Abstract
Purpose
Hand-assisted laparoscopic surgery (HALS) is an alternative to straight laparoscopy (LAP) in colorectal surgery. Many studies have compared the two in terms of efficacy, complications, and outcomes. This meta-analysis aims to uncover if there are any significant differences in conversion rates, operative times, body mass index (BMI), incision lengths, intraoperative and postoperative complications, and length of stay.
Methods
Comprehensive searches were performed on databases from their respective inceptions to 16 December 2021, with a manual search performed through Scopus. Randomized controlled trials (RCTs), cohort studies, and case series involving more than 10 patients were included.
Results
A total of 47 studies were found fitting the inclusion criteria, with 5 RCTs, 41 cohort studies, and 1 case series. Hand-assisted laparoscopic surgery was associated with lower conversion rates (odds ratio [OR] 0.41, 95%CI 0.28–0.60, p < 0.00001), shorter operative times (Mean Difference [MD] − 8.32 min, 95%CI − 14.05– − 2.59, p = 0.004), and higher BMI (MD 0.79, 95%CI 0.46–1.13, p < 0.00001), but it was also associated with longer incision lengths (MD 2.19 cm, 95%CI 1.66–2.73 cm, p < 0.00001), and higher postoperative complication rates (OR 1.15, 95%CI 1.06–1.24, p = 0.0004). Length of stay was not different in HALS as compared to Lap (MD 0.16 days, 95%CI − 0.06–0.38 days, p = 0.16, and intraoperative complications were the same between both techniques.
Conclusions
Hand-assisted laparoscopy is a suitable alternative to straight laparoscopy with benefits and risks. While there are many cohort studies comparing HALS and LAP, more RCTs would be needed for a better quality of evidence.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
The history of laparoscopic surgery spans back to the early 1900s, but only rapidly developed towards the end of the century [1]. As it matured, it offered an alternative to the traditional open surgeries, and provided benefits such as reduced intraoperative bleeding, postoperative pain, ileus, and hospital stay, at the expense of increased operative times [2].
Hand-assisted laparoscopic surgery (HALS), a variant of straight laparoscopic surgery, gained popularity in the mid-1990s with the introduction of hand-assisted devices [3]. It was seen as an alternative to straight laparoscopy (LAP) but with an easier learning curve, thus allowing an easier transition from open techniques to these minimally invasive methods [4].
Comparisons between HALS and LAP have been made in other surgical specialties [5], with the most recent reviews in the field of colorectal surgery published in 2008 by Aalbers [4] and a Cochrane Review by Moloo et al. [6] in 2010, which included 3 randomized controlled trials (RCTs). In the intervening years since, there have been many developments in operative techniques for both surgeries, in colorectal disease management, and consequently many new comparative studies have been performed.
This review aims to incorporate those studies in Aalbers’ and Moloo’s reports with the new advances published in the last decade. With the newly available data, it is important to provide clinicians with an updated, evidenced-based resource on which they can base their operative decisions.
Methods
Search strategy
An encompassing search with broad terms was performed on 16 December 2021 on the PreMEDLINE, MEDLINE, and Embase databases for articles related to HALS and LAP.
The following search protocol for MEDLINE is as follows:
-
1.
hand assisted laparoscopic surgery.mp. or exp Hand-Assisted Laparoscopy/
-
2.
hand port.mp.
-
3.
1 or 2
-
4.
Laparoscopic Surgery.mp. or exp Laparoscopy/
-
5.
3 and 4
Keywords were changed if necessary, for the equivalents in the other databases. Reference searches were also performed using Scopus, and all articles were collated and reviewed.
Study selection/inclusions and exclusions
Search results were screened independently by A.F. and Y.H. by their titles and abstracts according to a pre-determined set of inclusion and exclusion criteria, and disagreements were resolved by C.Y. In the interest of gathering as wide a dataset as possible, cohort studies and case series that had more than 10 patients and with quantifiable data were also included.
Inclusion criteria
-
1.
Studies that had a clearly documented data for population of HALS and LAP.
-
2.
Studies with quantified outcomes of any one or more of the following:
-
(a)
conversion rate,
-
(b)
operative time,
-
(c)
body mass index (BMI),
-
(d)
length of incision,
-
(e)
complication rate, and,
-
(f)
length of stay.
-
(a)
Exclusion criteria
-
1.
Studies that involved other specialties of surgery (e.g., urology or upper gastrointestinal surgery),
-
2.
In vitro models,
-
3.
Non-quantitative studies such as opinion pieces or letters or the editor,
-
4.
Conference abstracts, and,
-
5.
Non-English texts
Data extraction/data abstraction and analysis
Data from the included studies were extracted and compiled into tables comparing conversion rate, operative time, BMI, incision length, complication rate, and length of stay.
Conversion rate was defined as any deviation from the planned surgery, i.e., the data for LAP conversions includes cases that were converted into both HALS and open surgery.
Definitions of intraoperative and postoperative complications were flexible and varied between studies, hence all complication rates as reported and defined by the individual authors of each study were included. Only papers that clearly showed they were reporting their complication rates as any single complication happening per surgery were included. Papers that calculated their complication rates as a total number of complications among the number patients were excluded from this outcome measure analysis.
Data for continuous variables were collected as mean ± standard deviation (SD); however, in papers that reported a median and range, the formulae described in Hozo et al. [7] was used to estimate a mean ± SD for meta-analysis. For studies that reported their data as a median with an interquartile range, the formulae by Wan et al. [8] were used to estimate a mean ± SD.
Assessments of methodological quality
The Cochrane Collaboration tool for assessing risk of bias [9] was used to assess the methodological quality of RCTS. Cohort studies were evaluated using the Newcastle–Ottawa Scale (NOS) [10]. The scale consists of 8 questions and has a maximum possible score of 9 stars. All articles were evaluated independently by A.F. and Y.H., and disagreements were put to C.Y. for mediation.
Statistical analysis
All meta-analyses were performed using RevMan (version 5.3) [11], with a random effects analysis model used in all cases.
For continuous variables such as operative time, BMI, incision length, and length of stay, the mean difference (MD) was calculated using an inverse-variance (IV) statistical method and reported with 95% confidence intervals (95%CI). For dichotomous variables such as conversion rates and complication rates, an odds ratio (OR) was calculated using the Mantel–Haenszel statistical method and reported with 95%CI. A p-value of < 0.05 was predetermined to be the limit for statistical significance. An I2 value of greater than 60% was regarded as being heterogenous. Funnel plots were generated using RevMan to assess for publication bias.
Several papers reported their data as subgroups depending on disease or type of surgery (e.g., left hemicolectomies, right hemicolectomies). In these situations, an average mean and average standard deviation were calculated using the appropriate formulae.
RCTs were analyzed separately from the cohort studies/case studies. The MD or OR for each subgroup in each measure was reported individually, along with the overall value for the outcome.
Results
Selection results and characteristics of the studies
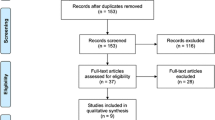
A total of 1352 records were obtained from the search after duplicates were removed. A further 1236 were removed after screening based on their title and abstract. The remaining 116 full-text articles were assessed for eligibility and 47 studies were included in this study [12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58]. A PRISMA flowchart was completed to illustrate this process (Fig. 1).
There were 5 RCTs [20, 27, 35, 48, 57] and 41 retrospective cohort studies[12,13,14,15,16,17,18,19, 21,22,23,24,25,26, 28,29,30,31,32,33,34, 36,37,38,39,40,41,42,43,44,45,46,47, 49,50,51,52, 54,55,56, 58], of which 8 were case-matched studies[17,18,19, 36, 38,39,40, 42, 58]. There was one case series [53] included in this review, as it had greater than 10 patients and data for each individual patient was recorded.
Methodological quality
The 5 RCTs showed a low to moderate risk of bias. This is largely due to it being impossible to conduct a double-blind trial in this context (Table 1). In general, the 5 RCTs had low risk of bias in their randomization processes and in their reporting of the outcomes. The 42 cohort studies scored an average of 5.40 stars out of 9 on the NOS (Table 2).
Publication bias
Funnel plots were generated with pseudo 95% confidence intervals [59, 60] for each outcome measured and are included in the appendix. In general, most measures showed an approximately symmetrical spread around the calculated mean difference or odds ratio, except for operative times which had more studies reporting a larger negative difference with a wider standard error (Supplementary figures, Fig. S1).
The effects of interventions
Conversion rate
HALS was overall associated with a lower conversion rate than LAP, with an OR of 0.41 (95%CI 0.28–0.60, p < 0.0001) (Fig. 2). Six studies reported nil conversions in either arm [34, 40, 41, 49, 50, 56]. For the purpose of full reporting, they were included in the analysis despite not impacting the results. There were no common factors identifiable between the studies with no conversions, with varying patient population sizes and conditions ranging from diverticular disease to carcinoma. A subgroup analysis of only the RCTs showed the difference was not significant amongst the 5 RCTs (OR 0.41, 95%CI 0.13–1.26, p = 0.12).
Operative time
Operative times were found to be overall slightly shorter In HALS than in LAP (MD -8.32 min, 95%CI − 14.05– − 2.59, p = 0.004) (Fig. 3). A subgroup analysis of the RCTs showed there was no significant difference between HALS and LAP. The 5 studies that showed the greatest difference between HALS and LAP were cohort studies, two of which focused exclusively on restorative proctocolectomy [41, 50], one on total abdominal colectomy [17], one on both total and subtotal abdominal colectomies [34], and one on low anterior resections [53].
BMI
BMI was analyzed as a pre-operative factor to see if it affected surgeon preference in choosing one operation over the other. HALS was overall associated with a higher BMI than LAP (MD 0.79, 95%CI 0.46–1.13, p < 0.00001) (Fig. 4). The study with the most significant MD of 3.40 [2.03, 4.77] was a retrospective cohort study focusing purely on diverticulitis [30], and it was noted that HALS was more often used in complex cases. The RCTs were not included in this pre-operative factor measure as the groups are randomized pre-operatively.
Incision length
Incision lengths were longer in HALS as compared to LAP (MD 2.19 cm, 95%CI 1.66–2.73 cm, p < 0.00001) (Fig. S2). All studies that had reported incision lengths were unanimous in reporting a longer mean incision length in HALS as compared to LAP; however, a subgroup analysis of the RCTs showed no significant difference (MD 1.04 cm, 95%CI − 0.52–2.61, p = 0.19). It should be noted that only 2 of the 5 RCTs reported incision length.
Complication rates
There was no significant difference in intraoperative complication rates (OR 1.13, 95%CI 0.59–2.20, p = 0.71) between HALS and LAP (Fig. S3). HALS was, however, associated with a higher postoperative complication rate than LAP (OR 1.15, 95%CI 1.06–1.24, p = 0.0004) (Fig. S4). Similarly, as with the other outcome measures, the RCTs did not show a significant difference between HALS or LAP in terms of postoperative complication rates.
Length of stay
Hospital stay was not significantly different between HALS and LAP (MD 0.16 days, 95%CI -0.06–0.38 days, p = 0.16) (Fig. S5). None of the included studies found any significant difference between HALS and LAP in length of stay.
Differences between malignant and non-malignant diseases
A separate subgroup analysis was performed by grouping studies on whether they exclusively included patients with malignant disease or non-malignant diseases (which includes diverticulitis, inflammatory diseases, and slow bowel transit). There was a statistically significant reduction in conversion rates in malignant disease but not so in non-malignant disease, although the effect sizes were similar (OR 0.37, p = 0.03 VS OR 0.30, p = 0.07 respectively). Interestingly, while there was a statistically significant reduction in the operative times for both malignant and non-malignant diseases, non-malignant cases appeared to have a significantly larger effect size (MD − 15.05 min vs MD -55.63 min respectively). There were no remarkable differences between incision length, length of stay, complication rates, and BMI when comparing malignant disease to non-malignant diseases (Fig. S6-S12).
Discussion
This review which incorporates 5 RCTs, 41 retrospective cohort studies, and 1 case series, shows that HALS has some advantages and disadvantages compared to LAP. The benefits of a lowered conversion rate and shortened operative times comes at the cost of an increased incision length and postoperative complication rates.
Operative times were found to be reduced in HALS, which is in line with the current literature that reports that HALS generally provides a reduced learning curve [38]. The tactile feedback helps the surgeon in spatial orientation and allows for a more efficient surgery. This is in line with the finding of a decreased conversion rate in HALS, as it allows for increased maneuverability in the abdominal cavity. One might expect a lower conversion rate to be associated with lowered intraoperative complication rates, but no significant difference between HALS and LAP was found for this outcome measure in our study.
Multiple studies have looked at HALS and LAP in the setting of obese patients, finding that HALS was associated with lower conversion rates in patients with high BMI [22]. The results of our study suggests that BMI may have been a factor in determining if a patient was planned for a HALS or a LAP in the included retrospective studies.
Incision lengths were found to be longer in HALS than in LAP, which is an expected result, as a longer incision is necessary to accommodate the hand port for the surgeon. The mean increase in incision length of 2.19 cm may also not be a clinically important result. Interestingly, the 2 RCTS [20, 27] that reported this outcome measure did not show significant difference in incision lengths between HALS and LAP when a subgroup analysis was performed.
HALS was however associated with an increased postoperative complication rate as compared to LAP surgeries. Similar to the previous point on BMI, this may perhaps be secondary to surgeon preference for HALS in more complicated surgical cases, hence predisposing HALS to more postoperative complications. Unfortunately, as most of the studies included in review are retrospective and non-randomized, this is a factor that cannot be accounted for. Nonetheless, this increased postoperative complication rate is not associated with a longer length of hospital stay, as the difference between HALS and LAP of 0.16 days (95%CI 0.06–0.38) was not statistically significant.
One consistent trend across the various outcome measures is that the RCTs did not show any significant difference between HALS and LAP when a subgroup analysis was performed. This could perhaps indicate that the perceived benefits and drawbacks are arising from inherent biases in the cohort studies (and the one case study). However, it should be noted that there are only 5 RCTs that fit our criteria, with 90% of our studies being non-randomized trials.
A strength of this study is its comprehensiveness — including any study that involves colorectal surgery, HALS, LAP, and fitting the rigorous selection criteria. The objective of this review is to study HALS and LAP as used in colorectal surgery, and not have it limited to particular techniques or conditions. However, this may have had an effect on diluting the results. It is possible that the above results between HALS and laparoscopy might differ depending on the procedure being performed. Similarly, the pathology in question might also influence the outcomes analyzed in this paper. More detailed reviews of the subgroups of malignant disease, inflammatory conditions, infective conditions, or emergency versus elective procedures, might yield some interesting results, but these are unfortunately beyond the scope of this paper.
On a similar note, a source of bias in this review is that most of the included studies are non-randomized. Several studies have noted within their cohorts that HALS was used more frequently in complex cases [13, 24], with Miyagaki et al. [30] finding in their study that HALS was used in more complex diverticular disease cases and was consequently associated with a higher postoperative complication rate and a longer length of stay.
Publication bias, on the other hand, is likely to be low in this review. Funnel plots for each outcome measure can be found in the supplementary figures (Fig. S1). Conversion rates, BMI, incision lengths, intraoperative and postoperative complication rates, and length of stay showed approximately symmetrical graphs, implying a lower risk of publication bias. In the study of operative times, however, the graph is slightly skewed towards having more publications that favor a shorter operating time for HALS, as well as more studies with a lower standard error in their reported mean difference in operative times.
Ultimately, there is a wealth of evidence included in this study, but more research will be required if a higher quality of evidence is desired. Perhaps one direction future studies could take would be to look at HALS versus LAP in specific patient subpopulations and for specific procedures, which can provide more a more granular guide for clinicians in decision-making. RCTs would ideally be the study of choice to reduce bias from preoperative factors influencing surgeon decisions for which procedure to perform.
Conclusion
HALS is a viable alternative to LAP for colorectal surgery. The decision for choosing one or the other depends on the weighing up the benefits of lower conversion rates and shorter operative times, with the risks of longer incision lengths and higher postoperative complication rates. There are possible sources of bias in the studies included in this review, and in the review itself, which may understate some results, and more RCTs need to be performed for a higher level of evidence.
References
Vecchio R, MacFayden BV, Palazzo F (2000) History of laparoscopic surgery. Panminerva Med 42(1):87–90
Schwenk W, Haase O, Neudecker J, Muller JM (2005) Short term benefits for laparoscopic colorectal resection. Cochrane Database Syst Rev (3):CD003145
Litwin DE, Darzi A, Jakimowicz J, Kelly JJ, Arvidsson D, Hansen P et al (2000) Hand-assisted laparoscopic surgery (HALS) with the HandPort system: initial experience with 68 patients. Ann Surg 231(5):715–723
Aalbers AG, Biere SS, van Berge Henegouwen MI, Bemelman WA (2008) Hand-assisted or laparoscopic-assisted approach in colorectal surgery: a systematic review and meta-analysis. Surg Endosc 22(8):1769–1780
Qian D, He Z, Hua J, Gong J, Lin S, Song Z (2014) Hand-assisted versus conventional laparoscopic splenectomy: a systematic review and meta-analysis. ANZ J Surg 84(12):915–920
Moloo H, Haggar F, Coyle D, Hutton B, Duhaime S, Mamazza J et al (2010) Hand assisted laparoscopic surgery versus conventional laparoscopy for colorectal surgery. Cochrane database of systematic reviews (Online) (10):CD006585
Hozo SP, Djulbegovic B, Hozo I (2005) Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol 5:13
Wan X, Wang W, Liu J, Tong T (2014) Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol 14:135
Higgins JP, Altman DG, Gotzsche PC, Juni P, Moher D, Oxman AD et al (2011) The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 343:d5928
Wells G, Shea B, O’Connell D, Peterson J, Welch V, Losos M et al (2021) The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Available from: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. Accessed 1 Sept 2022
Review Manager (RevMan) (2014) Version 5.3 ed. Copenhagen: The Nordic Cochrane Centre: The Cochrane Collaboration
Anderson J, Luchtefeld M, Dujovny N, Hoedema R, Kim D, Butcher J (2007) A comparison of laparoscopic, hand-assist and open sigmoid resection in the treatment of diverticular disease. Am J Surg 193(3):400–403
Bae SU, Choi YJ, Lee MK, Cho BS, Kang YJ, Park JS et al (2014) The role of hand-assisted laparoscopic surgery in a right hemicolectomy for right-sided colon cancer. Ann 30(1):11–17
Benlice C, Costedio M, Kessler H, Remzi FH, Gorgun E (2016) Comparison of straight vs hand-assisted laparoscopic colectomy: an assessment from the NSQIP procedure-targeted cohort. Am J Surg 212(3):406–412
Boushey RP, Marcello PW, Martel G, Rusin LC, Roberts PL, Schoetz DJ Jr (2007) Laparoscopic total colectomy: an evolutionary experience. Dis Colon Rectum 50(10):1512–1519
Chang YJ, Marcello PW, Rusin LC, Roberts PL, Schoetz DJ (2005) Hand-assisted laparoscopic sigmoid colectomy: helping hand or hindrance? Surg Endosc 19(5):656–661
Fichera A, Zoccali M, Felice C, Rubin DT (2011) Total Abdominal colectomy for refractory ulcerative colitis. surgical treatment in evolution. J Gastrointest Surg 15(11):1909–16
Gezen FC, Aytac E, Costedio MM, Vogel JD, Gorgun E (2015) Hand-Assisted versus straight-laparoscopic versus open proctosigmoidectomy for treatment of sigmoid and rectal cancer: a case-matched study of 100 patients. The Permanente journal 19(2):10–14
Gilmore BF, Sun Z, Adam M, Kim J, Ezekian B, Ong C et al (2016) Hand-Assisted laparoscopic versus standard laparoscopic colectomy: are outcomes and operative time different? J Gastrointest Surg 20(11):1854–1860
Darzi A (2000) Hand-assisted laparoscopic colorectal surgery. Surg Endosc 14(11):999–1004
Hassan I, You YN, Cima RR, Larson DW, Dozois EJ, Barnes SA et al (2008) Hand-assisted versus laparoscopic-assisted colorectal surgery: practice patterns and clinical outcomes in a minimally-invasive colorectal practice. Surg Endosc Other Interv Tech 22(3):739–743
Heneghan HM, Martin ST, Kiran RP, Khoury W, Stocchi L, Remzi FH et al (2013) Laparoscopic colorectal surgery for obese patients: decreased conversions with the hand-assisted technique. J Gastrointest Surg 17(3):548–554
Holubar SD, Privitera A, Cima RR, Dozois EJ, Pemberton JH, Larson DW (2009) Minimally invasive total proctocolectomy with brooke ileostomy for ulcerative colitis. Inflamm Bowel Dis 15(9):1337–1342
Jadlowiec CC, Mannion EM, Thielman MJ, Bartus CM, Johnson KH, Sardella WV et al (2014) Evolution of technique in performance of minimally invasive colectomies. Dis Colon Rectum 57(9):1090–1097
Larson DW, Boostrom SY, Cima RR, Pemberton JH, Larson DR, Dozois EJ (2010) Laparoscopic surgery for rectal cancer: short-term benefits and oncologic outcomes using more than one technique. Tech Coloproctol 14(2):125–131
Lee SW, Yoo J, Dujovny N, Sonoda T, Milsom JW (2006) Laparoscopic vs. hand-assisted laparoscopic sigmoidectomy for diverticulitis. Dis Col Rect 49(4):464–9
Marcello PW, Fleshman JW, Milsom JW, Read TE, Arnell TD, Birnbaum EH et al (2008) Hand-assisted laparoscopic vs. laparoscopic colorectal surgery: a multicenter, prospective, randomized trial. Dis Col Rect 51(6):818–26
Midura EF, Hanseman DJ, Davis BR, Johnson BL, Kuethe JW, Rafferty JF et al (2016) Laparoscopic sigmoid colectomy: are all laparoscopic techniques created equal? Surg Endosc Other Interv Tech 30(8):3567–3572
Milsom JW, De Oliveira JO, Trencheva KI, Pandey S, Lee SW, Sonoda T (2009) Long-term outcomes of patients undergoing curative laparoscopic surgery for mid and low rectal cancer. Dis Colon Rectum 52(7):1215–1222
Miyagaki H, Rhee R, Shantha Kumara HMC, Yan X, Njoh L, Cekic V et al (2016) Surgical treatment of diverticulitis: hand-assisted laparoscopic resection is predominantly used for complex cases and is associated with increased postoperative complications and prolonged hospitalization. Surg Innov 23(3):277–283
Moghadamyeghaneh Z, Carmichael JC, Mills S, Pigazzi A, Nguyen NT, Stamos MJ (2015) Hand-assisted laparoscopic approach in colon surgery. Journal of gastrointestinal surgery : official journal of the Society for Surgery of the Alimentary Tract 19(11):2045–2053
Myers EA, Feingold DL, Arnell TD, Njoh L, Cekic V, Jang JH et al (2014) The rate for the use of hand-assisted laparoscopic methods is directly proportional to body mass index. Surg Endosc Other Interv Tech 28(1):108–115
Nakajima K, Lee SW, Cocilovo C, Foglia C, Sonoda T, Milsom JW (2004) Laparoscopic total colectomy: Hand-assisted vs standard technique. Surg Endosc Other Interv Tech 18(4):582–586
Nakajima K, Nezu R, Hirota M, Nishida T (2010) The role of hand-assisted laparoscopic surgery in subtotal and total colectomy for Crohn’s colitis. Surg Endosc Other Interv Tech 24(11):2713–2717
Ng LWC, Tung LM, Cheung HYS, Wong JCH, Chung CC, Li MKW (2012) Hand-assisted laparoscopic versus total laparoscopic right colectomy: a randomized controlled trial. Colorectal Dis 14(9):e612–e617
Orcutt ST, Marshall CL, Balentine CJ, Robinson CN, Anaya DA, Artinyan A et al (2012) Hand-assisted laparoscopy leads to efficient colorectal cancer surgery. J Surg Res 177(2):E53–E58
Overbey DM, Cowan ML, Hosokawa PW, Chapman BC, Vogel JD (2017) Laparoscopic colectomy in obese patients: a comparison of laparoscopic and hand-assisted laparoscopic techniques. Surg Endoscop Other Intervent Tech 1–10
Ozturk E, Kiran RP, Remzi F, Geisler D, Fazio V (2010) Hand-assisted laparoscopic surgery may be a useful tool for surgeons early in the learning curve performing total abdominal colectomy. Colorectal Dis 12(3):199–205
Papaconstantinou HT, Sharp N, Thomas JS (2011) Single-incision laparoscopic right colectomy: a case-matched comparison with standard laparoscopic and hand-assisted laparoscopic techniques. J Am Coll Surg 213(1):72–80 discussion -2
Patel CB, Ragupathi M, Ramos-Valadez DI, Haas EM (2011) A three-arm (laparoscopic, hand-assisted, and robotic) matched-case analysis of intraoperative and postoperative outcomes in minimally invasive colorectal surgery. Dis Colon Rectum 54(2):144–150
Polle SW, van Berge Henegouwen MI, Slors JF, Cuesta MA, Gouma DJ, Bemelman WA (2008) Total laparoscopic restorative proctocolectomy: are there advantages compared with the open and hand-assisted approaches? Dis Colon Rectum 51(5):541–548
Pyo DH, Huh JW, Park YA, Cho YB, Yun SH, Kim HC et al (2016) A comparison of hand-assisted laparoscopic surgery and conventional laparoscopic surgery in rectal cancer: a propensity score analysis. Surg Endosc Other Interv Tech 30(6):2449–2456
Ringley C, Lee YK, Iqbal A, Bocharev V, Sasson A, McBride CL et al (2007) Comparison of conventional laparoscopic and hand-assisted oncologic segmental colonic resection. Surg Endosc 21(12):2137–2141
Rivadeneira DE, Marcello PW, Roberts PL, Rusin LC, Murray JJ, Coller JA et al (2004) Benefits of hand-assisted laparoscopic restorative proctocolectomy: a comparative study. Dis Colon Rectum 47(8):1371–1376
Roslani AC, Koh DC, Tsang CB, Wong KS, Cheong WK, Wong HB (2009) Hand-assisted laparoscopic colectomy versus standard laparoscopic colectomy: a cost analysis. Colorectal Dis 11(5):496–501
Shussman N, Brown MR, Johnson MC, Da Silva G, Wexner SD, Weiss EG (2013) Does nasogastric tube decompression get used less often with laparoscopic and hand-assisted compared with open colectomy? Surg Endosc Other Interv Tech 27(12):4564–4568
Sonoda T, Pandey S, Trencheva K, Lee S, Milsom J (2009) Longterm complications of hand-assisted versus laparoscopic colectomy. J Am Coll Surg 208(1):62–66
Targarona EM, Gracia E, Garriga J, Martinez-Bru C, Cortes M, Boluda R et al (2002) Prospective randomized trial comparing conventional laparoscopic colectomy with hand-assisted laparoscopic colectomy: applicability, immediate clinical outcome, inflammatory response, and cost. Surg Endosc Other Interv Tech 16(2):234–239
Tjandra JJ, Chan MKY, Yeh CH (2008) Laparoscopic- vs. hand-assisted ultralow anterior resection: a prospective study. Dis Colon Rect 51(1):26–31
Tsuruta M, Hasegawa H, Ishii Y, Endo T, Ochiai H, Hibi T et al (2009) Hand-assisted versus conventional laparoscopic restorative proctocolectomy for ulcerative colitis. Surg Laparos Endos Percut Tech 19(1):52–56
Vaccaro CA, Rossi GL, Quintana GO, Soriano ER, Vaccarezza H, Rubinstein F (2014) Laparoscopic colorectal resections: a simple predictor model and a stratification risk for conversion to open surgery. Dis Colon Rectum 57(7):869–874
Vogel JD, Lian L, Kalady MF, De Campos-Lobato LF, Alves-Ferreira PC, Remzi FH (2011) Hand-assisted laparoscopic right colectomy: how does it compare to conventional laparoscopy? Dis Colon Rectum 54(11):e277–e278
Yano H, Ohnishi T, Kanoh T, Monden T (2005) Hand-assisted laparoscopic low anterior resection for rectal carcinoma. J Laparoendosc Adv Surg Tech A 15(6):611–614
Yun HR, Cho YK, Cho YB, Kim HC, Yun SH, Lee WY et al (2010) Comparison and short-term outcomes between hand-assisted laparoscopic surgery and conventional laparoscopic surgery for anterior resections of left-sided colon cancer. Int J Colorectal Dis 25(8):975–981
Zhang GT, Zhang XD (2017) The Feasibility of hand-assisted laparoscopic and laparoscopic multivisceral resection compared with open surgery for locally advanced colorectal cancer. surgical laparoscopy, endoscopy and percutaneous techniques. 27(4):e57-e65
Chough I, Zaghiyan K, Ovsepyan G, Fleshner P (2019) It Is not just cosmesis: straight laparoscopy with stoma site extraction improves outcomes in ulcerative colitis patients undergoing total colectomy. Am Surg 85(10):1194–1197
Gulcu B, Isik O, Ozturk E, Yilmazlar T (2018) Hand-assisted laparoscopy: expensive but considerable step between laparoscopic and open colectomy. Surg Laparosc Endosc Percutan Tech 28(4):214–218
Wei M, Zhang X, Ma P, He W, Bi L, Wang Z (2018) Outcomes of open, laparoscopic, and hand-assisted laparoscopic surgeries in elderly patients with right colon cancers: A case-control study. Medicine (Baltimore) 97(35):e11907
Langan D, Higgins JP, Gregory W, Sutton AJ (2012) Graphical augmentations to the funnel plot assess the impact of additional evidence on a meta-analysis. J Clin Epidemiol 65(5):511–519
Sterne JAC, Harbord RM (2004) Funnel plots in meta-analysis. Stand Genomic Sci 4(2):127–141
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Poster presented at The American Society of Colon and Rectal Surgeons, 2019 ASCRS Annual Scientific Meeting. Cleveland, Ohio, USA. June 1–5, 2019 (poster presented on 3 June).
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Frois, A.O., Huang, Y. & Young, C.J. Hand-assisted versus straight laparoscopy for colorectal surgery — a systematic review and meta-analysis. Int J Colorectal Dis 37, 2309–2319 (2022). https://doi.org/10.1007/s00384-022-04272-x
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00384-022-04272-x