Abstract
Introduction
Currently, Vitamin D deficiency is a major public health problem and it affects more than one billion people worldwide. Vitamin D is crucial for bone mineralization and ossification. Patients with fractures need Vitamin D for the healing of their fractured bone. The current study was carried out to determine if there is change in the serum level of Vitamin–D associated with factors at early phase of fractured bone healing (ossification) process among adult fractured patients at University of Gondar teaching hospital, Northwest Ethiopia.
Methods
This facility-based prospective follow up study was conducted from March to June 2016. Data was collected by an interviewer, and pretested and structured questionnaires were used. Biological samples were collected to determine the serum level of vitamin–D in all subjects. In addition, X–Ray findings were used to determine the early phase of bone healing process. Data was entered into EPI INFO version 3.5.3 and analyzed using the Statistical Package for Social Sciences (SPSS) version 20. Both bivariate and multivariate logistic regression analysis was done to screen for factors associated with decreased serum levels of Vitamin–D. In the Multivariate regression analysis, those variables which had a P–value of <0.05 were considered as independently associated with change in serum level of Vitamin–D.
Results
A total of 118 adult patients with fractures participated in this study. The prevalence of patients’ with decreased serum levels of vitamin–D at post-test was 63.6% [95% CI; (0.551–0.720)]. Inadequate intake of milk and milk products in the 1st week of fracture [AOR = 95%CI: 0.20 (0.05–0.90)], Poor Dietary Diversity Score [AOR = 95% CI: 29.1 (2.27–371.65)], and ossified bone [AOR =95% CI: 4.10 (1.12–14.95)] showed statistically significant association with decreased serum level of Vitamin–D.
Conclusion and recommendations
Decreased serum level of Vitamin–D at early phase of fractured bone healing process was found in the majority of patients (>63%) raising concern for Vitamin D deficiency to be a significant public health problem in the study population. It was statistically associated with: poor dietary diversity score, in adequate intake of milk and milk products in the 1stone week of fracture and ossified (healed) bone. Introducing hospital based Vitamin–D supplementation and integrated with health and nutritional education is a vital intervention needed to improve serum levels of Vitamin–D.
Similar content being viewed by others
Background
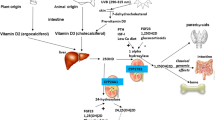
Vitamin–D is an essential micronutrient for the maintenance of bone quality and healing. It is actively involved in bone formation, mineralization, and maintenance of neuromuscular function [1]. It also regulates bone metabolism through activation of the Vitamin–D receptors found in osteoblasts [2]. Vitamin–D has a primary physiological role in maintaining extracellular calcium ion levels in the human body [3, 4].
Globally, Vitamin–D deficiency is a public health problem and it affects more than one billion people [5]. In developing nations, the magnitude of Vitamin–D deficiency ranges between 30 and 90% [6,7,8] and it is higher in older age and female gender [7]. Vitamin–D deficiency results from inadequate synthesis in the skin decreased dietary intake or impaired vitamin–D activation in the liver and kidney [9]. Low serum Vitamin–D levels of (25 [OH] D) in adults can precipitate or exacerbate osteopenia, osteoporosis, and cause osteomalacia and muscle weakness [10, 11]. It also causes impaired calcium absorption which can lead to growth retardation; skeletal abnormalities; and increases the risks of bone fractures [12, 13]. In its’ severe form, Vitamin–D deficiency leads to secondary hyperparathyroidism, bone loss, muscle weakness, and causes increased risk of many infections and chronic diseases (cancers and cardiovascular diseases) [14,15,16].
The cause of low serum levels of Vitamin–D is multi-factorial [17, 18]. Different settings around the world have identified multiple risk factors affecting levels of vitamin–D. For example; age and sex [18,19,20,21], socio-economic status [22,23,24], nutritional status (obese individual) [25], history of chronic and acute illness, repeated history of bone fracture [26], dietary diversity, clothing style, skin color [27], ethnicity, latitude (lower latitude), cultural practice [28], degree of physical activity (limiting outdoor) [27], geographical location and season (winter) [29] were significantly associated with decreased serum level of Vitamin–D.
Understanding the effect of low Vitamin D levels, and related risk factors, on the management of patients’ with fracture is critical. However, there is scarce knowledge of the effect of decreased serum levels of Vitamin–D during early phase of fractured bone healing process among patients with fractures in Ethiopia, particularly in the Northwest part of the country. Therefore, this study aimed to assess changes in serum level of Vitamin–D and its predictors at early phase of bone healing process among newly admitted fractured adult patients at University of Gondar teaching hospital, Northwest Ethiopia.
Method and materials
Study design and setting
This institutional based prospective follow up study was conducted from March–June 2015. The study was undertaken at Amhara regional state, University of Gondar teaching hospital. The hospital is located in Gondar, Northwest Ethiopia. It is located 738 km far from the capital city of Ethiopia, Addis Ababa at an elevation of 2215 m. The University of Gondar teaching hospital serves more than five million people. The services are predominantly delivered through the four major wards of Surgery, Internal medicine, Gynecology and Obstetrics, and Pediatrics with other sub specialty and minor units. Currently, the teaching hospital provides service for more than 20,000 surgical cases per year, among them are on average1450 patients with fractures annually. The majority of these patients are treated in different departments of the hospital like the emergency room, orthopedics, surgery, recovery, and the minor trauma unit.
Sample size determination, sampling procedure and study subjects
The required sample size for the study was determined using a single population proportion (P) formula with the following assumptions; the prevalence of change of serum level of Vitamin–Dwas taken as (P) = 50% and with the consideration of the following assumptions; a 95% confidence level, and 5% margin of error (d). At last, a minimum sample size of 118 was calculated after anticipating a 15% non–response rate. Systematic random sampling technique was employed to recruit study participants. The sampling interval was calculated by considering the average patient flow after reviewing surgical case registration book of the teaching hospital, for 2015. After determination of the sampling interval as 3.2, every 3rd fractured patient was included in the study. All patients aged ≥18 years with new traumatic bone fracture who visited the teaching hospital within 48 h after injury could participate in the study. Those patients with finger and non-traumatic fractures were not included in this study.
Data collection instrument and procedures
An interviewer administered, pretested and structured questionnaire was employed to collect the required information for this study. The questionnaire included socio-demographic and economic characteristics, health and dietary habits and other related characteristics. To check its consistency, the questionnaires were first prepared in English then translated to the local language (Amharic) and back to English by professional translators. A total of four health professionals (two Nurses as data collector, one laboratory technologist for biological sample analysis, and one Public Health Officer as supervisor) were recruited for the data collection process.
Biological samples to measure the serum level of 25hydroxy Vitamin–D (25–OHD) was collected within the first 48 h of fracture. Strict aseptic technique and a separate lancet for each study participants were used. A 5 ml of venous blood was carefully collected from the left hands of fractured patients. The serum was immediately frozen and stored between 2 °C and 8 °C until the laboratory test was conducted within 5 days. Serum concentration of 25-hydroxy Vitamin D (25–OH–D) status was defined by the mini Vitek Immune Diagnostic Assay System (VIDAS) machine. The change in serum level of Vitamin–D was classified into two categories as decreased and increased by considering 1 month’s follow up result from the baseline measurement. Decreased serum level of Vitamin–D was defined as a 25–OHD level of less than 50 nmol/L (20 ng/ml) [30].
X–ray (Radiography) findings were used to determine the site and healing process of fractured bone. Early phase of bone healing process was measured in the first 1 month of fracture. The healing process was defined as: healed bone, when the radiologist determined the fractured bone is ossified/calcified after 1 month of visit.
Nutritional status of the patient was assessed by taking anthropometric body measurement of height and weight. Based on the severity of fracture and type, two techniques were employed to take the height measurement of study subjects. Among a total of 118 patients; 73 were measured in a standing position with adult scale astormeter to the nearest 0.1 cm. The remaining 45 patients’ heights were measured in a recumbent position to the nearest 0.1 cm by using a board with an upright wooden base and a movable headpiece, on a flat surface. Weight of the patient was taken by an adult scale astor electronic weight scale with the calibration of 100 g unit. The scale was adjusted to zero before weighing every fractured patient. All study participants were without any shoes during the weight and height measurements.
A mathematical calculation of Body Mass Index (BMI) was used to determine the nutritional status of study subjects. Nutritional status was defined as poor nutritional status (when the BMI level of <18.5 kg/m2), good nutritional status (when BMI level was 18.5–24.9), Overweight (when BMI of 25–29.9), Obese (when BMI of ≥30) [31].
Dietary diversity score was determined by using a 24 h recall method. The patients were requested to list what they consumed in the previous 24 h of the survey. Dietary diversity score was then computed based on 9 food groups as recommended by FAO which are comprised of: grains, roots and tubers; legumes and nuts; dairy products; flesh foods (meat, fish, poultry and organ meats); eggs; vitamin A rich fruits and vegetables; other fruits and vegetables. Finally, dietary diversity scores (DDSs) were calculated; as poor dietary diversity score, (when the patient consumed ≤3 food groups), medium dietary diversity score (when the patient consumed 4–5 food groups) and high dietary diversity (when the patient consumed ≥6 food groups) in the previous 24 h of the survey [32].
The living standard of the household was computed using a composite indicator for urban and rural residents. The asset information was determined via a principal components analysis. The index was constructed using the selected household asset information for urban and for rural residents. The household wealth index was categorized into three categories (Poor, Middle and Rich) [33].
Data quality control
In order to ensure data quality, questionnaires were translated by professional translators. In addition, a 2 day training was given to recruited data collectors and supervisor. The training was focused on the objective of the study, technique of interview, how to collect the samples, and relevant ethical issues (respondent’s right, confidentiality of information etc.). Three days prior to the actual data collection, the data collection tool was pretested among 5% of study subjects out of the study area. During the pretest, the applicability of the procedures and tools were evaluated. ll questioners were checked for completeness, clarity and consistency by the supervisor. Furthermore, the investigators coordinated the overall activities of data collection process throughout the present study.
Data processing and analysis
Data were entered into EPI INFO version 3.5.3 and analyzed using the Statistical Package for Social Sciences (SPSS) version 20. Before analysis the data was cleaned thoroughly to check for errors during entry. Descriptive statistics, including frequencies and proportions were used to summarize the study variables. A binary logistic regression was used to investigate factors associated with decreased serum level of vitamin–D. Those predictor variables with a P–value of <0.2 in the bivariate analysis were exported to multivariate analysis to control the possible effect of confounders. The Adjusted Odds Ratio (AOR) at a 95% confidence interval and P–value of ≤0.05 was used to declare the strength of association with decreased serum level of Vitamin–D.
Results
Socio–demographic and clinical characteristics of the study participants
A total of 118 (103 male and 18 female) participants with a 100% of response rate were included in the study. The mean age of the study subjects were 36.87 year with a standard deviation (SD) of ±13.4 years. More than half (62.7%) of study subjects were rural dwellers. Orthodox religion accounted for 89.0% study participants. Seventy two (61.0%) subjects were farmers and 80 (67.8%) were married. The family size of the study subjects ranged from 1 to 5 with mean family size of 4.1 (Table 1).
Dietary intake and dietary diversity scores related characteristics
Teff, Maize and Sorghum were the three most common staple diets in the study area. Of 98.3% participants, The frequency of meals was <3 in 98.3% of participants prior to the first visit. More than 50 and 47.5% study participants reported that they had less than three meals per day and four meals per day, respectively, a month after the fracture. (Table 2).
A 24 h dietary recall technique was employed to determine the dietary diversity scores in the previous 24 h of the interview. During the time of first visit, nearly 60 % of study participants received poor dietary diversity scores. In contrast, the proportion of individuals with low dietary diversity scores was 24.6% 1 month after the fracture. (Fig. 1).
Co–morbidity and nutritional status related characteristics
Ten (8.5%) of the respondents had a history of a known chronic disease and were currently on medications during the study. Among them, three had both diabetes mellitus and hypertension, four had only hypertension, two had chronic asthma, and one had tuberculosis. Almost all patients with a chronic disease history received health and nutrition education for the dietary management of their chronic diseases.
Based on the mathematical calculation of body mass index (BMI), 89.0% study subjects had normal nutritional status in this study (Table 3).
Change in serum level of vitamin D
Nearly 70 % of study participants (69.49%) enrolled were treated as an inpatient and the rest as outpatients. There was a difference between the pre-tests and post-tests done at baseline and after 1 month (P–value 0.053) after the fracture. Serum levels of Vitamin D significantly decreased during the first month following a fracture in the majority of the study community [63.6%: 95% CI; (0.551–0.720)] compared to the base line.
Predictors of change in serum level of vitamin–D
In the bivariate logistic regression analysis age, place of residence, time of accident, milk consumption at first visit, religion, Dietary Diversity Scores (DDSs) at 1st visit, healed bone (ossification), Dietary Diversity Scores (DDSs) in the 1st week of a month and history of milk and milk products consumption in the 1st week of a month were significantly associated with decreased serum level of Vitamin–D. In the multivariable regression analysis, bone healing (ossified bone), milk and milk product consumption in the 1st week of a month and dietary diversity score in the 1st week of a month were listed out as strong predictor of decreased serum levels of Vitamin–D. But having chronic co morbidity has no any statistically significant association with change in serum level of Vitamin D. Since the P-value was > 0.2 in the bivarable model, it was not included in the multi-variable model. Accordingly, the likelihood of decreased serum levels of Vitamin–D was more than four times higher among patients who had ossification of the fractured bone than those who had not [AOR = 95%CI: 4.1 (1.12–14.9)]. The odds of decreased serum level of Vitamin–D was greater than 29 times among patients who had poor dietary diversity scores in 1st week than those who had high DDSs[(AOR = 95%CI: 29.05 (2.27, 371.65)]. Likewise, the odds of decreased serum level of Vitamin–D during early phase of bone healing process was 80% more likely among patients who didn’t consumed milk and milk products in the 1st week after fracture than those who had[AOR = 0.20; 95% CI (0.05, 0.90)] (Table 4).
Discussion
This study was aimed to determine if there is significant change in serum levels of Vitamin–D in conjunction with associated risk factors that may adversely affect the early phase of bone healing following a fracture. The prevalence of decreased serum levels of vitamin–D was 63.6% [95% CI; (0.551–0.720)]. Determinants of change in serum level of vitamin D were: inadequate intake of milk and milk products in the 1st week following a fracture, poor Dietary Diversity Score, and ossified bone.
Vitamin–D is essential micronutrient for bone healing and it is actively involved in bone formation and mineralization [1]. Since patients consumed more vitamins –D for the healing of their bone, the serum level of vitamin D becomes decreased. The prevalence of decreased Vitamin D levels (63.6%) were much higher than studies from Italy [21.6%] [34], India [32.3%] [35], and Singapore [34.5%] [23]. Variation among results could be due to the difference in skin color of study participants. Unlike other studies, the participnts of this study are dark–skinned individuals and they require greater duration of sunlight exposure than those light–skinned counterparts to synthesize comparable amounts of Vitamin–D [36, 37]. The other possible reason might be due to difference in geographical location of the study participants. African people have greater risks of low serum Vitamin–D levels [38,39,40,41,42] and, Vitamin–D deficiency is a common contributor of bone fracture [11]. Additionally, due to reduced sunlight exposure of hospitalized and institutionalized individuals due to fracture/disability, they might have higher risk of Vitamin–D insufficiency [17, 19, 33], which may further contribute to high prevalence of Vitamin–D deficiency. Moreover, there may be Vitamin–D supplementation and micronutrient fortification programs in other countries. Vitamin D supplementation, nutritional health education and fortification programs may help improve the serum Vitamin–D level and increase the healing process of fractured bone.
Ossified bone (healed bone) was statistically associated with decreased serum levels of Vitamin–D. Decreased serum level of Vitamin–D was higher among individuals with ossified bone than non ossified bone 1 month after fr. This result was congruent with a study done in Singapore [23] and Russia [43]. This was evidenced by the fact that Vitamin–D is critical to the bone healing pathway of fractured bones. It is important in the formation and mineralization of the callus; it acts directly on osteoblasts, stimulates the synthesis of osteocalcin, while also acting on osteoclasts to stimulate bone resorption [29, 44,45,46,47]. Due to its role in the formation and mineralization of the callus, serum level of Vitamin–D decreases when fractured bone heals [24]. Furthermore, Vitamin–D stimulates chondrogenesis by cell proliferation and promotion of matrix protein synthesis, during the fractured bone healing process [48,49,50], which can result in decreased serum levels of Vitamin–D.
Dietary diversity score in the previous 24 h of the survey was independently associated with change (often decrease) in serum level of Vitamin–D. Poor DDSs in the 1st week following a fracture increased the odds of decreased serum level of Vitamin–D. The result was similar in a study in six regions of the world (Asia, Europe, Latin America, Africa, North America, and Oceania) [51]. The possible reasons could be; dietary diversity scores in the previous 24 h of an individual positively correlated with adequate micronutrient density of the diet [52,53,54], including Vitamin–D. In addition, consumption of undiversified foods may increases inadequate intake of Vitamin–D rich food sources, contributing to decreased serum levels of Vitamin–D.
Inadequate intake of milk and milk products 1 month after fracture was one of the important risk factors affecting change in serum level of Vitamin–D. The result was in agreement with results from six regions of the world (Asia, Europe, Latin America, Africa, North America, and Oceania) [51]. Since Vitamin–D as the primary regulator of calcium absorption, this may be an important contributing cause for low levels of vitamin D [12]. Vitamin D influences calcium levels primarily by controlling the absorption of calcium from the intestine, through direct effects on bone and parathyroid hormone (PTH) secretion [13]. Decreased serum level of Vitamin–D has been linked to the increased levels in alkaline phosphatase and parathyroid hormone and decreased calcium levels [55]. This leads to decreased calcium absorption and ultimately the release of calcium from the bones. Even milk and milk products which are rich dietary sources of calcium, when Vitamin D is below20 nmol/L, this results in extremely decreased intestinal calcium absorption in the body [56].
The results of this study may applicable to many populations; however, the main limitation of this study lied in the cross–sectional nature of the study. Fortunately, the study noted important trends that can be used to design health and nutrition interventions to improve serum level of Vitamin–D among adult patients with fractures in Ethiopia and possibly around the world. More research is needed to apply important finding more broadly.
In conclusion, decreased serum level of Vitamin–D during early phase of bone healing process was high in the study community. Poor dietary diversity score, bone healing (ossification) status and inadequate intake of milk and milk products after 1 month following a fracture was significantly associated with decreased serum levels of Vitamin–D. Introducing hospital based Vitamin–D supplementation in conjunction with health and nutritional education is a vital intervention to improve serum levels of Vitamin–D and promote bone healing in the early phase following a fracture in order to maximize health and healing (ossification) process in the study area.
Abbreviations
- 25- OHD:
-
25- Hydroxyl Vitamin - D
- AOR:
-
Adjusted Odds Ratio
- BMI:
-
Body Mass Index
- DDS:
-
Dietary Diversity Score
- FAO:
-
Food and Agriculture Organization
- IPH:
-
Institute of Public Health
- PTH:
-
Parathyroid Hormone
- SPSS:
-
Statistical Package for Social Science
- VIDAS:
-
Vitek Immune Diagnostic Assay System
References
Gorter EA, Hamdy NA, Appelman-Dijkstra NM, Schipper IB. The role of vitamin D in human fracture healing. Bone. 2014;64:288–97.
Holick MF. Sunlight and vitamin D for bone health and prevention of autoimmune diseases, cancers and cardiovascular disease. Am J Clin Nutr. 2004;80:1678S–88S.
Oyen J, Apalset EM, Gjesdal CG, Brudvik C, Lie SA, Hove LM. Vitamin D inadequacy is associated with low-energy distal radius fractures: a case–control study. Bone. 2011;48(5):1140–5.
Mata-Granados JM, Cuenca-Acevedo JR, de Castro MDL, Holick MF, Quesada-Gómez JM. Vitamin–D insufficiency together with high serum levels of vitamin a increases the risk for osteoporosis in post–menopausal women. Clin Biochem. 2010;43(13–14):1064–8.
Plotnikoff GA, Quigley JM. Prevalence of severe Hypovitaminosis D in patients with persistent, nonspecific musculoskeletal pain. Mayo clinic. 2003;78:1463–70.
Todd CJ, Freeman CJ, Camilleri-Ferrante C, Palmer CR, Hyder A, Laxton CE, et al. Differences in mortality after fracture of hip: the east Anglian audit. BMJ. 1995;310(6984):904–8.
Folman Y, Gepstein R, Assaraf A, Liberty S. Functional recovery after operative treatment of femoral neck fractures in an institutionalized elderly population. J Bone Surg. 1994;75:454–6.
Di Monaco M, Castiglioni C, Vallero F, Di Monaco R, Tappero R. Appendicular lean mass does not mediate the significant association between vitamin D status and functional outcome in hip-fracture. Women Am Congress Rehabil Med. 2011;92(2):271–6.
Isaia G, Giorgino R, Rini GB, Bevilacqua M, Maugeri D, Adami S. Prevalence of hypovitaminosis D in elderly women in Italy: clinical consequences and risk factors. Osteoporos Int. 2003;14(7):577–82.
Bischoff-Ferrari HA, Giovannucci E, Willett W, Dietrich T, Dawson-Hughes B. Estimation of optimal serum concentrations of 25-hydroxyvitamin D for multiple health outcomes. Am J Clin Nutr. 2006;84(1):18–28.
Bischoff-Ferrari HA. Optimal serum 25-hydroxyvitamin D levels for multiple health outcomes sunlight, vitamin D and skin cancer. New York: Springer; 2008.
Parfitt AM, Gallagher JC, Heaney RP, Johnston CC, Neer R, GD. W. Vitamin D and bone health in the elderly. Am J Clin Nutr. 1982;36(5):1014-31.
Holick MF. Vitamin D: the underappreciated D-lightful hormone that is important for skeletal and cellular health. Curr Opin Endocrinol. 2002;9:87–98.
Wang TJ, Pencina MJ, Booth SL, Jacques PF, Ingelsson E, Lanier K, et al. Vitamin D deficiency and risk of cardiovascular disease. Circulation. 2008;117(4):503–11.
Penckofer S, Kouba J, Byrn M, Ferrans CE. Vitamin D and depression: where is all the sunshine. Ment Health Nurs. 2010;31(6):385–93.
Mathieu C, Gysemans C, Giulietti A, Bouillon R. Vitamin D and diabetes. Diabetologia. 2005;48(7):1247–57.
Brigg AD, Kuanu V, Greiller CL, Maclaughlin BD, Ramachandran M, Harris T, Timms PM, Venton TR, Vieth R, Norman AW, Griffiths CJ, Martineau AR. Longitudinal study of vitamin D metabolites after long bone fracture. J Bone Miner Res. 2013;28:1301–7.
Parchi P, Andreani L, Piolanti N, Niccolai F, Cervi V, Lisanti M. Effect of vitamin D in fracture healing in a child. Arch Osteaoporos. 2014;9:170.
Wolfi C, Englert S, Moghaddam AA, Zimmermann G, Schmidt-Gayk H, Honer B, Hogan A, Lehnhardt M, Grutzner PA, Kolios L. Time course of 25(OH)D3 vitamin D3 as well as parathyroid hormone during healing of patients with normal and low bone mineral density. BMC Musculo Skelet Disord. 2013;14:6.
Bogunovic L, Kim AD, Beamer BS, Nguyen J, Lane JM. Hypovitaminosis D in patients scheduled to undergo orthopaedic surgery: a single-center analysis. J Bone Joint Surg Am. 2010;92(13):2300–4.
Schunack W. Vitamin D3--a prodrug of different D3-hormones. Med Klin (Munich) 2006;1:20-4.
Smith JT, Halim K, Palms DA, Okike K, Bluman EM, Chiodo CP. Prevalence of vitamin D deficiency in patients with foot and ankle injuries. Foot Ankle Int. 2014;35(1):8–13.
Ramason R, Selvaganapathi N, Binte Ismail HN, Chin-Wong CW, Rajamoney NG, Chong SM. Prevalence of vitamin D deficiency in patients with hip fracture seen in an Orthogeriatric Service in Sunny Singapore. Geriatr Orthop Surg Rehabil. 2014;5(2):82–6.
Ettehad H, Mirbolook A, Mohammadi F, Mousavi M, Ebrahimi H, Shirangi A. Changes in the serum level of vitamin d during healing of tibial and femoral shaft fractures. Trauma Mon. 2014;19(1):25.
Puri S, Marwaha RK, Agarwal N, et al. Vitamin D status of apparently healthy schoolgirls from two different socioeconomic strata in Delhi: relation to nutrition and lifestyle. Br J Nutr. 2008;99:876–82.
El-Hajj Fuleihan G. Vitamin D deficiency in the Middle East and its health consequences. In: Holick MF, editor. Vitamin D: physiology, molecular biology, and clinical applications, 2nd edn. New Jersey: Humana Press; 2009.
Dawodu A, Dawson KP, Amirlak I, et al. Diet, clothing, sunshine exposure and micronutrient status of Arab infants and young children. Ann Trop Paediatr. 2001;21:39–44.
Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266–81.
Vieth R, Cole DE, Hawker GA, et al. Winter time vitamin D insufficiency is common in young Canadian women, and their vitamin D intake does not prevent it. Eur J Clin Nutr. 2001;55:1091–7.
Subj RHS. Excerpts from dietary reference values for food energy and nutrients for the United Kingdom. 1991.
WHO. World Health Organization body mass index (BMI) classification. 2013.
Kennedy G, Ballard T, Dop MC. Guidelines for measuring household and individual dietary diversity. United Nations: FAO; 2011. p. 56.
Rodrigo MD. Peak bone mass measured by phalangeal BMD and its association with nutritional status, socio-economic status and physical activity. Osteoporos Int. 2007.
Nuti R, Martini G, Valenti R, Gambera D, Gennari L, Salvadori S, et al. Vitamin D status and bone turnover in women with acute hip fracture. Clin Orthop Relat Res. 2004;422:208–13.
Dhanwal DK, Sahoo S, Gautam VK, Saha R. Hip fracture patients in India have vitamin D deficiency and secondary hyperparathyroidism. Osteoporos Int. 2013;24(2):553–7.
Holick MF, MacLaughlin JA, Doppelt SH. Regulation of cutaneous previtamin D3 photosynthesis in man: skin pigment is not an essential regulator. Science (New York). 1981;211(4482):590–3.
Clemens TL, Adams JS, Henderson SL, Holick MF. Increased skin pigment reduces the capacity of skin to synthesise vitamin D3. Lancet (London, Englad). 1982;1(8263):74–6.
De Torrent de la Jara G, Pecoud A, Favrat B. Musculoskeletal pain in female asylum seekers and hypo-vitaminosis D3. Br Med J. 2004;32:156–7.
Meyer HE, Falch JA, Sogaard AJ, Haug E. Vitamin D deficiency and secondary hyperparathyroidism and the association with bone mineral density. Bone. 2004;35:412–7.
Van der Meer IM, Boeke AJP, Lips P. Fatty fish and supplements are the greatest modifiable contributors to the serum 25-hydroxyvitamin D concentration in a multiethnic population. Clin Endocrinol. 2008;68:466–72.
Van der Meer IM, Karamali NS, Boeke AJP. High prevalence of vitamin D deficiency in pregnant non-western women in The Hague, Netherlands. Am J Clin Nutr. 2006;84:350–3.
Bakhtiyarova S, Lesnyak O, Kyznesova N, Blankenstein MA, Lips P. Vitamin D status among patients with hip fracture and elderly control subjects in Yekaterinburg. Russia Osteoporos Int. 2006;17:441–6.
Van der Mei IAF, Ponsonby AL, Engelsen O, et al. The high prevalence of vitamin D insufficiency across Australian populations. Environ Health Perspect. 2007;115:1132–9.
Seo EG, Einhorn TA, Norman AW. 24R,25-dihydroxyvitamin D3: an essential vitamin D3 metabolite for both normal bone integrity and healing of tibial fracture in chicks. Endocrinology. 1997;138(9):3864–72.
Holick MF. Resurrection of vitamin D deficiency and rickets. J Clin Invest. 2006;116(8):2062–72.
Guardia G, Parikh N, Eskridge T, Phillips E, Divine G, Rao DS. Prevalence of vitamin D depletion among subjects seeking advice on osteoporosis. Osteoporos Int 2008;19(1):13–9.
Binkley N, Novotny R, Krueger D, Kawahara T, Daida YG, Lensmeyer G. Low vitamin D status despite abundant sun exposure. J Clin Endocrinol Metab. 2007;92(6):2130–5.
Hovsepian S, Amini M, Aminorroay A, Amini P, Iraj B. Prevalence of vitamin D deficiency among adult population of Isfahan City. Iran J Health Popul Nutr. 2011;29(2):149–55.
Moussavi M, Heidarpour R, Aminorroaya A, Pournaghshband Z, Amini M. Prevalence of vitamin D deficiency in Isfahani high school students in 2004. Horm Res. 2005;64(3):144–8.
Talaei A, Yadegari N, Rafee M, Rezvanfar MR, Moini A. Prevalence and cut-off point of vitamin D deficiency among secondary students of Arak, Iran in 2010. Indian J Endocrinol Metab. 2012;16(5):786–90.
Mithal A, Wahl DA, Bonjour J-P, Burckhardt P, Dawson-Hughes B, Eisman JA, et al. Global vitamin D status and determinants of hypovitaminosis D. Osteoporos Int. 2009;20:1802–20.
Foote J, Murphy S, Wilkens L, Basiotis P, Carlson A. Dietary variety increases the probability of nutrient adequacy among adults. J Nutr. 2004;134:1779–85.
Mirmiran P, Azadbakht L, Esmaillzadeh A, Azizi F. Dietary diversity scores in adolescents- a good indicator of the nutritional adequacy of diets. Asia Pac J Clin Nutr. 2004;13(1):56–60.
Daniels M. Dietary diversity as a measure of the micronutrient adequacy of women’s diets. 2009.
Brinker MR, O’Connor DP, Monla YT. Metabolic and endocrine abnormalities in patients with nonunions. J Orthop Trauma. 2007;21:557–70.
Lips P. Vitamin D deficiency and secondary hyperparathyroidism in the elderly: consequences for bone loss and fractures and therapeutic implications. Endocrinology. 2001;22:477–501.
Acknowledgments
The authors would like to express our sincere gratitude to those study participants for their willingness to participate in this study. The authors’ heartfelt thanks also go to University of Gondar teaching hospital for giving the ethical clearance of this study.
Availability of data and materials
Data will be available upon request from the corresponding author.
Financial support
There was no financial support.
Author information
Authors and Affiliations
Contributions
YF designed the study, carried out statistical analysis, and thesis writes up process. SM, HW, and AT participated in proposal writing, reviewing, and approval. Finally, YF prepared the manuscript for publication. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Ethical clearance was obtained from the Institutional review boards of University of Gondar, Institute of Public Health (IPH). Permission letter for the next steps were secured form hospital administrative body. Written informed consent was obtained from each study participant after explaining the purpose of the study. The right of a participant to withdraw from the study at any time, without any precondition was disclosed unequivocally. Moreover, the confidentiality of information obtained was guaranteed by all data collectors and investigators using code numbers rather than personal identifiers and by keeping the questionnaire locked. Nutrition education and counseling was provided for all study participants after the data collection completed.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Fentaw, Y., Woldie, H., Mekonnen, S. et al. Change in serum level of vitamin D and associated factors at early phase of bone healing among fractured adult patients at University of Gondar teaching hospital, Northwest Ethiopia: a prospective follow up study. Nutr J 16, 54 (2017). https://doi.org/10.1186/s12937-017-0277-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12937-017-0277-y