Abstract
Background
Diabetic nephropathy (DN) contributes to end-stage renal failure. Microvascular injury resulted from reactive oxygen species is implicated in the pathogenesis of DN. Genetic polymorphism of Apolipoprotein E (APOE) influences the antioxidative properties of the protein. The relationship of APOE polymorphism with the risks of nephropathy in type 2 diabetes (T2DN) remains elusive.
Methods
An up-to-date meta-analysis was conducted on the basis of studies selected from PubMed, WanFang database, Embase, Vip database, Web of Science, Scopus, and CNKI database.
Results
A total of 33 studies conferring 3266 cases and 3259 controls were selected on the basis of criteria of inclusion and exclusion in this meta-analysis. For APOE alleles, the pooled odds ratio (OR) of ε2 vs. ε3 was 1.89 (95% confidence intervals [95% CI]: 1.49–2.38, P < 0.0001). With regard to APOE genotypes, ε2/ε2, ε2/ε3, and ε2/ε4 increased the risk of T2DN (ε2/ε2 vs. ε3/ε3: OR = 2.32, 95% CI: 1.52–3.56, P = 0.0001; ε2/ε3 vs. ε3/ε3: OR = 1.97, 95% CI: 1.50–2.59, P<0.0001; ε2/ε4 vs. ε3/ε3: OR = 1.69, 95% CI: 1.18–2.44, P = 0.0046).
Conclusions
This meta-analysis found that the APOE ε2 allele and the ε2-involved genotypes (ε2/ε2, ε2/ε3, and ε2/ε4) are the risk factors of T2DN.
Similar content being viewed by others
Background
Diabetic nephropathy (DN) contributes to end-stage renal failure [1]. Microvascular injury resulted from reactive oxygen species is implicated in the pathogenesis of DN [2, 3]. Elucidating risk factors of DN, such as genetic and environmental factors, is needed for controlling this disease.
Genetic factors complicated in DN etiology confer useful insights into the etiology of the disease [4]. Oxidative stress is also involved in the complex web of pathological events that confer susceptibility to DN [5, 6]. Excessive generation of reactive oxygen species (ROS) gives rise to imbalanced redox signaling, resulting in renal injury on the long term; moreover, oxidative stress is also linked to changes in the structure and function of apolipoprotein E (APOE), as its coding gene is implicated in DN pathology [7, 8]. Two single nucleotide polymorphisms (SNPs) (rs7412 and rs429358) existing on exon 4 of APOE gene combine to generate three major alleles: ε3 is characterized by cytosines in both positions, while substitution rs7412C > T defines ε2 and rs429358C > T determines ε4. The two SNPs confer APOE3 with arginine at residue 158 and cysteine on residue 112, APOE2 carrying cysteine on both positions, and APOE4 carrying arginine on both positions. Moreover, combinations of these alleles generate six APOE haplotypes (ε2/ε2, ε2/ε3, ε2/ε4, ε3/ε3, ε3/ε4, and ε4/ε4). Allele variation in ApoE locus accounts for 0–20% of ε2, 60–90% of ε3, and 10–20% of ε4, respectively [9]. Allele ε3 is accepted as “wild-type” as it is the most common, and ε2 and ε4 are variants. The association between the two SNPs and T2DN risk is conflicting. Lin et al. found that ε2 polymorphism increased the susceptibility to T2DN in Asian population [10]. ε2 carriers and ε3/ε4 genotype carriers had increasing risks of developing T2DN [11]. However, the differences in sample sizes, sample sources, disease status, genotyping method, and other uncontrolled factors generate the above disagreeing results.
Meta-analysis, featured in summarizing results quantitatively from a wide range of studies, is a powerful method of statistical analysis, increasing the sample size to reduce false-negative and false-positive associations caused by random errors. Notably, new studies on associations between APOE polymorphism and T2DN risks have been issued since Li et al. published their meta-analysis [12]. Therefore, an up-to-date meta-analysis was performed to further investigate the association by including these new published articles.
Methods
Articles search
The meta-analysis was conducted by searching the relative articles published before July 31, 2019 from PubMed, WanFang database, Embase, Vip database, Web of Science, Scopus, and CNKI database. The combinations of keywords were used for searching PubMed, Embase, Web of Science, Scopus were ([“APOE” OR “Apolipoprotein E”] AND [“Diabetic nephropathy”]). Furthermore, the equivalent Chinese keywords were utilized for searching the Chinese databases.
Inclusion/exclusion criteria
The articles selected in the meta-analysis were based on inclusion criteria (case–control design; type 2 DM with DN; and association of APOE with DN risks) and the exclusion criteria (case reports or reviews; duplicate reports; type 1 DM; and missing data of allele or genotype frequencies).
Data extraction and quality assessment
The information from the included articles was extracted, such as the last name of first author and data of APOE allele or genotype.
According to the Newcastle-Ottawa scale (NOS), the quality of the included articles was evaluated. If an included article met a condition, a score of one point was allocated; otherwise, no point (0 score) was allocated. Each of the included articles was awarded the sum of all points (total Quality Score) [13]. Moreover, the quality of these articles was evaluated by the two investigators (Zhaorui Cheng and Jikang Shi) independently. If an agreement for an included article was not reached by the two investigators, the third investigator (Shuang Qiu) settled inconformity finally. Low-quality articles were also selected to avoid selection bias.
Statistical analysis
Chi-square test of goodness of fit was used for evaluating Hardy–Weinberg equilibrium (HWE) for each included article among control groups, and HWE was rejected when P < 0.05. The strength of association between APOE polymorphisms and T2DN risks was assessed using Odds ratios (OR) and 95% confidence intervals (95% CI) owing to binary outcome variable. Both Chi-square test-based Q-statistic and quantified by I2-statistic were adopted to evaluate heterogeneity. Because genotype can represent the combined effect of alleles, the comparisons of APOE genotypes were performed. For heterogeneity between studies given by I squared > 50%, random-effect models were applied; otherwise, if I squared < 50%, fixed-effect models were used [14]. Subgroup analyses were conducted to find main heterogeneity sources. Meta-regression was carried out to further reveal heterogeneity sources and the contribution to heterogeneity. Sensitivity analysis was conducted to evaluate the stability of overall results. Publication bias was examined by funnel plots, and quantified using the Begg’s and Egger’s tests: P < 0.05 was considered significant publication bias [15]. Bonferroni correction was carried out in multiple comparison; thus, P < 0.025 was considered as statistically significant. R Studio (Version 1.1.383) (RStudio, Inc., MA, USA) for Windows was used for all data management and analyses.
Trial sequential analysis (TSA)
Dispersed data and repeated significance testing give rise to an increased risk of random error in traditional meta-analysis. TSA adjusts threshold for statistical significance, reducing the risk of type I error by required information size (RIS). In addition, TSA is used to estimate statistical reliability. In the meta-analysis, TSA software (TSA, version 0.9.5.5; Copenhagen Trial Unit, Copenhagen, Denmark, 2016) was used. The overall type I error was set at 5%, the statistical power was 80%, and the relative risk was reduced by 20% [16]. When the Z-curve crossed trial sequential monitoring boundary or RIS was reached, additional studies were not required; otherwise, additional studies were required.
Results
Characteristics of included articles
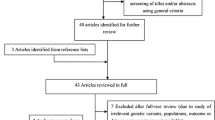
A total of 33 eligible articles were eventually chosen, after abstracts and full texts of 837 published articles originally collected were scrutinized according to the inclusion and exclusion criteria [17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49], thereby conferring 3266 cases and 3259 controls in this meta-analysis (Fig. 1) (Table 1).
Association of the APOE alleles with T2DN risks
A significant heterogeneity was found in ε2 vs. ε3 allele (I2 = 60%, P < 0.01) and in ε4 vs. ε3 allele (I2 = 66%, P < 0.01). Random-effects model was used in ε2 vs. ε3 (pooled OR = 1.89; 95% CI: 1.49–2.38; P<0.0001) (Fig. 2) and in ε4 vs. ε3 (pooled OR = 0.97; 95% CI: 0.77–1.22; P = 0.7948) (Fig. 3). Thus, ε2 allele is regarded as a risk factor of T2DN, and ε4 is not a protective factor.
Association between APOE genotypes and T2DN risks
There existed significant heterogeneity in ε2/ε3 and ε3/ε4 (ε2/ε3 vs. ε3/ε3: I2 = 54%, P < 0.01; ε3/ε4 vs. ε3/ε3: I2 = 70%, P<0.01), but not existed heterogeneity in ε2/ε2, ε2/ε4, and ε4/ε4 (ε2/ε2 vs. ε3/ε3: I2 = 0%, P = 0.47; ε2/ε4 vs. ε3/ε3: I2 = 17%, P = 0.22; ε4/ε4 vs. ε3/ε3: I2 = 0%, P = 0.49). The pooled OR of ε3/ε4 vs. ε3/ε3 was 0.98 (95% CI: 0.73–1.32; P = 0.9146), and that of ε4/ε4 vs. ε3/ε3 was 0.83 (95% CI: 0.53–1.28; P = 0.3904) (Figs. 4 and 5). For this reason, ε3/ε4 and ε4/ε4 did not show a protective effect on T2DN. However, ε2/ε2 and ε2/ε3 increased T2DN risk (ε2/ε2 vs. ε3/ε3: OR = 2.32, 95% CI: 1.52–3.56, P = 0.0001; ε2/ε3 vs. ε3/ε3: OR = 1.97, 95% CI: 1.50–2.59, P<0.0001) (Figs. 6 and 7), and ε2/ε4 genotype also increased T2DN risks significantly (ε2/ε4 vs. ε3/ε3: OR = 1.69, 95% CI: 1.18–2.44, P = 0.0046) (Fig. 8).
Subgroup analysis
For APOE alleles, when ε2 was compared with ε3, the association of increased T2DN risk was significant in Chinese population (OR = 2.04, 95% CI: 1.58–2.62); however, when ε4 was compared with ε3, the protective association of T2DN risk was significant in other population (OR = 0.68, 95% CI: 0.51–0.91) (Table 2). For APOE genotypes, the increased T2DN risks in Chinese population were identified for the genotypes (ε2/ε2 vs. ε3/ε3: OR = 2.74, 95% CI: 1.67–4.49; ε2/ε3 vs. ε3/ε3: OR = 2.09, 95% CI: 1.58–2.76; ε2/ε4 vs. ε3/ε3: OR = 1.64, 95% CI: 1.08–2.50). Whereas, ε3/ε4 genotype decreased T2DN risk in other population (ε3/ε4 vs. ε3/ε3: OR = 0.61, 95% CI: 0.44–0.84), but ε4/ε4 genotype were not associated with T2DN risk in neither of the populations (Table 2). The source of heterogeneity was not found using meta-regression analysis, although each factor decreased overall heterogeneity.
Sensitivity analysis and publication bias
Results of sensitivity analysis in this meta-analysis revealed that there was no individual article influencing the corresponding pooled ORs and 95% CIs (Table 3 and Table 4), indicating that results of this meta-analysis are robust.
Beggʼs funnel plot and Eggerʼs test identified that significant publication bias was not found between either allele and either genotype and T2DN risk (all P>0.05). (Supplementary Figure S1).
Trial sequential analysis
With regard to the relationship of ε2 with T2DN risks and for the relationship of the genotypes (ε2/ε2, ε2/ε3, and ε2/ε4) with T2DN risks, the sample size reached RIS, and the Z-curve crossed the trial sequential monitoring boundary (Supplementary Figure S2). For the relationship of the ε4/ε4 genotype with T2DN risks, the sample size reached RIS (Supplementary Figure S3). For the relationship of ε4 with T2DN risks and for the relationship of the ε3/ε4 genotype with T2DN risks, the sample size and Z curve were not up to the requirements (Supplementary Figure S3).
Discussion
This meta-analysis further investigated the association between the APOE polymorphism and T2DN risks using up-to-date data, indicating that ε2 allele may increase T2DN risks; moreover, ε2/ε2, ε2/ε3, and ε2/ε4 genotypes increase T2DN risks. The ε2 allele and the ε2-involved genotypes may confer the association of APOE polymorphism with T2DN risk.
Meta-analyses between ε2/ε3/ε4 of APOE and DN risks have been performed to recognize the function of variants in APOE. In 2011, Li et al. found that ε2 increases T2DN risk in patients with diabetes [50]. In 2014, Lin et al. also showed that ε2 polymorphism increased the susceptibility to T2DN in Asian population [10]. In 2015, Li et al. validated that ε2 may act as promotion factors of nephropathy in type 2 diabetes, but ε4 is not associated with T2DN risk [12]. This meta-analysis further corroborated that the ε2 allele and the ε2-involved genotypes may confer the association of APOE genetic polymorphism with T2DN risk. Additionally, the association of ε2 with increased T2DN risks was further identified in Chinese population, and ε4 and ε3/ε4 genotype were associated with decreased T2DN risks in other population.
Heterogeneity affects interpretations of results [51]. Although the source was not pinpointed, each separate factor did decrease the overall heterogeneity. Sensitivity analyses and TSA were further performed to assess the robustness of the deductions, reflecting a reliable conclusion.
Oxidative stress affects APOE via amino acid residues 112 and 158, suggesting that oxidative stress may be a source of heterogeneity [52]. Reduced glutathione provides major antioxidative activity; however, glutathione levels were remarkably reduced in patients with DN compared with those in patients with diabetes and healthy controls [53]. The meta-analysis documented the relationship of ε2 allele and the genotypes (ε2/ε2, ε2/ε3, and ε2/ε4) with T2DN risk, suggesting that APOE2 in patients with T2DN cannot balance oxidative stress involved in T2DN progress, and oxidative stress may generate heterogeneity in patients with T2DN.
APOE is interfered by oxidative stress in structure and function. APOE contains two domains (the low-density-lipoprotein receptor [LDLR] binding region [residues 136–150] and the principal lipoprotein-binding region [residues 244–272]), highlighting the implication of the LDLR-binding region of APOE in DN progress. The affinity of APOE3 to LDLR is similar to that of APOE4; however, the binding ability of APOE2 is significantly lower [54]. Moreover, the cysteine-to-arginine substitution in APOE2 at position 158 affects LDLR-binding activity by forming of a new salt bridge between Arg150 and Asp154, further affecting the interaction between APOE2 and LDLR [55]. Thus, oxidative stress interferes the structure and function of APOE by dysregulating the affinity of APOE to LDLR possibly, and the dysregulation of LDLR correlates with DN risk directly [56]. Furthermore, renal lipid accumulation is observed in human DN [57], and knockout of ApoE increases foam cell-rich soft plaques and aggressive renal dysfunction in mice substantially [58].
Study strengths and limitations
There are some strengths in this study. First, the up-to-date articles were collected extensively, rendering this study more statistical power to draw valid conclusion on this issue. Second, TSA was the first utilized to evaluate the association of APOE genetic polymorphism with T2DN risk, conferring reliable evidence to reach the conclusion.
Some limitations exist in this study. First, the main source of heterogeneity was not identified, although subgroup analysis and regression analysis were conducted, and further studies based on larger sample size and multiple ethnicity and region are required. Moreover, the other factors, which could contribute to heterogeneity, are not retrieved. Second, data of oxidative stress status, which possibly reflects renal injury more directly than APOE genetic polymorphism, are not available in literatures. Third, the case-control design could prove an association, rather than a causal relationship, thereby needing prospective cohort studies in future.
Conclusion
In conclusion, the ε2 allele and the ε2-involved genotypes (ε2/ε2, ε2/ε3, and ε2/ε4) may confer the association of APOE genetic polymorphism with T2DN risk. Investigations of oxidative stress status in blood of patients with T2DN are necessary for giving more insight into the association. Elucidating the risk factors of T2DN would be meaningful for the mechanism and control of the disease.
Availability of data and materials
All data generated or analysed during this study are included in this published article.
Abbreviations
- DM:
-
Diabetes mellitus
- DN:
-
Diabetic nephropathy
- T2DN:
-
Nephropathy in type 2 diabetes
- ROS:
-
Reactive oxygen species
- APOE:
-
Apolipoprotein E
- SNPs:
-
Single nucleotide polymorphisms
- NOS:
-
Newcastle-Ottawa scale
- HWE:
-
Hardy–Weinberg equilibrium
- OR:
-
Odds ratios
- 95% CI:
-
95% confidence intervals
- TSA:
-
Trial sequential analysis
- RIS:
-
Required information size
- LDLR:
-
Low-density-lipoprotein receptor.
References
Gross JL, de Azevedo MJ, Silveiro SP, Canani LH, Caramori ML, Zelmanovitz T. Diabetic nephropathy: diagnosis, prevention, and treatment. Diabetes Care. 2005;28:164–76.
Vlassara H, Uribarri J. Advanced glycation end products (AGE) and diabetes: cause, effect, or both? Curr Diab Rep. 2014;14:453.
Volpe CMO, Villar-Delfino PH, Dos Anjos PMF, Nogueira-Machado JA. Cellular death, reactive oxygen species (ROS) and diabetic complications. Cell Death Dis. 2018;9:119.
Wei L, Xiao Y, Li L, Xiong X, Han Y, Zhu X, et al. The susceptibility genes in diabetic nephropathy. Kidney Dis (Basel). 2018;4:226–37.
Miranda-Diaz AG, Pazarin-Villasenor L, Yanowsky-Escatell FG, Andrade-Sierra J. Oxidative stress in diabetic nephropathy with early chronic kidney disease. J Diabetes Res. 2016;2016:7047238.
Beisswenger PJ, Drummond KS, Nelson RG, Howell SK, Szwergold BS, Mauer M. Susceptibility to diabetic nephropathy is related to dicarbonyl and oxidative stress. Diabetes. 2005;54:3274–81.
Jofre-Monseny L, Minihane AM, Rimbach G. Impact of apoE genotype on oxidative stress, inflammation and disease risk. Mol Nutr Food Res. 2008;52:131–45.
Araki S. APOE polymorphism and diabetic nephropathy. Clin Exp Nephrol. 2014;18:230–3.
Liu CC, Kanekiyo T, Xu H, Bu G. Apolipoprotein E and Alzheimer disease: risk, mechanisms and therapy. Nat Rev Neurol. 2013;9:106–18.
Lin YJ, Pan JL, Jiang MJ, Tan JH, Zhong W, Gong TK, et al. Apo E gene polymorphism affects development of type 2 diabetic nephropathy in Asian populations, especially in east Asians: an updated meta-analysis. Med Sci Monit. 2014;20:1596–603.
Guan J, Zhao HL, Baum L, Sui Y, He L, Wong H, et al. Apolipoprotein E polymorphism and expression in type 2 diabetic patients with nephropathy: clinicopathological correlation. Nephrol Dial Transplant. 2009;24:1889–95.
Li T, Shi Y, Yin J, Qin Q, Wei S, Nie S, et al. The association between lipid metabolism gene polymorphisms and nephropathy in type 2 diabetes: a meta-analysis. Int Urol Nephrol. 2015;47:117–30.
Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25:603–5.
Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–60.
Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088–101.
Wetterslev J, Jakobsen JC, Gluud C. Trial sequential analysis in systematic reviews with meta-analysis. BMC Med Res Methodol. 2017;17:39.
Horita K, Eto M, Makino I. Apolipoprotein E2, renal failure and lipid abnormalities in non-insulin-dependent diabetes mellitus. Atherosclerosis. 1994;107:203–11.
Eto M, Horita K, Morikawa A, Nakata H, Okada M, Saito M, et al. Increased frequency of apolipoprotein epsilon 2 allele in non-insulin dependent diabetic (NIDDM) patients with nephropathy. Clin Genet. 1995;48:288–92.
Kimura H, Suzuki Y, Gejyo F, Karasawa R, Miyazaki R, Suzuki S, et al. Apolipoprotein E4 reduces risk of diabetic nephropathy in patients with NIDDM. Am J Kidney Dis. 1998;31:666–73.
Zhang XT, Zou DJ, Li H, Yu L, Fan YX. Study on apolipoprotein E polymorphism in type II diabetes mellitus patients with nephropathy. Chin J Nephrol. 1999;15:77–81.
Xiang G, Xia B, He Y. The relationship of Apo E2 and renal insufficiency lipid levels in NIDDM. Zhonghua Yi Xue Za Zhi. 1999;79:339–41.
Ha SK, Park HS, Kim KW, Kim SJ, Kim DH, Kim JH, et al. Association between apolipoprotein E polymorphism and macroalbuminuria in patients with non-insulin dependent diabetes mellitus. Nephrol Dial Transplant. 1999;14:2144–9.
Akarsu E, Pirim I, Capoglu I, Kaya H, Akcay G, Unuvar N. A relation between the apolipoprotein E genotypes and microalbuminuria in type 2 diabetes mellitus. Turk J Med Sci. 2000;31:59–64.
Dai QF, Wen YH. A study on the relationship between Apo E levels and genotypes in diabetic nephropathy. Modern J Integr Tradit Chin West Med. 2000;9:2321–2.
Shen HL, Liu LM, Xiang KS, Long CM, Weng Q, Xu J. Relationship between ApoE gene polymorphism and type 2 diabetes mellitus with its nephropathy in Chinese. Chinese J Diab. 2002;10:4–6.
Zhang L, Zhu DF, Li Y, Yang MG. Study on apolipoprotein E polymorphism of type 2 diabetes mellitus with nephropathy. Acta Universitatis Medicinalis Anhui. 2002;37:286-9.
Liu L, Xiang K, Zheng T, Zhang R, Li M, Li J. Co-inheritance of specific genotypes of HSPG and ApoE gene increases risk of type 2 diabetic nephropathy. Mol Cell Biochem. 2003;254:353–8.
Park JS, Nam JY, Kim CS, Kim DM, Cho MH, Park J, et al. Apolipoprotein E genetic polymorphism and diabetic Microangiopathy in type 2 diabetic patients. J Kor Diabetes Assoc. 2004;28:511–20.
Liu SY, Ruan YX. Association between nephropathies of type 2 diabetes and Apo E polymorphism. Chin J Nephrol. 2004;20:295–6.
Xiong B, Ning YY, Zhu XZ. Relativity between apolipoprotei E, fatty acid binding 2 polymorphism and type 2 diabetes mellitus patients with nephropathy. Clin Focus. 2005;20:367–70.
Hua F, Liu WQ, Hu WL, Tang Y. Association between Apo E polymorphism and type 2 diabetic nephropathy. J Soochow University (Medical Science Edition). 2006;26:837–8.
Guo Q, Liu J. Association of Apo-E gene polymorphism with biochemical and lipid metabolism parameters in patients with diabetic nephropathy of Hui and Han populations in Gansu Province. Chin J Endocrinol Metab. 2006;22:333–7.
Ng MC, Baum L, So WY, Lam VK, Wang Y, Poon E, et al. Association of lipoprotein lipase S447X, apolipoprotein E exon 4, and apoC3 -455T>C polymorphisms on the susceptibility to diabetic nephropathy. Clin Genet. 2006;70:20–8.
Zhang GW, Zhang AZ, Xu ZF. Study on the relationship between apolipoprotein E (Apo E) gene polymorphism and Chinese patients with type 2 diabetes mellitus and diabetogenous nephropathy (DN). Zhejiang Clin Med J. 2007;9:735–6.
Pan SZ, Chen XJ, Yang LY, Lin JY, Xu J. Relationship between PON2, ApoE gene polymorphisms and type 2 diabetic nephropathy. China Med. 2007;2:385–8.
Ilhan N, Kahraman N, Seckin D, Ilhan N, Colak R. ApoE gene polymorphism on development of diabetic nephropathy. Cell Biochem Funct. 2007;25:527–32.
Kwon MK, Rhee SY, Chon S, Oh S, Woo JT, Kim SW, et al. Association between apolipoprotein E genetic polymorphism and the development of diabetic nephropathy in type 2 diabetic patients. Diabetes Res Clin Pract. 2007;77(Suppl 1):S228–32.
Leiva E, Mujica V, Elematore I, Orrego R, Diaz G, Prieto M, et al. Relationship between Apolipoprotein E polymorphism and nephropathy in type-2 diabetic patients. Diabetes Res Clin Pract. 2007;78:196–201.
Rouzi X, Fan Y, Musa H, Qiqige. The correlation between genetic polymorphism of Apo-E and diabetes in Uygur and Han populations. J Xinjiang Med Univ. 2008;31:1178–80.
Erdogan M, Eroglu Z, Biray C, Karadeniz M, Cetinkalp S, Kosova B, et al. The relationship of the apolipoprotein E gene polymorphism Turkish type 2 diabetic patients with and without nephropathy. J Endocrinol Investig. 2009;32:219–22.
Xiang Q, Wu YB, Song DP, Liu H, Wang YM, Chen B, et al. The study of the association of apolipoprotein E (ApoE) gene polymorphism with diabetic nephropathy in type 2 diabetic patients. Chin J Diabetes. 2010;18:185–6.
Reis KA, Ebinc FA, Koc E, Demirci H, Erten Y, Guz G, et al. Association of the angiotensinogen M235T and APO E gene polymorphisms in Turkish type 2 diabetic patients with and without nephropathy. Ren Fail. 2011;33:469–74.
Sun L, Wang SL, Shi XH, Yang Z. Interactions between APOE and MTHFR mutations is associated with the risk for type 2 diabetic nephropathy. J Med Mol Biol. 2013;10:95–9.
Satirapoj B, Supasyndh O, Dispan R, Punpanich D, Tribanyatkul S, Choovichian P. Apolipoprotein E genetic polymorphisms and the development of nephropathy in type 2 diabetes. J Med Assoc Thail. 2013;96:1119–26.
Wang Y, Xiao ZH, Huang P. Studies on relationship between apolipoprotein E genotype and diabetic nephropathy in Chinese Han population of Guangdong province. J Pract Med. 2014;30:3090–2.
Luo ES, Yang QF, Li X. Analysis on the association between Apolipoprotein E gene polymorphism and renal complications in Chinese type 2 diabetic patients. J Qiqihar Univ Med. 2016;37:3130–2.
Atta MI, Abo Gabal K, El-Hadidi K, Swellam M, Genina A, Zaher NF. Apolipoprotein E genotyping in Egyptian diabetic nephropathy patients. IUBMB Life. 2016;68:58–64.
Jiang Y, Ma L, Han C, Liu Q, Cong X, Xu Y, et al. Effects of Apolipoprotein E isoforms in diabetic nephropathy of Chinese type 2 diabetic patients. J Diabetes Res. 2017;2017:3560920.
Karimoei M, Pasalar P, Mehrabzadeh M, Daneshpour M, Shojaee M, Forouzanfar K, et al. Association between apolipoprotein E polymorphism and nephropathy in Iranian diabetic patients. Saudi J Kidney Dis Transpl. 2017;28:997–1002.
Li Y, Tang K, Zhang Z, Zhang M, Zeng Z, He Z, et al. Genetic diversity of the apolipoprotein E gene and diabetic nephropathy: a meta-analysis. Mol Biol Rep. 2011;38:3243–52.
Munafo MR, Flint J. Meta-analysis of genetic association studies. Trends Genet. 2004;20:439–44.
Miyata M, Smith JD. Apolipoprotein E allele-specific antioxidant activity and effects on cytotoxicity by oxidative insults and beta-amyloid peptides. Nat Genet. 1996;14:55–61.
Singh K, Singh G. Alterations in some oxidative stress markers in diabetic nephropathy. J Cardiavasc Disease Res. 2017;8:24–7.
Weisgraber KH, Innerarity TL, Mahley RW. Abnormal lipoprotein receptor-binding activity of the human E apoprotein due to cysteine-arginine interchange at a single site. J Biol Chem. 1982;257:2518–21.
Dong LM, Parkin S, Trakhanov SD, Rupp B, Simmons T, Arnold KS, et al. Novel mechanism for defective receptor binding of apolipoprotein E2 in type III hyperlipoproteinemia. Nat Struct Biol. 1996;3:718–22.
Zhang Y, Ma KL, Liu J, Wu Y, Hu ZB, Liu L, et al. Dysregulation of low-density lipoprotein receptor contributes to podocyte injuries in diabetic nephropathy. Am J Physiol Endocrinol Metab. 2015;308:E1140–8.
Herman-Edelstein M, Scherzer P, Tobar A, Levi M, Gafter U. Altered renal lipid metabolism and renal lipid accumulation in human diabetic nephropathy. J Lipid Res. 2014;55:561–72.
Buzello M, Tornig J, Faulhaber J, Ehmke H, Ritz E, Amann K. The apolipoprotein e knockout mouse: a model documenting accelerated atherogenesis in uremia. J Am Soc Nephrol. 2003;14:311–6.
Acknowledgements
We thank all the participants of the study.
Funding
Our study was supported by the funds from the National Natural Science Foundation of China (81573230), the Organizational Management Model and Construction of Evaluation Index System for Comprehensive Demonstration Base of Prevention and Control Technology of Major Chronic Disease in Northeast China (2018YFC1311600).
Author information
Authors and Affiliations
Contributions
Conception and design: Yi Cheng, Yawen Liu, Jikang Shi and Zhaorui Cheng. Provision of study materials: Jikang Shi, Zhaorui Cheng, Yunkai Liu, Shuang Qiu, and Yong Li. Collection and assembly of data: Jikang Shi, Zhaorui Cheng, Heran Cui, Yulu Gu, Yaxuan Ren, He Zhang, and Qian Zhao. Data analysis and interpretation: Jikang Shi, Zhaorui Cheng, Yichun Qiao, Helin Sun, and Yueyang Hu. Manuscript writing: Jikang Shi and Zhaorui Cheng. Revised the language/article: All authors. Final approval of manuscript: All authors.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Additional file 1: Figure S1.
Funnel plot of the association between ApoE gene polymorphism and nephropathy in type 2 diabetes. (A) ε2 allele (B) ε4 allele (C) ε2/ε2 genotype (D) ε2/ε3 genotype (E) ε2/ε4 genotype (F) ε3/ε4 genotype (G)ε4/ε4 genotype. Figure S2. Trial sequential analysis of the association between ApoE gene polymorphism and nephropathy in type 2 diabetes. (A) ε2 allele; (B) ε2/ε2 genotype; (C) ε2/ε3 genotype; (D) ε2/ε4 genotype. Figure S3. Trial sequential analysis of the association between ApoE gene polymorphism and nephropathy in type 2 diabetes. (A) ε4 allele; (B) ε3/ε4 genotype; (C) ε4/ε4 genotype.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Shi, J., Cheng, Z., Qiu, S. et al. ε2 allele and ε2-involved genotypes (ε2/ε2, ε2/ε3, and ε2/ε4) may confer the association of APOE genetic polymorphism with risks of nephropathy in type 2 diabetes: a meta-analysis. Lipids Health Dis 19, 136 (2020). https://doi.org/10.1186/s12944-020-01307-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12944-020-01307-6