Abstract
Objective
The objective of this study is to systematically assess the clinical efficacy of hand-assisted laparoscopic surgery (HALS) and laparoscopic right colectomy (LRC).
Methods
The randomized controlled trials (RCTs) and non-RCTs were collected by searching electronic databases (Pubmed, Embase, and the Cochrane Library). The outcomes included intraoperative outcomes, postoperative outcomes, postoperative morbidity, and oncologic outcomes. Meta-analysis was performed using of RevMan 5.3 software.
Results
A total of five studies involving 438 patients were finally included, with 202 cases in HALS group and 236 cases in LRC group. Results of meta-analysis showed that there was no statistical difference between HALS and LRC in terms of conversion rate, length of hospital stay, reoperation rate, postoperative morbidity, and oncologic outcomes. The operative time was 6.5 min shorter in HALS group; however, it was not a clinically significant difference. Although the incision length was longer in HALS, it did not influence the postoperative recovery.
Conclusions
HALS can be considered an alternative to LRC which combines the advantages of open as well as laparoscopic surgery.
Similar content being viewed by others
Background
Since the first report of laparoscopic colectomy by Jacobs et al. [1] in the early 1990s, laparoscopic surgery has been established in the treatment of colorectal diseases. Laparoscopic colorectal surgery could achieve similar oncological outcomes compared with open surgery [2, 3], and it has certain potential advantages, include less blood loss, faster postoperative recovery, less pain, and less wound-related complications [4,5,6,7]. Although its benefits have been well documented, laparoscopic right colectomy (LRC) has still not been widely used [8, 9]. This is probably due to the fact that the procedure is technically difficult, lacking of tactile feedback, and time-consuming and needs sufficient operative volume to ascend the learning curve [10,11,12].
Hand-assisted laparoscopic surgery (HALS) was introduced to simplify the procedure while preserving the clinical advantages of minimally invasive surgery [13]. Comparing with LRC, HALS decreases the learning curve by restoring tactile feedback, and the intracorporeal hand can be used to blunt dissection, retraction, and rapid control of bleeding. On the other hand, HALS can result in a larger incision and a more invasive procedure and interfere with the field of vision [14].
To date, it is controversial as to whether HALS or LRC is preferred in the treatment of colorectal diseases. Proponents believe HALS facilitates minimally invasive colorectal resection for patients with complicated situation, such as obese patients and individuals with a bulky tumor. Critics believe that HALS discourages the use of laparoscopic methods and results in notably longer incisions.
There are several studies comparing HALS and LRC, but no single study provides evidence which procedure is better. Therefore, a meta-analysis is carried out comparing HALS and LRC.
Methods
The review was performed based on the PRISM (Preferred Reporting Items for Systematic Reviews and Meta-analyses) statement [15].
Study search
Electronic databases (Pubmed, Embase, and the Cochrane Library) were searched without limits of date and language. The search terms were as follows: (“hand-assisted laparoscopic” OR “HALS”) AND (“laparoscopic” OR “laparoscopic-assisted” OR “conventional laparoscopy”) AND (“right hemicolectomy” OR “right colectomy”). Additionally, the reference of included studies and related reviews were searched for potentially eligible trials. The search was performed on January 15, 2017.
Inclusion and exclusion criteria
Inclusion criteria are as follows: (1) patients undergoing right colectomy; (2) clinical studies that compared HALS versus LRC on surgical outcomes; (3) be randomized controlled trials (RCTs) or non-RCTs, no language restrictions; (4) studies were required to report on at least one of the outcomes of interest as following: intraoperative outcomes, postoperative outcomes, postoperative morbidity and oncologic outcomes.
Exclusion criteria are as follows: (1) full-test cannot be obtained and (2) no outcome data of interest.
Study selection
Two reviewers independently selected studies according to the inclusion and exclusion criteria, and disagreement was resolved through discussion. The EndNote X7 software was used to remove duplicate records, then the titles and abstracts were screened to identify potential articles, and full texts were retrieved and reviewed to decide whether the studies were eligible.
Data extraction
Two reviewers independently extracted the following data into preformatted table: (1) Study characteristics: first author, year of publication, study design, country, and histologic diagnosis; (2) Baseline data of patients: number of cases, mean age, sex ratio, body mass index (BMI), and American Society of Anesthesiologists (ASA) score; (3) Intraoperative outcomes: operative time, incision length, and conversion rate; (4) Postoperative outcomes: length of hospital stay and reoperation rate; (5) Postoperative morbidity: postoperative overall complications, wound infection, anastomotic leak, and ileus; (6) Oncologic outcomes: number of harvested lymph node, recurrence rate, and death rate.
Quality assessment
The quality of included RCTs was assessed by using the Cochrane Collaboration’s risk of bias tool [16], including the following domains: selection bias, performance bias, detection bias, attrition bias, reporting bias, and other bias. The quality of included non-RCTs was assessed by using the Newcastle–Ottawa Scale (NOS) [17], including three main items: patient selection, comparability, and exposure of HALS and LRC groups. The scale used a star system, which ranged from 0 to 9 stars, and studies were considered as high quality if achieving 7 stars or more. The assessments were performed by two reviewers separately, with inconsistency resolved by discussion.
Statistical analysis
The meta-analysis was performed using RevMan 5.3 software. For categorical variables or continuous variables, odds ratios (ORs) or weighted mean differences (WMDs) with the corresponding 95% confidence interval (CI) were used as the summary statistic respectively, and P < 0.05 was considered statistically significant. If the studies reported continuous variables as medians with ranges, we assumed that the mean is equal to the medians and estimated the standard deviation (SD) as range/4 (samples ≤ 70) or range/6 (samples > 70) [18]. The homogeneity among the included studies was assessed using the I 2 statistic and the x 2 test. The fixed effects model was performed for studies with low heterogeneity (I 2 ≤ 50% and P > 0.05). In the presence of significant heterogeneity (I 2 > 50% or P < 0.05), the random effect model was used to pool the data. The pooled results were expressed by forest plots, and funnel plot was used to estimate for publication bias from a fixed effects model.
Results
Studies selection
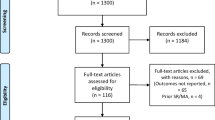
A total of 88 studies were retrieved by the primary search, and five clinical studies were eligible for the meta-analysis eventually [19,20,21,22,23], involving 438 patients of whom 202 in HALS group and 236 in LRC group. Figure 1 shows the detailed process of the studies selection. Among the included trials, three trials [19, 20, 22] compared malignant cases between HALS and LRC, and two trials [21, 23] compared malignant and benign cases, whose sample size ranged from 58 to 127. The study characteristics and baseline data of patients are listed in Table 1.
Quality judgments of studies
The RCT [20] was of high risk of bias, and the four non-RCTs [19, 21,22,23] achieved NOS score of 7 or more, which showed the included studies were high quality. The quality assessment is detailed in Table 2.
Operative time
All five studies involving 438 patients reported available data on the operative time, and low heterogeneity was observed among the trails (I 2 = 4%, P = 0.38). Meta-analysis result showed that operative time was 6.5 min shorter in HALS compared with LRC (WMD = − 6.52; 95% CI − 13.02, − 0.03; P = 0.05; Fig. 2). However, it was not a clinically significant difference.
Incision length
Three studies involving 251 patients reported available data on the incision length, and low heterogeneity was observed among the trails (I 2 = 41%, P = 0.18). Meta-analysis result showed that HALS was associated with longer incision length compared with LRC (WMD = 2.02; 95% CI 1.61, 2.43; P < 0.0001; Fig. 3).
Conversion rate
All five studies involving 438 patients reported available data on the conversion rate, and low heterogeneity was observed among the trails (I 2 = 0%, P = 0.79). Meta-analysis result showed no significant difference between HALS and LRC in conversion rate (OR = 1.26; 95% CI 0.60, 2.67; P = 0.54; Fig. 4).
Length of hospital stay
All five studies involving 438 patients reported available data on the length of hospital stay, and low heterogeneity was observed among the trails (I 2 = 0%, P = 0.43). Meta-analysis result showed no significant difference between HALS and LRC in length of hospital stay (WMD = 0.07; 95% CI − 0.19, 0.33; P = 0.59; Fig. 5).
Reoperation rate
Three studies involving 283 patients reported available data on the reoperation rate, and low heterogeneity was observed among the trails (I 2 = 0%, P = 0.44). Meta-analysis result showed no significant difference between HALS and LRC in reoperation rate (OR = 0.83; 95% CI 0.18, 3.81; P = 0.81; Fig. 6).
Postoperative overall complications
All five studies involving 438 patients reported available data on the postoperative overall complications, and low heterogeneity was observed among the trails (I 2 = 0%, P = 0.79). Meta-analysis result showed no significant difference between HALS and LRC in postoperative overall complications (OR = 1.04; 95% CI 0.64, 1.68; P = 0.89; Fig. 7).
Wound infection
All five studies involving 438 patients reported available data on the wound infection, and low heterogeneity was observed among the trails (I 2 = 0%, P = 0.94). Meta-analysis result showed no significant difference between HALS and LRC in wound infection (OR = 0.89; 95% CI 0.43, 1.86; P = 0.76; Fig. 8).
Anastomotic leak
Four studies involving 343 patients reported available data on the anastomotic leak, and low heterogeneity was observed among the trails (I 2 = 0%, P = 0.45). Meta-analysis result showed no significant difference between HALS and LRC in anastomotic leak (OR = 0.89; 95% CI 0.16, 5.05; P = 0.90; Fig. 8).
Ileus
Three studies involving 320 patients reported available data on the ileus, and low heterogeneity was observed among the trails (I 2 = 0%, P = 0.78). Meta-analysis result showed no significant difference between HALS and LRC in ileus (OR = 1.64; 95% CI 0.75, 3.59; P = 0.21; Fig. 8).
Number of lymph node harvested
All five studies involving 438 patients reported available data on the number of lymph node harvested, and significant heterogeneity was observed among the trails (I 2 = 55%, P = 0.06). Meta-analysis result showed no significant difference between HALS and LRC in number of lymph node harvested (WMD = − 0.13; 95% CI − 3.51, 3.24; P = 0.94; Fig. 9).
Recurrence and death rate
Three studies involving 253 patients reported available data on the recurrence and death rate, and low heterogeneity was observed among the trails (I 2 = 0%, P > 0.88). Meta-analysis result showed no significant difference between HALS and LRC in recurrence rate (OR = 0.90; 95% CI 0.37, 2.19; P = 0.81; Fig. 10) and death rate (OR = 1.01; 95% CI 0.48, 2.16; P = 0.97; Fig. 10).
Assessment of publication bias
Funnel plot analysis was performed on the postoperative overall complications. As showed, there was low risk of publication bias in our meta-analysis (Fig. 11).
Discussion
Jacobs et al. [1] reported the first laparoscopic colectomy in the early 1990s. Comparing with open surgery, laparoscopic colorectal surgery has certain potential advantages [4,5,6,7], as well as similar oncological outcomes [2, 3]. HALS is a hybrid procedure which allows the surgeon to insert the non-dominant hand into the abdomen through a special hand-access device while still maintaining the pneumoperitoneum [24]. Comparing with LRC, HALS decreases the learning curve by restoring tactile feedback, and the intracorporeal hand can be used to blunt dissection, retraction, and rapid control of bleeding. It is controversial as to whether HALS or LRC is preferred at present. There were two previously published systematic reviews on the subject comparing HALS and laparoscopic approach in colorectal surgery [25, 26], rather than comparing HALS and LRC. Therefore, a meta-analysis is carried out.
The meta-analysis that compares HALS and LRC showed that there was no statistical difference between HALS and LRC in terms of conversion rate, length of hospital stay, reoperation rate, postoperative morbidity, and oncologic outcomes. The operative time was 6.5 min shorter in HALS group; however, it was not a clinically significant difference. Although the incision length was longer in HALS, it did not influence the postoperative recovery.
The one previously published systematic review showed the operative time was shorter in HALS comparing with laparoscopic approach [25]. Meijer et al. [27] found that “surgical action efficiency” ratio was 0.55 for HALS and 0.71 for laparoscopic colorectal surgery, which indicated HALS was more efficient. Leblanc et al. [28] performed sigmoid colectomy on an augmented reality simulator and showed HALS reduced operative time by accelerating colonic mobilization and anastomosis. In our meta-analysis, the operative time was 6.5 min shorter in HALS group and was the same as Aalbers et al. reported [25]. Pandya et al. [29] reported the most common reasons for conversion were diverticular inflammation, intraabdominal adhesions, and need for distal rectal resection. Biondi et al. [30] showed conversion occurred mostly because of invasion to adjacent structures, bulky tumor, and adhesions, and converted patients had worse survival than laparoscopic completed patients. It was worth noting that laparoscopic colorectal surgery could convert to HALS or laparotomy [31,32,33], which indicated HALS was more suitable for complicated situation.
The incision length of HALS was longer in this meta-analysis, but it did not influence the postoperative recovery in terms of length of hospital stay, reoperation rate, and postoperative morbidity. Marcello et al. [32] reported a multicenter prospective randomized trial and showed postoperative recovery was similar between HALS and laparoscopic colorectal surgery. Regarding the number of trocars used, HALS is performed using a hand port and two to three trocars. On the other hand, LRC is performed using four to five trocars. The hand port in HALS can restore the tactile sensation and is used as an extraction site. LRC makes an extra small incision to extract the specimen at the end of operation. Both the port site (in HALS) and the extraction site (in LRC) are potential sites for future ventral hernia, and Bae et al. [19] showed there was no statistical difference between HALS and LRC.
Radical surgery of malignant tumor included enough distance of resection margin and number of lymph nodes harvested. There was evidence that positive circumferential margin in rectal cancer was adverse effect of recurrence [34], and Chang et al. [35] reported that survival would increase when a greater lymph node was resected in the patients of II or III colon cancer. Our pooled results showed the oncologic outcomes have no statistical difference between HALS and LRC with respect to number of harvested lymph node, recurrence rate, and death rate.
With regard to cost, Qiu et al. [22] reported the total costs of HALS were less than those of LRC. HALS used conventional staplers rather than expensive laparoscopic staplers, and the trocars were less used in HALS; thus, the total costs could be partly offset in spite of the hand device being expensive. Roslani et al. [36] performed a comprehensive cost analysis and found the total costs were similar between HALS and laparoscopic colorectal surgery.
There were limitations in this meta-analysis. (1) Only one RCT [20] was available to include. Because of the considerable clinical heterogeneity of non-RCTs, the power of pooled results may be declined. (2) The sample size was great difference, and patients came from different countries among the included studies, but the quality of included studies was satisfying. (3) The pooled result of harvested lymph node number existed significant statistical heterogeneity, thus reduced the dependability of evidence.
Conclusions
In conclusion, comparing with LRC, the incision length was longer in HALS, but it did not influence the postoperative recovery. Although the operative time was 6.5 min shorter in HALS, it was not a clinically significant difference. There was no statistical difference in terms of conversion rate, length of hospital stay, reoperation rate, postoperative morbidity, and oncologic outcomes between HALS and LRC. Therefore, HALS can be considered an alternative to LRC which combines the advantages of open as well as laparoscopic surgery. Additional multicenter randomized controlled trials are necessary to fully compare the clinical outcomes between HALS and laparoscopic procedure for right colectomy.
Abbreviations
- ASA:
-
American Society of Anesthesiologists
- BMI:
-
Body mass index
- HALS:
-
Hand-assisted laparoscopic surgery
- LRC:
-
Laparoscopic right colectomy
- M/Fe:
-
Male/female
- NA:
-
Not available
- No:
-
Number
- RCT:
-
Randomized controlled trial
References
Jacobs M, Verdeja JC, Goldstein HS. Minimally invasive colon resection (laparoscopic colectomy). Surg Laparosc Endosc. 1991;1(3):144–50.
Huscher CG, Bretagnol F, Corcione F. Laparoscopic colorectal cancer resection in high-volume surgical centers: long-term outcomes from the LAPCOLON Group Trial. World J Surg. 2015;39:2045–51.
Jayne DG, Thorpe HC, Copeland J, et al. Five-year follow-up of the Medical Research Council CLASICC trial of laparoscopically assisted versus open surgery for colorectal cancer. Br J Surg. 2010;97:1638–45.
Veldkamp R, Kuhry E, Hop WC, et al. Laparoscopic surgery versus open surgery for colon cancer: short-term outcomes of a randomised trial. Lancet Oncol. 2005;6:477–84.
Clinical Outcomes of Surgical Therapy Study Group. A comparison of laparoscopically assisted and open colectomy for colon cancer. N Engl J Med. 2004;350:2050–9.
Bonjer HJ, Deijen CL, Abis GA, et al. A randomized trial of laparoscopic versus open surgery for rectal cancer. N Engl J Med. 2015;372:1324–32.
Biondi A, Grosso G, Mistretta A, et al. Laparoscopic-assisted versus open surgery for colorectal cancer: short- and long-term outcomes comparison. J Laparoendosc Adv Surg Tech A. 2013;23(1):1–7.
Robinson CN, Chen GJ, Balentine CJ, et al. Minimally invasive surgery is underutilized for colon cancer. Ann Surg Oncol. 2011;18:1412–8.
Steele SR, Brown TA, Rush RM, et al. Laparoscopic vs open colectomy for colon cancer: results from a large nationwide population-based analysis. J Gastrointest Surg. 2008;12:583–91.
Reichenbach DJ, Tackett AD, Harris J, et al. Laparoscopic colon resection early in the learning curve: what is the appropriate setting? Ann Surg. 2006;243:730–5.
Miskovic D, Ni M, Wyles SM, et al. Learning curve and case selection in laparoscopic colorectal surgery: systematic review and international multicenter analysis of 4852 cases. Dis Colon Rectum. 2012;55:1300–10.
Biondi A, Grosso G, Mistretta A, et al. Laparoscopic vs. open approach for colorectal cancer: evolution over time of minimal invasive surgery. BMC Surg. 2013;13(S2):S12.
Boland JP, Kusminsky RE, They EH. Laparoscopic mini-laparotomy with manipulation: the middle path. Minim Invasive Ther. 1993;2:63–7.
Targarona EM, Gracia E, Rodriguez M, et al. Hand-assisted laparoscopic surgery. Arch Surg. 2003;138(2):133–41.
Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535.
Higgins JP, Altman DG, Gotzsche PC, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928.
Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25(9):603–5.
Hozo SP, Benjamin B, Hozo I. Estimating the mean and variance from the median range, and the size of a sample. BMC Med Res Methodol. 2005;5:13.
Bae SU, Park JS, Choi YJ, et al. The role of hand-assisted laparoscopic surgery in a right hemicolectomy for right-sided colon cancer. Ann Coloproctol. 2014;30(1):11–7.
Ng LW, Tung LM, Cheung HY, et al. Hand-assisted laparoscopic versus total laparoscopic right colectomy: a randomized controlled trial. Color Dis. 2012;14(9):e612–7.
Papaconstantinou HT, Sharp N, Thomas JS. Single-incision laparoscopic right colectomy: a case-matched comparison with standard laparoscopic and hand-assisted laparoscopic techniques. J Am Coll Surg. 2011;213(1):72–80.
Qiu HZ, Xu L, Niu BZ, et al. Hand-assisted laparoscopic versus laparoscopic-assisted right hemicolectomy: a clinical controlled study. Chinese J Gastrointest Surg. 2011;14(7):545–8.
Vogel JD, Lian L, Kalady MF, et al. Hand-assisted laparoscopic right colectomy: how does it compare to conventional laparoscopy? J Am Coll Surg. 2011;212(3):367–72.
Bemelman WA, Ringers J, Meijer DW, et al. Laparoscopic-assisted colectomy with the Dexterity™ Pneumo Sleeve. Dis Colon Rectum. 1996;39:S59–61.
Aalbers AG, Biere SS, van Berge Henegouwen MI, et al. Hand-assisted or laparoscopic-assisted approach in colorectal surgery: a systematic review and meta-analysis. Surg Endosc. 2008;22:1769–80.
Moloo H, Haggar F, Coyle D, et al. Hand assisted laparoscopic surgery versus conventional laparoscopy for colorectal surgery. Cochrane Database Syst Rev. 2010;10:CD006585.
Meijer DW, Bannenberg JJ, Jakimowicz JJ. Hand-assisted laparoscopic surgery: an overview. Surg Endosc. 2000;14:891–5.
Leblanc F, Delaney CP, Ellis CN, et al. Hand-assisted versus straight laparoscopic sigmoid colectomy on a training simulator: what is the difference? World J Surg. 2010;34:2909–14.
Pandya S, Murray JJ, Coller JA, et al. Laparoscopic colectomy: indications for conversion to laparotomy. Arch Surg. 1999;134:471–5.
Biondi A, Grosso G, Mistretta A, et al. Predictors of conversion in laparoscopic-assisted colectomy for colorectal cancer and clinical outcomes. Surg Laparosc Endosc Percutan Tech. 2014;24(1):e21–6.
Myers EA, Feingold DL, Arnell TD, et al. The rate for the use of hand-assisted laparoscopic methods is directly proportional to body mass index. Surg Endosc. 2014;28:108–15.
Marcello PW, Fleshman JW, Milsom JW, et al. Hand-assisted laparoscopic vs. laparoscopic colorectal surgery: a multicenter, prospective, randomized trial. Dis Colon Rectum. 2008;51:818–28.
Targarona EM, Gracia E, Garriga J, et al. Prospective randomized trial comparing conventional laparoscopic colectomy with hand-assisted laparoscopic colectomy. Surg Endosc. 2002;16:234–9.
Wiggers T, van de Velde CJ. The circumferential margin in rectal cancer: recommendations based on the Dutch Total Mesorectal Excision Study. Eur J Cancer. 2002;38:973–6.
Chang GJ, Rodriguez-Bigas MA, Skibber JM, et al. Lymph node evaluation and survival after curative resection of colon cancer: systematic review. J Natl Cancer Inst. 2007;99:433–41.
Roslani AC, Koh DC, Tsang CB, et al. Hand-assisted laparoscopic colectomy versus standard laparoscopic colectomy: a cost analysis. Color Dis. 2009;11:496–501.
Acknowledgements
Not applicable.
Funding
This work was supported by Social Development Program from Shenyang Science and Technology Bureau, China (No. F14-158-9-35). The sponsoring organization was not involved in the study design, data analysis, or interpretation.
Availability of data and materials
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
Author information
Authors and Affiliations
Contributions
GSW and JPZ designed the study. GSW wrote the manuscript. GSW and WWS performed the literature review, extraction of the data, and analysis of the pooled data. MD and JPZ reviewed and revised the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Wang, G., Zhou, J., Sheng, W. et al. Hand-assisted laparoscopic surgery versus laparoscopic right colectomy: a meta-analysis. World J Surg Onc 15, 215 (2017). https://doi.org/10.1186/s12957-017-1277-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12957-017-1277-2