Abstract
Background
CAP (Community acquired pneumonia) is frequent, with a high mortality rate and a high burden on health care systems. Development of predictive biomarkers, new therapeutic concepts, and epidemiologic research require a valid, reproducible, and quantitative measure describing CAP severity.
Methods
Using time series data of 1532 patients enrolled in the PROGRESS study, we compared putative measures of CAP severity for their utility as an operationalization. Comparison was based on ability to correctly identify patients with an objectively severe state of disease (death or need for intensive care with at least one of the following: substantial respiratory support, treatment with catecholamines, or dialysis). We considered IDSA/ATS minor criteria, CRB-65, CURB-65, Halm criteria, qSOFA, PSI, SCAP, SIRS-Score, SMART-COP, and SOFA.
Results
SOFA significantly outperformed other scores in correctly identifying a severe state of disease at the day of enrollment (AUC = 0.948), mainly caused by higher discriminative power at higher score values. Runners-up were the sum of IDSA/ATS minor criteria (AUC = 0.916) and SCAP (AUC = 0.868). SOFA performed similarly well on subsequent study days (all AUC > 0.9) and across age groups. In univariate and multivariate analysis, age, sex, and pack-years significantly contributed to higher SOFA values whereas antibiosis before hospitalization predicted lower SOFA.
Conclusions
SOFA score can serve as an excellent operationalization of CAP severity and is proposed as endpoint for biomarker and therapeutic studies.
Trial registration
clinicaltrials.gov NCT02782013, May 25, 2016, retrospectively registered.
Similar content being viewed by others
Background
CAP (Community acquired pneumonia) is one of the most frequent infectious diseases worldwide, contributing to more than a quarter million hospital admissions per year in Germany [1, 2]. Almost half of all patients with severe CAP developed sepsis, with a total mortality of about 13% [3]. Thus, there is an urgent need to develop new therapeutic strategies against CAP and for early detection of a severe disease course.
Observational studies are crucial for research on molecular pathomechanistic concepts and identification of new biomarkers, complementing efforts of experimental work in vitro and in vivo. Eventually, clinical trials are necessary to prove the effects of new therapies or the utility of new diagnostic or prognostic markers [4]. For both, observational studies and clinical trials, high quality endpoints are essential. When overall mortality is relatively low, measures of disease severity at different time points may be an important alternative [5]. Such an operationalization should be objectively assessable since clinical judgment may under- or overestimate severity of CAP [6, 7]. The operationalization should be of quantitative nature, capture relevant pathomechanistic aspects, and largely conform to clinical judgment. The goal of our analyses presented here was to identify such an operationalization for CAP severity of hospitalized patients.
Common endpoint for studies of hospitalized CAP is short-term mortality. Expansion of this endpoint by specific intensive care treatment is becoming accepted [5, 7,8,9,10]. A number of scores are described in the literature, which may serve as candidates for operationalization of CAP severity. These comprise scores initially developed to predict CAP or sepsis outcome (e.g. IDSA/ATS minor criteria [11], C(U)RB65 [12, 13], Halm [5, 14], PSI [15], SCAP [9], and SMART-COP [7]) as well as scores designed to longitudinally describe disease severity (e.g. qSOFA [16], SIRS [17], and SOFA [18]). Despite these efforts to develop clinical scoring systems, assessment of CAP severity still mainly relies on sound clinical judgment, which however is observer dependent and qualitative, therefore not suitable for quantitative analyses [19]. Thus, the current lack of a validated operationalization of CAP severity throughout the course of treatment in the hospital may hamper clinical research [20]. Analyses of well-defined and deeply characterized cohorts of CAP patients are required to improve this situation.
The ongoing PROGRESS study is conducted as a large-scale multi-centric observational study of hospitalized patients with CAP to establish a comprehensive database for high quality clinical and molecular research [21]. We use this database to evaluate the above-mentioned scoring systems regarding their potential to objectively operationalize CAP severity. The aim of this analysis is to identify suitable endpoints for subsequent biomarker research.
Methods
Study subjects
Analyses in this manuscript were based on data of 1532 subjects recruited in the PROGRESS study (clinicaltrials.gov: NCT02782013). Details on study design and procedures can be found elsewhere [21]. In short, PROGRESS is a multicenter observational study with biomaterial asservation of CAP patients admitted to hospitals in Germany and Austria (Fig. 1). The major aim of the PROGRESS study is to identify clinical and molecular-genetic factors determining or predicting severe disease courses in a hypothesis free manner. A total sample size of 3000 is envisaged.
Here, we analyze data of PROGRESS patients from the first half of recruitment, with the aim to develop a suitable operationalization of CAP severity as endpoint for later molecular-genetic research.
Patients of the PROGRESS study were enrolled at general wards (75%), intensive care units (ICU, 13%), and emergency departments (10%). Data were collected by trained study nurses and documented using standardized web-based case report forms (eCRF). The protocol was approved by the ethics committee of the University of Jena (2403–10/08) and by locally responsible ethics committees for each study site. Requirements of the Declaration of Helsinki [22] and the ICH-GCP guideline [23] were met.
All study participants suffered from confirmed CAP and were at least 18 years of age. Written informed consent was obtained from patients or their legal representatives. CAP was defined as working diagnosis of CAP provided by the enrolling physician, pulmonary infiltrate detected by chest X-ray, and at least two of the following five symptoms: 1) fever, 2) cough, 3) purulent sputum, 4) shortness of breath or need for respiratory support, or 5) crackling or rales on auscultation, dullness to percussion, or bronchial breathing.
Hospital acquired pneumonia was avoided by exclusion of patients hospitalized due to pneumonia for more than 48 h before enrollment and patients hospitalized for any other reason within the last 28 days. PROGRESS focuses on immune competent patients, thus excluding immune-compromising comorbidities and treatments. For details on exclusion criteria and their frequencies see Additional file 1. A screening log was used to estimate the frequency of exclusion criteria and other reasons for non-participation [21]. Detection of pathogens relied on tests carried out in routine care: blood culture, culture of respiratory materials, antigen tests for S. pneumoniae and L. pneumophila, and Influenza rapid tests. Results were documented as positive if so considered by the treating physician.
Study-specific assessments
Patient baseline data comprised socio-demographic, anthropometric, and anamnestic information. Risk factors for pneumonia such as smoking history, previous antibiotic use, tube feeding, and mild or newly administered immunosuppression not counting as exclusion criteria were recorded [24, 25].
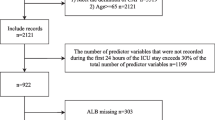
Initial assessment at enrollment (d0) was followed by four study visits (d1 through d4). On d0 through d4, vital parameters (e.g. heart & respiratory rate, body temperature), oxygenation parameters (e.g. blood gas analysis, pulse oximetry), markers for organ function (e.g. bilirubin, creatinine, Glasgow coma scale, use of catecholamines), and other relevant parameters (e.g. detected pathogens, antibiotic treatment, ventilation, treatment on ICU) were assessed and documented and blood samples were taken. Documentation was completed by follow-up at days 28, 180, and 360 post enrollment. A flow chart of our study is shown in Fig. 2. Exclusion criteria and their frequencies can be found in Additional file 1.
Primary endpoint
As mortality in the PROGRESS cohort is low, a composite primary endpoint (PE) was used for comparison of score’s ability to operationalize CAP severity. PE was defined as death within 28 days by any cause, or transfer to ICU. To ensure that transfer to ICU was not solely due to considerations other than deterioration of CAP, we required new CAP-specific treatment in addition to ICU transfer: Substantial respiratory support (ventilation, extracorporeal oxygenation, oxygen supplementation ≥6 l per minute, except for patients with home ventilation), treatment with catecholamines (any dose of adrenalin, epinephrine, noradrenalin, norepinephrine, dopamine, or dobutamine), or dialysis (except for patients with chronic kidney disease). Similar concepts have been proposed before [5, 7,8,9,10, 26].
Candidate scores for operationalization of CAP severity
The literature was reviewed for established pneumonia and sepsis related outcome and severity scores with potential for operationalization of CAP severity. Of these, we selected scores for which data required for calculation are available in PROGRESS at least at the day of enrolment. If comparison of highly similar scores was available in the literature, only the superior score was selected. Scores defined for clearly different settings or which were too similar to our primary endpoint were excluded.
Statistical analysis
Association of severity scores and primary endpoint
Performance of selected scores in describing current CAP severity was assessed by receiver operating characteristics (ROC) for detecting presence (cases) vs. absence (controls) of PE at d0. Area under ROC curves, Youden-statistics (sensitivity+specificity-1), and corresponding confidence intervals were calculated. Differences between areas under ROC curves for different scores were tested for significance. Inspired by Fine et al., missing parameters were replaced by uncritical values to accommodate score calculation [27]. For time series analyses of SOFA and PE, missing parameters were imputed by “last observation carried forward”.
Prediction performance of scores is compared by calculating net reclassification improvements (NRI) of cases and controls separately [28]. The NRI is the difference of relative prediction improvement and prediction deterioration when comparing two prediction models. As an example, NRI = 0.1 implies that the difference of patients better classified by model 1 and patients better classified by model 2 is 10%, i.e. the statistics measures the net improvement of prediction.
Comparison with other cohorts
To assess the generalizability of our results, we compare our PROGRESS cohort with the general population of hospitalized CAP patients in Germany based on the 2014 AQUA report and with the GenIMS study which is similar to our study [2, 29]. Comparisons were performed using standard descriptive methods and tests.
Analysis of pre-clinical risk factors
We analyzed the impact of known risk factors of disease severity and progression on SOFA score at enrollment, namely age, sex, BMI, smoking status, pack-years, and antibiotic treatment in the five days before hospitalization [7, 24, 25, 30, 31]. We performed uni- and multivariate linear regression analyses and tested for interaction.
All calculations were performed using the statistical software package R [32].
Results
Cohort description
Baseline characteristics and comparison with other cohorts
Of the 1532 subjects from the PROGRESS study considered here, 902 (59%) were male. Mean age of all subjects was 59 years. Further patient characteristics are shown in Table 2, comprising baseline risk factors (e.g. nursery home residency, smoking history), chronic comorbidities, clinical state at enrollment, treatment, and outcome. For 994 patients (65%) results of microbiological analysis were available, with 103 testing positive for Streptococcus pneumoniae (10.4% of those tested).
We compared our PROGRESS study with the similar GenIMS study [29] and the overall population of hospitalized CAP patients in Germany described in the annual AQUA report [2]. Percentage of males was roughly similar in all three samples but PROGRESS patients were younger. Risk factors like nursing home residency and chronic bed confinement were less common in PROGRESS. Comparison of clinical characteristics is shown in Table 2.
Primary endpoint
28-day mortality was low in our cohort (N = 35, 2.3%). Therefore, most PE cases were caused by “qualified ICU”, i.e. stay on ICU with at least one of the following treatments: Ventilation (N = 112), catecholamines (N = 54), oxygen supplementation with at least 6 l/min (N = 51), extracorporeal oxygenation (N = 4), or dialysis (N = 7). More than one criterion may be fulfilled concurrently. In total N = 155 (10.1%) patients suffered the endpoint. See Fig. 3 for overlap of treatments and for distribution of PE occurrence over time.
Primary Endpoint (PE) in the PROGRESS study. a Distribution of observed PE states across study events (adm = admission to hospital, d0 = enrollment, d1 = study visit 1, d2 = study visit 2, d3 = study visit 3, d4 = study visit 4, d4+ = time between study visit 4 and 28d follow-up. b Frequencies of specific treatments qualifying for the PE
Candidate severity scores
Review of the literature led to selection of the following scores as candidates for operationalization of CAP severity: IDSA/ATS minor criteria [11], CRB-65 [12], CURB-65 [13], Halm [5, 14], PSI [15], qSOFA [16], SIRS [17], SCAP [9], SMART-COP [7], and SOFA [18]. An overview including considered deterioration of organ functions is provided in Table 1.
All selected scores assess cardiopulmonary function and the central nervous system, but differ in their original purpose, coverage of other organ systems, and specific parameters used. For PSI, CRB-65, and CURB-65 lower score values were more prevalent in our study (see in Additional file 1: Figures S2 & S3). For SIRS, about 80% of PROGRESS patients were assigned to the second class (sepsis) while the first and third classes were allocated in a ratio of about 2:1. Descriptive statistics for IDSA/ATS minor criteria, Halm, SCAP, SMART-COP, and SOFA are also provided in Table 2. Again, most patients show lower score values. The distribution of SOFA scores at enrollment (and at subsequent study visits) is shown in Additional file 1: Figure S4, with the highest frequency for two SOFA points.
Scores for operationalization of CAP severity
Score comparison at enrollment
To evaluate their potential for operationalization of CAP severity, we compared ten established scores for their ability to identify PE at d0.
Figure 4a displays corresponding ROC curves, showing a clearly superior diagnostic value of the SOFA score, indicated by largest AUC and maximum Youden index (for further details see Table 3). All comparisons of SOFA with the other scores were statistically significant. Second and third highest AUC values were achieved by IDSA/ATS minor criteria and SCAP, respectively. Regarding NRI, SOFA is superior in classification of both, cases (with PE) and controls (without PE). For instance, for SOFA compared to second placed IDSA/ATS minor criteria, NRI for cases is 0.28 and for controls 0.17 (see Additional file 1: Table S1 for comparison of all scores versus the null model (guessing) and for the comparison of SOFA with the alternative scores)
Performance of scores regarding PE prediction. a Receiver operating characteristics for severity scores at enrollment. b Percentage of patients with PE in dependence on severity scores: Severity scores were rescaled to the unit interval for this purpose. For SOFA we pooled scores > 10, for Halm > 5 and for SMART-COP > 8 in order to deal with sparsely filled score classes. For SCAP, quintiles were used
At the upper end of score values, only SOFA and IDSA/ATS minor criteria (> 80%), followed by SMART-COP (> 60%), accumulated a larger fraction of PE patients, compared to less than 40% for the other scores (Fig. 4b). At lower values, all scores showed similar proportions of PE patients.
Performance of SOFA score during course of disease and dependence on risk factors
To evaluate the value of the SOFA score for operationalization of CAP severity at different time points, longitudinal data of the PROGRESS study were considered. ROC analyses performed separately for time of enrollment (d0) and each study visit (d1 through d4) showed uniformly high diagnostic power (Fig. 5a). All ROC curves achieved AUC values above 90%. The contribution of SOFA sub-scores to the overall SOFA score is shown in Fig. 5b for all time points. As expected due to the large fraction of patients with less severe CAP, the pulmonary SOFA sub-score contributed most. The proportion was somewhat lower for the time of enrollment and increases thereafter. Overall, SOFA decreases with time and extrapulmonary sub-scores improved prior to the pulmonary sub-score. The second largest contributor to the SOFA score was the kidney sub-score with a mean contribution of 9.2%.
Time series data of SOFA. a ROC analysis of SOFA for different study time points: Diagnostic power is similar for all time points; b Contribution of SOFA sub-scores for day of enrollment and study visits. As expected, the pulmonary SOFA sub-score has the largest impact, which even increases during the course of therapy. Displayed numbers refer to the percentage of the pulmonary SOFA sub-score
We analyzed the discriminative power of the SOFA in dependence on age and comorbidities. Regarding age, we observed a uniform performance across age groups (AUC: 0.94 (< 40 years), 0.97 (40–59 years), 0.93 (60–79 years), 0.95 (≥80 years). Performance is only slightly reduced in patients with comorbidities (AUC = 0.97 without comorbidities, AUC = 0.93 with comorbidities).
Risk factors influencing SOFA score at admission
Since the SOFA score emerged as a strong candidate for operationalization of CAP severity, we analyzed known CAP risk factors regarding their influence on the score at enrollment. Univariate regression models showed significance for age (beta = 0.034, p = 2.0 × 10− 30), sex (beta = − 0.77, p = 5.6 × 10− 12, higher SOFA in males), pack-years (beta = 0.020, p = 4.6 × 10− 11), and antibiotic treatment prior to hospitalization (beta = − 0.94, p = 2.7 × 10− 14). BMI (beta = 0.017, p = 0.058) and current smoking (beta = − 0.17, p = 0.17) were not significant.
All four univariate significant factors remained significant in multivariate analysis (age: beta = 0.029, p = 4.8 × 10− 23, sex: beta = − 0.50, p = 2.5 × 10− 6, pack-years: beta = 0.0085, p = 4.2 × 10− 3, antibiotic treatment prior to hospitalization: beta = − 0.51, p = 1.2 × 10− 5). None of the possible bivariate interaction terms were significant, indicating independent and additive effects. The multivariate model explains 14% of the total variance of SOFA. As expected, high age, male sex, and pack-years were predictors for more severe disease. Patients with antibiotic treatment prior to hospitalization tended to have lower initial SOFA scores.
Discussion
Operationalizing severity of community-acquired pneumonia is important for risk management and biomarker development. It is a major goal of our study to analyze, which of several scores best represents clinical decision-making and can serve as operationalization of CAP severity. Therefore, we compared clinical scores established to describe disease severity or predict outcome in pneumonia or sepsis. SOFA outperformed all competing scores and showed uniform diagnostic power during the course of CAP. In agreement with the literature, we identified age, sex, pack-years, and antibiotic treatment prior to hospital admission as major factors influencing initial SOFA scores [7, 24, 25, 30, 31].
Our strategy to develop a good operationalization of disease severity was to compare the discriminative power of clinical scores with respect to an objectively severe disease state defined by 28-day mortality or qualified intensive care treatment [5, 7,8,9,10]. We used such a combined endpoint in our study since mortality was low. The combined endpoint is very likely to be better predictable than mortality alone since available scores typically use parameters assessed by clinicians to decide on necessity of intensive care, while mortality may depend on circumstances not completely covered. This could explain somewhat lower prediction performance reported in the literature [33].
A literature search revealed a variety of possible candidate scores for operationalizing CAP severity (see Table 1). Although all of them are related to infectious diseases, there are some differences with respect to their original clinical purpose: CURB-65, CRB-65, and PSI were designed to predict mortality of CAP [12, 13, 15]. SIRS was developed to generally distinguish infection, sepsis, and severe sepsis, so that one can expect that it has discriminative power to distinguish mild from severe disease courses in CAP [17]. IDSA/ATS criteria were developed to identify patients requiring treatment on ICU. In contrast, the SOFA score is not originally defined to predict outcome, but to describe the sequence of complications in distinct organs [18]. However, it is considered to be useful for the prediction of outcome in critically ill patients [34]. The qSOFA is proposed as a simplified version of SOFA and was introduced into the new sepsis-3 definition [16]. Halm score is proposed as a summary of criteria of clinical stability. It outperformed CURB-65, IDSA/ATS stability criteria, and CRP regarding prediction of mortality of CAP patients and other severe disease outcomes [5, 14]. SMART-COP is proposed as an improvement of PSI and CURB-65 to predict requirement of intensive care for pneumonia patients [7]. Finally, SCAP was developed to predict critical time courses of pneumonia [9]. A Cochrane meta-analysis shows that IDSA/ATS minor criteria, SCAP, and SMART-COP are superior to PSI and CURB-65 in predicting ICU admission and intensive care treatment of CAP patients (SOFA was not considered) [35].
Several scores were not considered in our present analysis: Expanded-CURB shows superiority in predicting 30-day mortality compared to PSI, SMART-COP, and A-DROP [36]. However, the score requires LDH and albumin, which were not available for most of our patients. A-DROP was not considered in our study due to high similarity with CURB-65 [24]. MEWS and NEWS are shown to be superior compared to qSOFA in predicting adverse outcomes but are intended as emergency scores not fitting our study population [37]. IDSA/ATS major criteria were not considered since they consist of ventilation status and septic shock, very close to our compound endpoint. Instead, we considered the IDSA/ATS minor criteria [11] which were recently proposed to predict need for treatment on ICU [8].
Discriminative power of the scores was assessed by ROC analysis and NRI regarding the presence of our primary endpoint, PE. It revealed that the SOFA score clearly outperformed all considered alternatives. IDSA/ATS minor criteria were only somewhat inferior –probably due to their similarity with SOFA but lesser quantitativeness. Alternatives following with larger distance were SCAP and SMART-COP, followed by Halm, PSI, and CURB-65. SIRS and qSOFA performed worst. Although we focused on cross-sectional analysis, our results were in line with the literature mostly studying future events: A similar performance of CURB-65, CRB-65, and PSI regarding 30-day mortality was observed by several authors in different contexts [7, 36, 38, 39] and in a meta-analysis [35]. We observed similar AUCs for our PE as observed in these studies. Comparable performances of SIRS and qSOFA were found in a population of infected patients regarding an endpoint similar to our PE [37]. Reduced performance of qSOFA compared to SOFA has been shown [33, 40]. Improvement of CRB-65 by adding oxygenation was observed by Kolditz et al. for another German CAP population [8]. Thus, a general trend appears to emerge that SOFA-like scores, covering the current state of the lung and extrapulmonary organs prone to deterioration during CAP, such as IDSA/ATS minor criteria, SMART-COP, SCAP, or Halm perform better in identifying a severe state of CAP (for an overview of organs covered by each score see Table 1). The major advantage of SOFA in our analysis was that it had superior discriminative power in the upper range of the score where the alternatives do not achieve sufficiently high positive predictive values, with IDSA/ATS minor criteria and SMART-COP as exceptions (see Fig. 4b). Of note, the SOFA score also showed discriminative power for low values, comparable to the alternatives. SOFA showed a uniform discriminative power across study days, age groups, and patients with or without comorbidities. Using SOFA, a higher percentage of patients was better classified regarding PE in comparison to the other scores as assessed by the NRI (see Additional file 1: Table S1).
Our study has some limitations. All analyses were performed on data from the PROGRESS study, a multi-center, prospective, longitudinal observational study of hospitalized CAP patients [21] which was designed for identification of potential biomarkers by analysis of several omics layers, including genetic, transcriptomic, and proteomic features, while minimizing potential confounders. Thus, the study population was younger and less comorbid in comparison to the general population of hospitalized CAP-patients in Germany [2]. Lower mortality was observed in PROGRESS, which cannot be explained by the younger age alone (analysis not shown). For our analyses presented here on operationalization of CAP severity, this limits generalizability of results to some extent and requires replication of our results in representative patient cohorts. Potentially, this patient selection may favor prediction of PE rather than mortality. However, our results were independent of patient’s age and only slightly influenced by comorbidities.
Conclusion
The performance of the SOFA score in operationalizing CAP severity might not come as a complete surprise. Parameters corresponding to its elements are commonly assessed by clinicians to decide on status and further treatment of patients. However, as pointed out in the literature, there is no formalization of this process so far. Based on the analysis of data from the PROGRESS study, we recommend the SOFA score to assess disease severity of hospitalized CAP patients. It has the potential to greatly facilitate clinical studies due to its (semi)quantitative nature. In particular, we consider the SOFA score a suitable endpoint for biomarker research questions as addressed in the PROGRESS study. However, we must acknowledge that applicability of our findings to the general CAP population remains to be shown in a less selected patient cohort.
Abbreviations
- A-DROP:
-
Age, Dehydration, Respiratory failure, Orientation disturbance, systolic blood Pressure
- AQUA:
-
Institut für angewandte Qualitätsförderung und Forschung im Gesundheitswesen
- ATS:
-
American Thoracic Society
- AUC:
-
Area under the curve
- BMI:
-
Body Mass Index
- CAP:
-
Community Acquired Pneumonia
- CRB-65:
-
Confusion, Respiratory rate, Blood pressure, age ≥ 65 years
- CRP:
-
C-reactive protein
- CURB-65:
-
Confusion, Urea, Respiratory rate, Blood pressure, age ≥ 65 years
- d0, d1, d2, d3, d4:
-
PROGRESS study day 0 (day of enrollment) and days 1 through 4 (study visits)
- eCRF:
-
Electronic case report form
- GenIMS:
-
Genetic and Inflammatory Markers of Sepsis study
- ICH-GCP:
-
International Council for Harmonization of Technical Requirements for Pharmaceuticals for Human Use Good Clinical Practice
- ICU:
-
Intensive Care Unit
- IDSA:
-
Infectious Diseases Society of America
- IDSA/ATSmc:
-
IDSA/ATS minor criteria
- MEWS:
-
Modified Early Warning Score
- NEWS:
-
National Early Warning Score
- NRI:
-
Net Reclassification Improvement
- PE:
-
Primary Endpoint
- PROGRESS:
-
Prospective observational study on hospitalized community acquired pneumonia
- PSI:
-
Pneumonia Severity Index, also known as Fine-Score
- qSOFA:
-
quick SOFA (Sepsis-related Organ Failure Assessment)
- ROC:
-
Receiver Operating Characteristic
- SCAP:
-
Severe CAP
- SIRS:
-
Systemic Inflammatory Response Syndrome
- SMART-COP:
-
Systolic blood pressure, Multilobar chest radiography involvement, low Albumin level, high Respiratory rate, Tachycardia, Confusion, poor Oxygenation, and low arterial pH
- SOFA:
-
Sequential Organ Failure Assessment Score
References
WHO 2016. WHO report on global burden of disease 2000–2012, update 2014. http://www.who.int/healthinfo/global_burden_disease/en/. Accessed 9 Feb 2016.
AQUA - Institut für angewandte Qualitätsförderung und Forschung im Gesundheitswesen GmbH. PNEU – PNEU - Ambulant erworbene Pneumonie. 2015. https://www.sqg.de/downloads/Bundesauswertungen/2014/bu_Gesamt_PNEU_2014.pdf. Accessed 9 Feb 2016.
Dremsizov T, Clermont G, Kellum JA, Kalassian KG, Fine MJ, Angus DC. Severe sepsis in community-acquired pneumonia: when does it happen, and do systemic inflammatory response syndrome criteria help predict course? Chest. 2006;129:968–78.
Musher DM, Thorner AR. Community-acquired pneumonia. N Engl J Med. 2014;371:1619–28.
Akram AR, Chalmers JD, Taylor JK, Rutherford J, Singanayagam A, Hill AT. An evaluation of clinical stability criteria to predict hospital course in community-acquired pneumonia. Clin Microbiol Infect. 2013;19:1174–80.
Bello S, Vilá M, Torres A. Cardiovascular and inflammatory biomarkers for defining the prognosis of CAP. Clin Pulm Med. 2015;22:114–22.
Charles PGP, Wolfe R, Whitby M, Fine MJ, Fuller AJ, Stirling R, et al. SMART-COP: a tool for predicting the need for intensive respiratory or vasopressor support in community-acquired pneumonia. Clin Infect Dis. 2008;47:375–84.
Kolditz M, Ewig S, Schutte H, Suttorp N, Welte T, Rohde G. Assessment of oxygenation and comorbidities improves outcome prediction in patients with community-acquired pneumonia with a low CRB-65 score. J Intern Med. 2015;278:193–202.
Espana PP, Capelastegui A, Gorordo I, Esteban C, Oribe M, Ortega M, et al. Development and validation of a clinical prediction rule for severe community-acquired pneumonia. Am J Respir Crit Care Med. 2006;174:1249–56.
Vincent J-L. Endpoints in sepsis trials: more than just 28-day mortality? Crit Care Med. 2004;32:S209–13.
Mandell LA, Wunderink RG, Anzueto A, Bartlett JG, Campbell GD, Dean NC, et al. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis. 2007;44(Suppl 2):S27–72.
Ewig S, Welte T. CRB-65 for the assessment of pneumonia severity: who could ask for more? Thorax. 2008;63:665–6.
Lim WS. Defining community acquired pneumonia severity on presentation to hospital: an international derivation and validation study. Thorax. 2003;58:377–82.
Halm EA, Fine MJ, Marrie TJ, Coley CM, Kapoor WN, Obrosky DS, Singer DE. Time to clinical stability in patients hospitalized with community-acquired pneumonia: implications for practice guidelines. JAMA. 1998;279:1452–7.
Fine MJ, Auble TE, Yealy DM, Hanusa BH, Weissfeld LA, Singer DE, et al. A prediction rule to identify low-risk patients with community-acquired pneumonia. N Engl J Med. 1997;336:243–50.
Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, et al. The third international consensus definitions for Sepsis and septic shock (Sepsis-3). JAMA. 2016;315:801–10.
Reinhart K, Brunkhorst FM, Bone H-G, Bardutzky J, Dempfle C-E, Forst H, et al. Prävention, Diagnose, Therapie und Nachsorge der Sepsis. 2010. https://sepsis-hilfe.org/fileadmin/user_upload/pdf/leitlinien_s-2k_sepsis.pdf. Accessed 18 Apr 2016.
Vincent JL, Moreno R, Takala J, Willatts S, de Mendonça A, Bruining H, et al. The SOFA (Sepsis-related organ failure assessment) score to describe organ dysfunction/failure. On behalf of the working group on Sepsis-related problems of the European society of intensive care medicine. Intensive Care Med. 1996;22:707–10.
Steel HC, Cockeran R, Anderson R, Feldman C. Overview of community-acquired pneumonia and the role of inflammatory mechanisms in the immunopathogenesis of severe pneumococcal disease. Mediat Inflamm. 2013;2013:490346.
Kolditz M, Ewig S, Höffken G. Management-based risk prediction in community-acquired pneumonia by scores and biomarkers. Eur Respir J. 2013;41:974–84.
Ahnert P, Creutz P, Scholz M, Schutte H, Engel C, Hossain H, et al. PROGRESS - prospective observational study on hospitalized community acquired pneumonia. BMC Pulm Med. 2016;16:108.
World Medical Association Declaration of Helsinki. Recommendations guiding physicians in biomedical research involving human subjects. Cardiovasc Res. 1997;35:2–3.
European Medicines Agency. ICH Topic E6 (R1) Guideline for Good Clinical Practice: NOTE FOR GUIDANCE ON GOOD CLINICAL PRACTICE CPMP/ICH/135/95. 2002. https://www.ema.europa.eu/documents/scientific-guideline/ich-e6-r1-guideline-good-clinical-practice_en.pdf. Accessed 9 Feb 2016.
Shindo Y, Ito R, Kobayashi D, Ando M, Ichikawa M, Shiraki A, et al. Risk factors for drug-resistant pathogens in community-acquired and healthcare-associated pneumonia. Am J Respir Crit Care Med. 2013;188:985–95.
Feldman C, Anderson R. Cigarette smoking and mechanisms of susceptibility to infections of the respiratory tract and other organ systems. J Inf Secur. 2013;67:169–84.
Kolditz M, Ewig S, Klapdor B, Schutte H, Winning J, Rupp J, et al. Community-acquired pneumonia as medical emergency: predictors of early deterioration. Thorax. 2015;70:551–8.
Fine MJ, Singer DE, Hanusa BH, Lave JR, Kapoor WN. Validation of a pneumonia prognostic index using the MedisGroups comparative hospital database. Am J Med. 1993;94:153–9.
Leening MJG, Vedder MM, Witteman JCM, Pencina MJ, Steyerberg EW. Net reclassification improvement: computation, interpretation, and controversies: a literature review and clinician's guide. Ann Intern Med. 2014;160:122–31.
Kellum JA, Kong L, Fink MP, Weissfeld LA, Yealy DM, Pinsky MR, et al. Understanding the inflammatory cytokine response in pneumonia and sepsis: results of the Genetic and Inflammatory Markers of Sepsis (GenIMS) Study. Arch Intern Med. 2007;167:1655–63.
Remington LT, Sligl WI. Community-acquired pneumonia. Curr Opin Pulm Med. 2014;20:215–24.
Torres A, Peetermans WE, Viegi G, Blasi F. Risk factors for community-acquired pneumonia in adults in Europe: a literature review. Thorax. 2013;68:1057–65.
R Core Team. R: a language and environment for statistical Computing. 2015. http://www.R-project.org/. Accessed 9 Feb 2016.
Raith EP, Udy AA, Bailey M, McGloughlin S, MacIsaac C, Bellomo R, Pilcher DV. Prognostic accuracy of the SOFA score, SIRS criteria, and qSOFA score for in-hospital mortality among adults with suspected infection admitted to the intensive care unit. JAMA. 2017;317:290–300.
Ferreira FL, Bota DP, Bross A, Mélot C, Vincent JL. Serial evaluation of the SOFA score to predict outcome in critically ill patients. JAMA. 2001;286:1754–8.
Marti C, Garin N, Grosgurin O, Poncet A, Combescure C, Carballo S, Perrier A. Prediction of severe community-acquired pneumonia: a systematic review and meta-analysis. Crit Care. 2012;16:R141.
J-l L, Xu F, Zhou H, X-j W, L-x S, R-q L, et al. Expanded CURB-65: a new score system predicts severity of community-acquired pneumonia with superior efficiency. Sci Rep. 2016;6:22911.
Churpek MM, Snyder A, Han X, Sokol S, Pettit N, Howell MD, Edelson DP. Quick Sepsis-related Organ Failure Assessment, Systemic Inflammatory Response Syndrome, and Early Warning Scores for Detecting Clinical Deterioration in Infected Patients outside the Intensive Care Unit. Am J Respir Crit Care Med. 2017;195(7):906–11.
Chalmers JD, Singanayagam A, Akram AR, Mandal P, Short PM, Choudhury G, et al. Severity assessment tools for predicting mortality in hospitalised patients with community-acquired pneumonia. Systematic review and meta-analysis. Thorax. 2010;65:878–83.
Morris A. ACP Journal Club. Review: CURB65, CRB65, and pneumonia severity index similarly predict mortality in community-acquired pneumonia. Ann Intern Med. 2011;154:13.
Dorsett M, Kroll M, Smith CS, Asaro P, Liang SY, Moy HP. qSOFA Has Poor Sensitivity for Prehospital Identification of Severe Sepsis and Septic Shock. Prehosp Emerg Care. 2017;21(4):489–97.
Acknowledgements
We thank the patients and their relatives and legal guardians very much for participation in the PROGRESS study and The PROGRESS Study Group for recruiting and data collection. We also like to thank Kerstin Wieland for programming of database and eCRF and for supporting data integration, Marlies Herold for data and study management, Kay Stötzer for biobanking, Hartwig Schütte for former study management, Christoph Engel for contributions regarding conception of the PROGRESS study, and Tobias Welte for supporting initiation of the PROGRESS project.
The PROGRESS Study Group comprised:
Stefan, Angermair, Charité - Universitätsmedizin Berlin, Benjamin Franklin, Klinik für Anästhesiologie und Intensivtherapie, Hindenburgdamm 30, 12203 Berlin, Stefan.Angermair@charite.de
Christoph, Arntzen, Krankenhaus Angermünde, Klinik f. Innere Medizin / Pneumologie, Rudolf-Breitscheid-Str. 37, 16278 Angermünde, arntzen@krankenhaus-angermuende.de
Lorenz, Balke, Universitätsklinikum Schleswig-Holstein - Campus Kiel, Medizin 1, Arnold-Heller-Straße 3, 24105 Kiel, Lorenz.Balke@uksh.de
Robert, Bals, Universitätsklinikum des Saarlandes, Innere Medizin V, Kirrbergerstr. Gebäude 91, 66421 Homburg/Saar, robert.bals@uks.eu
Michael, Benzke, Charité - Universitätsmedizin Berlin, Medizinische Klinik m. S. Infektiologie und Pneumologie, Chariteplatz 1, 10117 Berlin, michael.benzke@charite.de
Ayhan, Berber, Mathias-Spital Rheine, Klinik für Pneumologie und Thoraxonkologie, Med.Klinik V, Frankenburgstr. 31, 48431 Rheine, pneumologie-sek@Mathias-Spital.de
Frank, Bloos, Universitätsklinikum Jena, Klinik für Anästhesiologie und Intensivtherapie, Erlanger Allee 101, 07747 Jena, Frank.Bloos@med.uni-jena.de
Martin, Buchenroth, Evangelische Kliniken Bonn, Betriebsstätte Johanniter Krankenhaus, Innere Medizin II, Johanniterstraße 3-5, 53113 Bonn, Martin.Buchenroth@ek-bonn.de
Lea, Deterding, Universität Leipzig, Innere Medizin, Neurologie und Dermatologie, Pneumologie / 62-2, Liebigstr. 20, 04103 Leipzig, Lea.Deterding@medizin.uni-leipzig.de
Nicolas, Dickgreber, Mathias-Spital Rheine, Klinik für Pneumologie und Thoraxonkologie, Med.Klinik V, Frankenburgstr. 31, 48431 Rheine, n.dickgreber@Mathias-Spital.de
Oleg, Dmitriev, Christliches Krankenhaus Quakenbrück e. V., Med. Klinik (Abtl. Pneumologie, Allergologie, Schlafmedizin), Danziger Str. 2, 49610 Quakenbrück, h.druckmiller@ckq-gmbh.de
Hermann, Druckmiller, Christliches Krankenhaus Quakenbrück e. V., Med. Klinik (Abtl. Pneumologie, Allergologie, Schlafmedizin), Danziger Str. 2, 49610 Quakenbrück, h.druckmiller@ckq-gmbh.de
Holger, Flick, LKH-Univ. Klinikum Graz, UKIM Pulmologie, Auenbruggerplatz 1, 8036 Graz - Austria, holger.flick@medunigraz.at
Ulrike, Föllmer, Charité - Universitätsmedizin Berlin, Medizinische Klinik m. S. Infektiologie und Pneumologie, Augustenburgerplatz 1, 13353 Berlin, ulrike.foellmer@charite.de
Julia, Freise, Medizinische Hochschule Hannover, Klinik für Pneumologie, Carl-Neuberg-Str. 1, 30652 Hannover, freise.julia@mh-hannover.de
Carmen, Garcia, Charité - Universitätsmedizin Berlin, CCM, Medizinische Klinik m. S. Infektiologie und Pneumologie, Chariteplatz 1, 10117 Berlin, carmen.garcia@charite.de
Sven, Gläser, Vivantes Klinikum Spandau, Kard., Pneum. und kons. Intensivmedizin, Neue Bergstraße 6, 13585 Berlin, sven.glaeser@vivantes.de
Christian, Grah, Gemeinschaftskrankenhaus Havelhöhe, Kardio-Pneumologie, Kladower Damm 221, 14089 Berlin, cgrah@me.com
Simone, Hamberger, Kliniken d. Main-Taunus-Kreises, Klinik f. Pneumologie u. Allg. Innere Medizin, Lindenstr. 10, 65719 Hofheim, shamberger@kliniken-mtk.de
Karsten, Hartung, Lungenklinik Ballenstedt/Harz gGmbH, Ev. Fachkrankenhaus f. Lungenkrankheiten, Robert-Koch-Str. 26-27, 06493 Ballenstedt, K.Hartung@lungenklinik-ballenstedt.de
Barabara, Hauptmeier, Berufsgenossenschaftl. Universitätsklinikum Bergmannsheil GmbH, Klinik f. Pneumologie, Allergologie u. Schlafmedizin, Bürkle-de-la-Camp Platz 1, 44789 Bochum, barbara.schlosser@ruhr-uni-bochum.de
Matthias, Held, Klinikum Würzburg Mitte-Standort MissioKlinik gGmbH, Medizinische Klinkik m. S. Pneumologie u. Beatmungsmedizin, Salvatorstr. 7, 97074 Würzburg, matthias.held@missioklinik.de
Frederik, Hempel, Klinikum Dortmund gGmbH, Medizinische Klinik (Pneumologie / Infektiologie), Münsterstraße 240, 44145 Dortmund, Frederik.Hempel@klinikumdo.de
Iris, Hering, Diakoniekrankenhaus Rotenburg(Wümme)gGmbH, Zentrum für Pneumologie, Elise-Averdieck-Str. 17, 27356 Rotenburg/Wümme, hering@diako-online.de
Carola, Hobler, Kliniken d. Main-Taunus-Kreises, Klinik f. Pneumologie u. Allg. Innere Medizin, Lindenstr. 10, 65719 Hofheim, chobler@kliniken-mtk.de
Andreas, Hocke, Charité - Universitätsmedizin Berlin, Medizinische Klinik m. S. Infektiologie und Pneumologie, Molekulare Bildgebung und Immunregulation, Chariteplatz 1, 10117 Berlin, Andreas.Hocke@charite.de
Ursula, Hoffmann, Universitätsmedizin Mannheim, Studienkoordinierungszentrum, 1. Medizinische Klinik, Theodor-Kutzer-Ufer 1-3, 68167 Mannheim, Ursula.Hoffmann@umm.de
Charite ICU-Teams, Charité - Universitätsmedizin Berlin, Medizinische Klinik m. S. Infektiologie und Pneumologie, Campus Virchowklinikum, Pneumologische Ambulanz, Augustenburgerplatz 1, 13353 Berlin, petra.creutz@charite.de
Henning, Kahnert, Vivantes Klinikum Spandau, Kard., Pneum. und kons. Intensivmedizin, Neue Bergstraße 6, 13585 Berlin, henning.kahnert@vivantes.de
Oliver, Kanwar, Evangelische Kliniken Bonn, Betriebsstätte Johanniter Krankenhaus, Innere Medizin II, Johanniterstraße 3-5, 53113 Bonn, oliver.kanwar@ek-bonn.de
Lena, Kappauf, Evangelisches Krankenhaus Kalk gGmbH, Innere Medizin / Pneumologie, Buchforststr. 2, 51103 Köln, kappauf@evkk.de
Charlotte, Keller, Charité - Universitätsmedizin Berlin, CCM, Medizinische Klinik m. S. Infektiologie und Pneumologie, Chariteplatz 1, 10117 Berlin, charlotte-louisa.keller@charite.de
Nils, Keller, Klinikum St. Georg gGmbH, Klinik für Infektions-/Tropenmedizin und Nephrologie, Delitzscher Straße 141, 04129 Leipzig, Nils.Kellner@sanktgeorg.de
Walter, Knüppel, Krankenhaus Bad Arolsen GmbH, Innere Medizin (Herz-, Kreislauf- u. Lungendiagnostik), Große Allee 50, 34454 Bad Arolsen, w.knueppel@skhba.de
Eva, Koch, Universität Leipzig, Innere Medizin, Neurologie und Dermatologie, Pneumologie / 62-2, Liebigstr. 20, 04103 Leipzig, Eva.Koch@medizin.uni-leipzig.de
Martin, Kolditz, Universitätsklinikum Carl Gustav Carus, TU Dresden, Medizinische Klinik 1 - Abteilung Pneumologie, Fetscherstraße 74, 01307 Dresden, Martin.Kolditz@uniklinikum-dresden.de
Christine, Krollmann, Krankenhaus München-Neuperlach, Klinik für Kardiologie, Pneumologie und Internistische Intensivmedizin, Oskar-Maria-Graf-Ring 51, 81737 München, christine.krollmann@klinikum-muenchen.de
Cornelia, Kropf-Sanchen, Universitätsklinikum Ulm, Studienzentrale Innere II, Pneumologie, Albert-Einstein-Allee 23, 89081 Ulm, cornelia.kropf@uniklinik-ulm.de
Josefa, Lehmke, Vivantes Humboldt-Klinikum, Kardiologie und kons. Intensivmedizin, Am Nordgraben 2, 13509 Berlin, josefa.lehmke@vivantes.de
Christian, Lensch, Universitätsklinikum des Saarlandes, Innere Medizin V, Kirrbergerstr. Gebäude 91, 66421 Homburg/Saar, christian.lensch@uks.eu
Andreas, Liebrich, St. Vincenz und Elisabeth Hospital, Innere Medizin, An der Goldgrube 11, 55130 Mainz, a-liebrich@kkmainz.de
Achim, Lies, Vivantes Netzwerk f. Gesundheit GmbH Vivantes Klinikum Neukölln, Klinik f. Innere Med. - Pneumologie u.Infektiologie - Thoraxzentrum, Rudower Str. 48, 12351 Berlin, achim.lies@vivantes.de
Katrin, Ludewig, Universitätsklinikum Jena, Klinik für Anästhesiologie und Intensivtherapie, Erlanger Allee 101, 07747 Jena, katrin.ludewig@med.uni-jena.de
Lena-Maria, Makowski, Universitätsklinikum Münster, Innere Medizin, Intensivmedizin, Albert-Schweizer-Campus 1, Gebäude A 1, 48149 Münster, lena.makowski@ukmuenster.de
Phillipp, Mayer, Krankenhaus München-Neuperlach, Klinik für Kardiologie, Pneumologie und Internistische Intensivmedizin, Oskar-Maria-Graf-Ring 51, 81737 München, philipp.mayer@klinikum-muenchen.de
Brigitte, Mayer, Klinken Heidenheim, Medizinische Klinik II, Schloßhaustraße 100, 89522 Heidenheim, brigitte.mayer@kliniken-heidenheim.de
Agata, Mikolajewska, Charité - Universitätsmedizin Berlin, Medizinische Klinik m. S. Infektiologie und Pneumologie, Chariteplatz 1, 10117 Berlin, Agata.Mikolajewska@charite.de
Anne, Moeser, Universitätsklinikum Jena, Zentrum für Infektionsmedizin und Krankenhaushygiene, Erlanger Allee 101, 07747 Jena, anne.moeser@med.uni-jena.de
Thomas, Müller, Kliniken d. Main-Taunus-Kreises, Klinik f. Pneumologie u. Allg. Innere Medizin, Lindenstr. 10, 65719 Hofheim, tmueller@kliniken-mtk.de
Michaela, Niebank, Charité - Universitätsmedizin Berlin, Medizinische Klinik m. S. Infektiologie und Pneumologie, Chariteplatz 1, 10117 Berlin, michaela.niebank@charite.de
Markus, Niesen, Vivantes Klinikum Spandau, Kard., Pneum. und kons. Intensivmedizin, Neue Bergstraße 6, 13585 Berlin, markus.niesen@vivantes.de
Tim, Oqueka, Universitätsklinikum Hamburg Eppendorf, Onkologisches Zentrum, Pneumologische Studienzentrale, Ost 24, Raum 15, Martinistraße 52, 20246 Hamburg, t.oqueka@uke.de
Wulf, Pankow, Vivantes Netzwerk f. Gesundheit GmbH Vivantes Klinikum Neukölln, Klinik f. Innere Med. - Pneumologie u.Infektiologie - Thoraxzentrum, Rudower Str. 48, 12351 Berlin, wulf.pankow@vivantes.de
Judith, Pannier, Städt. Klinikum Dessau, Innere Medizin, Auenweg 38, 06847 Dessau-Roßlau, judith.pannier@klinikum-dessau.de
Claus, Peckelsen, Städtisches Klinikum München GmbH - Klinikum Harlaching, Klinik für Akut- und Internistische Intensivmedizin, Sanatoriumsplatz 2, 81545 München, claus.peckelsen@klinikum-muenchen.de
Mathias, Plauth, Städt. Klinikum Dessau, Innere Medizin, Auenweg 38, 06847 Dessau-Roßlau, mathias.plauth@klinikum-dessau.de
Mathias, Pletz, Universitätsklinikum Jena, Zentrum für Infektionsmedizin und Krankenhaushygiene, Erlanger Allee 101, 07747 Jena, mathias.pletz@med.uni-jena.de
Jan, Pluta, Krankenhaus Angermünde, Klinik f. Innere Medizin / Pneumologie, Rudolf-Breitscheid-Str. 37, 16278 Angermünde, Jan.Pluta@gmx.de
Kalina, Popkirova, Klinikum Dortmund gGmbH, Medizinische Klinik (Pneumologie / Infektiologie), Münsterstraße 240, 44145 Dortmund, Kalina.Popkirova@klinikumdo.de
Jessica, Rademacher, Medizinische Hochschule Hannover, Klinik für Pneumologie, Carl-Neuberg-Str. 1, 30652 Hannover, Rademacher.Jessica@mh-hannover.de
Mirja, Ramke, Charité - Universitätsmedizin Berlin, CCM, Medizinische Klinik m. S. Infektiologie und Pneumologie, Chariteplatz 1, 10117 Berlin, mirja.ramke@charite.de
Felix, Rosenow, Universitätsklinikum Münster, Innere Medizin, Intensivmedizin, Albert-Schweizer-Campus 1, Gebäude A 1, 48149 Münster, felix.rosenow@ukmuenster.de
Stefan, Rüdiger, Universitätsklinikum Ulm, Studienzentrale Innere II, Pneumologie, Z.Hd. Polte/Bachbauer/Er, Zimmer 3354/AWT: 23, Albert-Einstein-Allee 23, 89081 Ulm, stefan.ruediger@uniklinik-ulm.de
Bernhard, Ruf, Klinikum St. Georg gGmbH, Klinik für Infektions-/Tropenmedizin und Nephrologie, Delitzscher Straße 141, 04129 Leipzig, Bernhard.Ruf@sanktgeorg.de
Jan, Rupp, Universitätsklinikum Schleswig-Holstein - Campus Lübeck, Med. Klinik III (Pneumologie), Ratzeburger Allee 160, 23538 Lübeck, jan.rupp@uksh.de
Bernhard, Schaaf, Klinikum Dortmund gGmbH, Medizinische Klinik (Pneumologie / Infektiologie), Chefsekretariat, Münsterstraße 240, 44145 Dortmund, bernhard.schaaf@klinikumdo.de
Tom, Schaberg, Diakoniekrankenhaus Rotenburg(Wümme)gGmbH, Zentrum für Pneumologie, Elise-Averdieck-Str. 17, 27356 Rotenburg/Wümme, Schaberg@diako-online.de
Marianne, Schelle, Städt. Klinikum Dessau, Innere Medizin, Auenweg 38, 06847 Dessau-Roßlau, marianne.schelle@klinikum-dessau.de
Patrick, Schmidt-Schridde, Städtisches Klinikum München GmbH - Klinikum Harlaching, Klinik für Akut- und Internistische Intensivmedizin, Sanatoriumsplatz 2, 81545 München, schmidt-schridde@gmx.de
Galina, Schott, Christliches Krankenhaus Quakenbrück e. V., Med. Klinik (Abtl. Pneumologie, Allergologie, Schlafmedizin), Danziger Str. 2, 49610 Quakenbrück.
Barbara, Schröder, Klinikum Würzburg Mitte-Standort MissioKlinik gGmbH, Medizinische Klinkik m. S. Pneumologie u. Beatmungsmedizin, Salvatorstr. 7, 97074 Würzburg, barbara.schroeder@missioklinik.de
Tetyana, Shchetynska-Marinova, Universitätsmedizin Mannheim, Studienkoordinierungszentrum, 1. Medizinische Klinik, Theodor-Kutzer-Ufer 1-3, 68167 Mannheim, Tetyana.Shchetynska-Marinova@umm.de
Michael, Simpfendörfer, St.Vincentius-Kliniken gAG, Med. Klinik IV / Pneumologie, Südendstr. 32, 76137 Karlsruhe, michael.simpfendoerfer@vincentius-ka.de
Thomas, Spinner, Krankenhaus München-Neuperlach, Klinik für Kardiologie, Pneumologie und Internistische Intensivmedizin, Oskar-Maria-Graf-Ring 51, 81737 München, thomas.spinner@klinikum-muenchen.de
Norbert, Suttorp, Charité - Universitätsmedizin Berlin, Medizinische Klinik m. S. Infektiologie und Pneumologie, Chariteplatz 1, 10117 Berlin, norbert.suttorp@charite.de
Dorina, Thiemig, Vivantes Netzwerk f. Gesundheit GmbH Vivantes Klinikum Neukölln, Klinik f. Innere Med. - Pneumologie u.Infektiologie - Thoraxzentrum, Rudower Str. 48, 12351 Berlin, dorina.thiemig@vivantes.de
Daniel, Thomas-Rüddel, Universitätsklinikum Jena, Klinik für Anästhesiologie und Intensivtherapie, Erlanger Allee 101, 07747 Jena, DANIEL.THOMAS@med.uni-jena.de
Markus, Unnewehr, Klinikum Dortmund gGmbH, Medizinische Klinik (Pneumologie / Infektiologie), Münsterstraße 240, 44145 Dortmund, markus.unnewehr@klinikumdo.de
Barbara, Wagener, Lungenklinik Ballenstedt/Harz gGmbH, Ev. Fachkrankenhaus f. Lungenkrankheiten, Robert-Koch-Str. 26-27, 06493 Ballenstedt, B.Wagener@lungenklinik-ballenstedt.de
Gudrun, Wakonigg, LKH-Univ. Klinikum Graz, UKIM Pulmologie, Auenbruggerplatz 20, 8036 Graz - Austria, gudrun.wakonigg@medunigraz.at
Deborah, Wehde, Berufsgenossenschaftl. Universitätsklinikum Bergmannsheil GmbH, Klinik f. Pneumologie, Allergologie u. Schlafmedizin, Bürkle-de-la-Camp Platz 1, 44789 Bochum, deborah.wehde@rub.de
Hubert, Wirtz, Universität Leipzig, Innere Medizin, Neurologie und Dermatologie, Pneumologie / Studiensekretariat, Liebigstr. 20, 04103 Leipzig, Hubert.Wirtz@medizin.uni-leipzig.de
Funding
The PROGRESS study is funded by the German Federal Ministry of Education and Research (BMBF), grant numbers 01KI07110 (Giessen), 01KI07111 (Jena), 01KI07113 (Leipzig), 01KI07114 (Berlin), 01KI1010I (Leipzig), and 01KI1010D (Greifswald). Additional funding is provided by the German Center for Lung Research (DZL, grant number 82DZLJ19A2).BMBF and DZL have no influence on the design of the study, on collection, analysis, and interpretation of data, or on writing the manuscript.
Availability of data and materials
The datasets generated and/or analyzed during the current study are not publicly available due to limitations of patient’s informed consent. However, data are available from the PROGRESS consortium via the corresponding author on reasonable request for pneumonia-related research.
Author information
Authors and Affiliations
Consortia
Contributions
PA, MK, FMB, HH, TC, ML, NS, and MS contributed to the study design. PA, PC, and NS contributed to the study management. PA contributed to the data management. MK contributed to biobanking and clinical chemistry. KH, FS, and MS contributed to the data analysis. PA, PC, MK, HH, MB, UV, TC, MW, ML, NS, and MS contributed to the discussion. PA, PC, FS, and MS contributed to the writing of the manuscript. All authors read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
In this manuscript, data from the PROGRESS study (clinicaltrials.gov: NCT02782013) were used. PROGRESS was approved by the ethics committee of the University of Jena (2403–10/08) and by locally responsible ethics committees of each study center. All participants or their legal guardians gave written informed consent for participation in the study. Requirements of the Declaration of Helsinki [22] and the ICH-GCP guideline [23] were met.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Additional file
Additional file 1:
Exclusion Criteria For screened patients fulfilling inclusion criteria but not enrolled in the study, exclusion criteria and their frequencies were documented. Candidate Scores for Operationalization of CAP Severity Additional details on scores considered here as candidates for operationalization of CAP severity. Figure S1. Age distribution in the PROGRESS cohort in comparison with AQUA. PROGRESS patients are younger than the overall CAP population described in the AQUA report. Figure S2. Distribution of CRB-65 on d0 in comparison with AQUA. PROGRESS patients appear to have less severe disease at enrollment than the overall CAP population described in AQUA. Lower age is partly responsible for this effect. Figure S3. Distribution of PSI on d0 in comparison with GenIMS. There appears to be a larger fraction of patients with less severe disease and lower mortality risk in PROGRESS compared to patients in GenIMS. Figure S4. Distribution of SOFA scores at different time points (d0 = enrollment, d1 = study visit 1, d2 = study visit 2, d3 = study visit 3, d4 = study visit 4). SOFA scores 7 to 24 were pooled. According to study protocol, patients with initially high disease severity were not subjected to study visits. Therefore, at visit d0 a clear shift towards higher scores was observed. Overall, there appears to be a general trend towards improved SOFA scores over time. However, a few patients still have increased SOFA values at later time points. Table S1. We present net reclassification improvement (NRI) for cases and controls induced by the scores compared with the null model (guessing) and corresponding NRIs of SOFA compared to the other scores. SOFA is superior to all other scores for both, cases and controls. (DOCX 156 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Ahnert, P., Creutz, P., Horn, K. et al. Sequential organ failure assessment score is an excellent operationalization of disease severity of adult patients with hospitalized community acquired pneumonia – results from the prospective observational PROGRESS study. Crit Care 23, 110 (2019). https://doi.org/10.1186/s13054-019-2316-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13054-019-2316-x