazole), 1626 (CONH str., amide), 707 (C–Br str., Ar–Br); 1H-NMR: 7.29–7.69 (m, 12H, ArH), 4.58 (s, 2H, –CH2S), 4.62 (s, 2H, –NCH2), 8.24 (s, 1H, N=CH–Ar), 7.91 (s, 1H, –NH); 13C-NMR: 170.6, 165.1, 151.1, 144.9, 141.5, 139.8, 133.1, 131.7, 131.3, 127.8, 124.9, 123.4, 123.1, 119.2, 113.7, 110.9, 33.4, 29.5; MS ES + (ToF): m/z 521 [M++1]; CHN: Calc. C24H18BrN5O2S: C, 55.39; H, 3.49; N, 13.46; Found: C, 55.43; H, 3.42; N, 13.52.
Compound 21
IR: 3051 (C–H str., aromatic), 1453 (C=C str., aromatic), 1654 (C=N, N=CH str.), 1246 (C–N str.), 686 (C–S str., CH2S), 1159 (C–O–C str. of oxazole), 1619 (CONH str., amide), 3160 (N–H str., indole); 1H-NMR: 7.03–7.76 (m, 12H, ArH), 4.58 (s, 2H, –CH2S), 4.63 (s, 2H, –NCH2), 7.51 (s, 1H, N=CH–Ar), 7.01 (s, 1H, –NH); 13C-NMR: 169.4, 164.1, 151.1, 143.9, 141.7, 139.8, 136.9, 132.9, 130.3, 125.3, 124.9, 123.9, 122.5, 122.5, 120.4, 119.7, 119.3, 113.7, 111.1, 110.9, 32.2, 29.6; MS ES + (ToF): m/z 481 [M++1]; CHN: Calc. C26H20N6O2S: C, 64.98; H, 4.20; N, 17.49; Found: C, 65.07; H, 4.25; N, 17.52.
Compound 22
IR: 3054 (C–H str., aromatic), 1453 (C=C str., aromatic), 1665 (C=N, N=CH str.), 1245 (C–N str.), 746 (C–S str., CH2S), 1202 (C–O–C str. of oxazole), 1624 (CONH str., amide), 1570 (conjugation); 1H-NMR: 7.29–7.76 (m, 13H, ArH), 4.58 (s, 2H, –CH2S), 4.61 (s, 2H, –NCH2), 7.51 (s, 1H, N=CH–Ar), 7.01 (s, 1H, –NH); 13C-NMR: 170.1, 164.8, 151.1, 141.7, 139.1, 138.6, 135.7, 128.7, 128.7, 126.9, 124.9, 124.4, 119.3, 113.7, 110.9, 33.3, 29.5; MS ES + (ToF): m/z 468 [M++1]; CHN: Calc. C26H21N5O2S: C, 66.79; H, 4.53; N, 14.98; Found: C, 66.71; H, 4.58; N, 15.05.
Compound 23
IR: 3079 (C–H str., aromatic), 1460 (C=C str., aromatic), 1687 (C=N, N=CH str.), 1241 (C–N str.), 707 (C–S str., CH2S), 1186 (C–O–C str. of oxazole), 1617 (CONH str., amide), 1570 (C=N str., Pyridine); 1H-NMR: 7.410–8.83 (m, 12H, ArH), 4.59 (s, 2H, –CH2S), 4.63 (s, 2H, –NCH2), 7.49 (s, 1H, N=CH–Ar), 7.413 (s, 1H, –NH); 13C-NMR: 170.8, 165.4, 150.1, 144.3, 141.7, 139.8, 132.8, 124.8, 124.4, 119.2, 113.7, 110.9, 33.3, 29.5; MS ES + (ToF): m/z 443 [M++1]; CHN: Calc. C23H18N6O2S: C, 62.43; H, 4.10; N, 18.99; Found: C, 62.49; H, 4.15; N, 19.05.
Compound 24
IR: 3057 (C–H str., aromatic), 1454 (C=C str., aromatic), 1671 (C=N, N=CH str.), 1243 (C–N str.), 709 (C–S str., CH2S), 1198 (C–O–C str. of oxazole), 1623 (CONH str., amide), 1570 (C–H str., thiophene); 1H-NMR: 7.06–8.37 (m, 8H, Ar–H), 4.62 (s, 2H, CH2–S), 4.59 (s, 2H, –NCH2), 8.26 (s, 1H, N=CH–Ar), 7.07 (s, 1H, –NH), {7.08 (d, 1H, CH), 7.54 (t, 1H, CH), 7.84 (d, 1H, CH) of thiophene ring}; 13C-NMR: 170, 164.8, 151.1, 141.7, 138.8, 127.7, 125.3, 124.9, 124.4, 119.2, 113.7, 110.8, 33.3, 29.6; MS ES + (ToF): m/z 448 [M++1]; CHN: Calc. C22H17N5O2S2: C, 59.04; H, 3.83; N, 15.65; Found: C, 59.14; H, 3.85; N, 15.58.
Compound 25
IR: 2919 (C–H str., aromatic), 1454 (C=C str., aromatic), 1661 (C=N, N=CH str.), 1244 (C–N str.), 739 (C–S str., CH2S), 1171 (C–O–C str. of oxazole), 1626 (CONH str., amide), 1572 (C–H str., thiophene); 1H-NMR: 6.90–8.40 (m, 8H, Ar–H), 3.72 (s, 2H, CH2–S), 4.62 (s, 2H, –NCH2), 8.26 (s, 1H, N=CH–Ar), 7.20 (s, 1H, NH), {7.55 (d, 1H, CH), 7.77 (d, 1H, CH) of thiophene ring}, 2.52 (s, 3H, –CH3); 13C-NMR: 169.8, 164.6, 151.1, 141.7, 139.1, 137.4, 127.3, 125.3, 124.9, 124.4, 119.2, 113.7, 110.8, 33.4, 28.9, 13.4; MS ES + (ToF): m/z 462 [M++1]; CHN: Calc. C23H19N5O2S2: C, 59.85; H, 4.15; N, 15.17; Found: C, 59.91; H, 4.22; N, 15.23.
Compound 26
IR: 3046 (C–H str., aromatic), 1457 (C=C str., aromatic), 1663 (C=N, N=CH str.), 1249 (C–N str.), 746 (C–S str., CH2S), 1197 (C–O–C str. of oxazole), 1618 (CONH str., amide), 3214 (–OH); 1H-NMR: 6.85–7.77 (m, 12H, ArH), 4.58 (s, 2H, –CH2S), 4.63 (s, 2H, –NCH2), 8.26 (s, 1H, N=CH–Ar), 7.89 (s, 1H, –NH), 4.6 (s, 1H, –OH); 13C-NMR: 170.1, 164.8, 151.1, 141.7, 139.8, 132.8, 131.1, 124.9, 124.4, 119.2, 118.5, 113.7, 110.9, 33.1, 29.5; MS ES + (ToF): m/z 458 [M++1]; CHN: Calc. C24H19N5O3S: C, 63.01; H, 4.19; N, 15.31; Found: C, 63.08; H, 4.11; N, 15.38.
Biological study
Antimicrobial activity
Tube dilution method [21] was used for the determination of minimum inhibitory concentration (MIC) of the synthesized derivatives (1–26) using ofloxacin and fluconazole as standard drugs against seven microbial species i.e. B. subtilis (MTCC-441), E. coli (MTCC-443), P. aeruginosa (MTCC-424), S. typhi (MTCC-98), K. pneumoniae (MTCC-530),
Candida albicans (MTCC-227) and A. niger (MTCC-281). Double strength nutrient broth I.P. (bacteria) or sabouraud dextrose broth I.P. (fungi) was used for the preparation of the serial dilution of test and standard compounds [23]. Dimethyl sulfoxide (DMSO) was used for the preparation of stock solution of test and standard compounds. The concentrations of 50, 25, 12.5, 6.25, 3.125 and 1.562 µg/ml were obtained by doing further progressive dilutions. The samples were incubated at 37 ± 1 °C for 24 h (bacteria), at 25 ± 1 °C for 7 days (A. niger) and at 37 ± 1 °C for 48 h (C. albicans), respectively and the results were recorded in terms of MIC. The lowest concentration of the compounds under evaluation that showed no signs of microbial growth of in the tube was the MIC. A control was performed with the test medium supplemented with DMSO at the same dilutions as used in the study to ensure that the solvent had no effect on the bacterial growth.
Anticancer activity
Human colorectal carcinoma [HCT-116 (ATCC (American Type Culture Collection) CCL-247)] cancer cell line was used for the determination of anticancer activity of the prepared derivatives using 2-(3-diethyl-amino-6-diethylazaniumylidene-xanthen-9-yl)-5-sulfobenzene-sulfonate (SRB) assay. In this study, trichloroacetic acid was used for fixing the cells and then staining was done for 30 min with 0.4% (w/v) sulforhodamine B mixed with 1% acetic acid. Five washes of 1% acetic acid solution helped in discarding the unbound dye and protein-bound dye was extracted with 10 mM unbuffered tris base solution for confirmation of optical density at 570 nm in a computer-interfaced, 96-well microtiter plate reader [22].
Conclusion
A series of new benzoxazole derivatives was prepared and its chemical structure was confirmed by 1H/13C NMR, Mass and IR studies. All the derivatives were further evaluated for antibacterial, antifungal and anticancer activity and it was observed that the compounds 1, 10, 13, 16, 19, 20 and 24 displayed the best activity against various microbial species in comparison to reference drug ofloxacin and fluconazole. In case of anticancer activity, the compound 4 was the most active whereas compounds 6 and 26 had activity closer to the reference drug, 5-fluorouracil.
References
Tenover FC (2006) Mechanisms of antimicrobial resistance in bacteria. Am J Med 119(6A):S3–10
Chilumula NR, Gudipati R, Ampati S, Manda S, Gadhe D (2010) Synthesis of some novel methyl-2-(2-(arylideneamino)oxazol-4-ylamino)benzoxazole-5-carboxylate derivatives as antimicrobial agents. Int J Chem Res 1(2):1–6
Ram Prasad S, Saraswathy T, Niraimathi V, Indhumathi B (2012) Synthesis, characterization and antimicrobial activity of some hetero benzocaine dervivatives. Int J Pharm Pharm Sci 4(5):285–287
Kamal A, Dastagiri D, Ramaiah MJ, Reddy JS, Bharathi EV, Reddy MK, Sagar MP, Reddy TL, Pushpavalli S, Bhadra MP (2011) Synthesis and apoptosis inducing ability of new anilino substituted pyrimidine sulfonamides as potential anticancer agents. Eur J Med Chem 46(12):5817–5824
Sun X, Suo J, Yan J (2016) Immunotherapy in human colorectal cancer: challenges and prospective. World J Gastroenterol 22(28):6362–6372
Ryu CK, Lee RY, Kim NY, Kim YH, Song AL (2009) Synthesis and antifungal activity of benzo[d]oxazole-4,7-diones. Bioorg Med Chem Lett 19(20):5924–5926
Aiello S, Wells G, Stone EL, Kadri H, Bazzi R, Bell DR, Stevens MFG, Matthews CST, Bradshaw D, Westwell AD (2008) Synthesis and biological properties of benzothiazole, benzoxazole, and chromen-4-one analogues of the potent antitumor agent 2-(3,4-dimethoxyphenyl)-5-fluorobenzothiazol. J Med Chem 51(16):5135–5139
Sondhi SM, Singh N, Kumar A, Lozach O, Meijer L (2006) Synthesis, anti-inflammatory, analgesic and kinase (CDK-1, CDK-5 and GSK-3) inhibition activity evaluation of benzimidazole/benzoxazole derivatives and some Schiff’s bases. Bioorg Med Chem 14(11):3758–3765
Davidson JP, Corey EJ (2003) First enantiospecific total synthesis of the antitubercular marine natural product pseudopteroxazole revision of assigned stereochemistry. J Am Chem Soc 125(44):13486–13489
Katsura Y, Inoue Y, Nishino S, Tomoi M, Itoh H, Takasugi H (1992) Studies on antiulcer drugs. III. Synthesis and antiulcer activities of imidazo[1,2-a]pyridinylethylbenzoxazoles and related compounds. A novel class of histamine H2-receptor antagonists. Chem Pharm Bull 40(6):1424–1438
Benazzouz A, Boraud T, Dubedat P, Boireau A, Stutzmann JM, Gross C (1995) Riluzole prevents MPTP-induced parkinsonism in the rhesus monkey: a pilot study. Eur J Pharmacol 284(3):299–307
Smith P, Ward DN (2011) Heterocyclic benzoxazole compositions as inhibitors of hepatitis c virus. US 2012/0208856_01
Yasuo S, Megumi Y, Satoshi Y, Tomoko Midori I, Tetsutaro N, Kokichi S, Fukio K (1998) Benzoxazole derivatives as novel 5-HT3 receptor partial agonists in the Gut. J Med Chem 41(16):3015–3021
Sun LQ, Chen J, Bruce M, Deksus JA, Epperson JR, Takaki K, Johnson G, Iben L, Mahle CD, Ryan E, Xu C (2004) Synthesis and structure–activity relationship of novel benzoxazole derivatives as melatonin receptor agonists. Bioorg Med Chem Lett 14(14):3799–3802
Razavi H, Palaninathan SK, Powers ET, Wiseman RL, Purkey HE, Mohamedmohaideen NN, Deechongkit S, Chiang KP, Dendle MTA, Sacchettini JC, Kelly JW (2003) Benzoxazoles as transthyretin amyloid fibril inhibitors: synthesis, evaluation, and mechanism of action. Angew Chem Int Ed 42(24):2758–2761
Sessions EH, Yin Y, Bannister TD, Weiser A, Griffin E, Pocas J, Cameron MD, Ruiz C, Lin L, Schürer SC, Schröter T, LoGrasso P, Feng Y (2008) Benzimidazole- and benzoxazole-based inhibitors of Rho kinase. Bioorg Med Chem Lett 18(24):6390
Kumar D, Kumar NM, Sundaree S, Johnson EO, Shah K (2010) An expeditious synthesis and anticancer activity of novel 4-(3´-indolyl)oxazole. Eur J Med Chem 45(3):1244–1249
Babul reddy A, Hymavathi RV, Narayana Swamy G (2013) A new class of multi-substituted oxazole derivatives: synthesis and antimicrobial activity. J Chem Sci 125(3):495–509
Padmavathi V, Kumara CP, Venkatesh BC, Padmaja A (2011) Synthesis and antimicrobial activity of amido linked pyrrolyl and pyrazolyl-oxazoles, thiazoles and imidazoles. Eur J Med Chem 46(11):5317–5326
Sączewski F, Stencel A, Bieńczak AM, Langowska KA, Michaelis M, Werel W, Hałasa R, Reszka P, Bednarski PJ (2008) Structure activity relationships of novel heteroaryl-acrylonitriles as cytotoxic and antibacterial agents. Eur J Med Chem 43(9):1847–1857
Cappuccino JC, Sherman N (1999) Microbiology-a laboratory manual. Addison Wesley, California, p 263
Skehan P, Storeng R, Scudiero D, Monks A, McMahon J, Vistica D, Warren JT, Bokesch H, Kenney S, Boyd MR (1990) New colorimetric cytotoxicity assay for anticancer-drug screening. J Natl Cancer Inst 82(13):1107–1112
The Indian Pharmacopoeia Commission (2007) Pharmacopoeia of India, vol Ӏ. Controller of publication, ministry of health department, Govt. of India, New Delhi, p 37
Authors’ contributions
The designing, synthesis, antimicrobial activity and spectral analysis of the prepared benzoxazole derivatives was done by the authors BN, SK and ST. The anticancer evaluation of synthesized compounds was carried out by the authors KR, SAAS, SML and VM. All authors read and approved the final manuscript.
Acknowledgements
The authors wish to extend their gratitude to the Head, Department of Pharmaceutical Sciences, M. D. University, Rohtak for providing necessary facilities to carry out this research work.
Competing interests
The authors declare that they have no competing interests.
Ethics approval and consent to participate
Not applicable.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Kakkar, S., Tahlan, S., Lim, S.M. et al. Benzoxazole derivatives: design, synthesis and biological evaluation. Chemistry Central Journal 12, 92 (2018). https://doi.org/10.1186/s13065-018-0459-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13065-018-0459-5