Abstract
Background
Until now the exact biochemical processes during healing of metaphyseal fractures of healthy and osteoporotic bone remain unclear. Especially the physiological time courses of 25(OH)D3 (Vitamin D) as well as PTH (Parathyroid Hormone) the most important modulators of calcium and bone homeostasis are not yet examined sufficiently. The purpose of this study was to focus on the time course of these parameters during fracture healing.
Methods
In the presented study, we analyse the time course of 25(OH)D3 and PTH during fracture healing of low BMD level fractures versus normal BMD level fractures in a matched pair analysis. Between March 2007 and February 2009 30 patients older than 50 years of age who had suffered a metaphyseal fracture of the proximal humerus, the distal radius or the proximal femur were included in our study. Osteoporosis was verified by DEXA measuring. The time courses of 25(OH)D3 and PTH were examined over an eight week period. Friedmann test, the Wilcoxon signed rank test and the Mann-Withney U test were used as post-hoc tests. A p-value ≤ 0.05 was considered significant.
Results
Serum levels of 25(OH)D3 showed no differences in both groups. In the first phase of fracture healing PTH levels in the low BMD level group remained below those of the normal BMD group in absolute figures. Over all no significant differences between low BMD level bone and normal BMD level bone could be detected in our study.
Conclusions
The time course of 25(OH)D3 and PTH during fracture healing of patients with normal and low bone mineral density were examined for the first time in humans in this setting and allowing molecular biological insights into fracture healing in metaphyseal bones on a molecural level. There were no significant differences between patients with normal and low BMD levels. Hence further studies will be necessary to obtain more detailed insight into fracture healing in order to provide reliable decision criteria for therapy and the monitoring of fracture healing.
Similar content being viewed by others
Background
Low serum levels of 25(OH)D3 are suggested to be associated with reduced BMD (bone mineral density) [1, 2]. Lips et al. found reduced levels of serum 25(OH)D3 to be common amongst women with osteoporosis [3]. The diminishing ability of the skin to synthesize 25(OH)D3 with advancing age or a poor diet are just are some reasons [4, 5].
Individuals with vitamin D deficiency are more likely to fall and to lose muscle mass [6]. Chronic insufficiency of 25(OH)D3 leads to secondary hyperparathyroidism with increased bone turnover, bone loss and thus a higher fracture risk [4]. As a result, guidelines for the management of postmenopausal osteoporosis typically include recommendations for its supplementation [7, 8].
During fracture repair, circulating levels of 25(OH) D3 increase in white leghorn chickens due to an increase in CYP24A1 activity (25-dihydroxyvitamin D3 24-hydroxylase, degrades 1.25-(OH)2D to calcitroic acid) [9]. Serum 1.25-(OH)2D levels after sustaining a fracture have only been measured in a few studies with humans [10]. Significantly reduced levels were found 28 hours after sustaining the fracture.
There are only few studies, observing the time course of 25(OH)D3 during the fracture healing, neither in healthy nor in osteoporotic bone. Meller et al. investigated on it and amongst others also on PTH in a population of 13 young adult patients without osteoporosis and long bone fractures [11] and in a population of 41 geriatric patients with proximal femur fractures [12].
The second major mediator of bone remodelling is the parathyroid hormone (PTH). PTH regulates serum calcium levels by acting mainly on bones and kidneys. Two important functions e.g. are stimulating of renal calcium re-absorption and bone resorbtion when calcium levels are low [13]. The intermittent administration of human parathyroid hormone is to date the only clinically approved bone anabolic therapy for osteoporotic patients of both genders [14].
The 84-amino-acid polypeptide hormone is an essential regulator of calcium homeostasis [15]. PTH and PTHrP (PTH-related protein) indirectly activate osteoclasts leading to increased bone resorption [16]. The hypercalcemic effect of PTH lies, in part, in its effect on the nephron enhancing renal calcium reabsorption and, in part, in bone resorption [17].
PTH infused continuously reduces BMD, increases levels of bone resorption marker TRAP-5b and raises serum calcium levels [17, 18]. An intermittent application of PTH that permits complete serum clearence between doses [19] increases 1.25-(OH)2D and BMD, raises levels of bone formation marker osteocalcin without producing hypocalcaemia. 1.25-(OH)2D complements this PTH effect and may therefore facilitate an optimal skeletal response to PTH. This indicates that a full response requires an active 1.25-(OH)2D synthesizing system [17].
Some clinical studies indicate that a daily subcutaneous injection of PTH stimulates the development of new bone, increases bone mass in patients with osteoporosis [20] and reduces the incidence of fractures in postmenopausal women [21].
Administered during fracture healing PTH promotes bone healing in healthy and osteoporotic rats, when it is applied during early stage of healing without having any adverse systemic effect [22].
So far there is no data on the endogenic behaviour of PTH during fracture repair of healthy or osteoporotic bones. In our study, we therefore examined the time courses of 25(OH)D3 and PTH during healing of metaphyseal fractures of normal and low BMD level long bones.
Methods
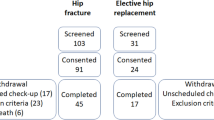
Between March 2007 and February 2009 all patients over 50 years of age who had suffered a metaphyseal fracture (distal radius-, proximal humerus- and proximal femur fracture) with an obligatory indication for surgical stabilization were included in this study. Exclusion criteria were defined as: polytrauma, significant soft tissue injury, high grade open fracture, > 24h mechanical ventilation after surgery, dialysis patients, long-term therapy with immunosuppressants such as corticosteroids, collagenosis, chronic inflammatory bowel disease, haematological disorders, malignant neoplasms and metabolic bone diseases (osteomalcia, hyperparathyreodism, Paget’s disease).
In all patients X-rays of the fractured region and also of the lumbar spine were performed in an anterior-posterior and lateral view. X-rays of the lumbar spine were used to exclude morphological changes of the vertebrae leading to wrong values in DXA bone density measurements. To monitor the clinical course of fracture healing, follow-up X-rays of the fractured site were performed after 4 and 8 weeks, and also one year after osteosynthetic stabilization.
BMD was examined on the lumbar spine, the entire hip on both sides and the hip subregions using dual energy X-ray absorptiometry DXA (Lunar iDPX, GE Medical Systems Germany, Solingen, Germany) based on Encore TM Version II.X software. One year after surgery follow-up DXA measurements were performed. Using normative data for young adult white people, BMD was categorized as normal, low bone mineral density, or osteoporosis, as defined by the World Health Organization. Participants with a T-score ≤ −2.5 SD were categorized as having osteoporosis.
Laboratory analysis was performed the morning after injury in fasting state. Among typical routine laboratory values, 25(OH)D3 and parathyroid hormone PTH were examined. Quantitative measurement was detected using chemiluminescence immunoassay “Access Ostase assay 37300” (Beckman Coulter™, Brea, USA) and electrochemiluminescence immunoassay ECLIA® (Roche, Basel, Switzerland). The laboratory values were followed-up one, four and eight weeks after suregry. The following kits where used:
Vitamin D3/25(OH)D3:
cobas® 25 Hydroxyvitamin D3 03314847 190, Roche
Diagnostics GmbH, D-68298 Mannheim
PTH (Draft):
cobas® Parathyroid hormone – PTH, intact 11972103
160, Roche Diagnostics GmbH, D-68298 Mannheim
Patients with low BMD levels did not receive osteoporosis medication in this period.
The study was designed as an epidemiological case–control study. After receiving the results from DXA-measuring, patients were matched in osteoporotic and nonosteoporotic groups. Further matching-criteria were: age (+/- 5 years), sex (male, female), fracture localization (proximal humerus, distal radius, proximal femur), fracture type (according to AO-classification; A-, B- or C-type fractures) and surgical technique (plate, intramedular nail). Thus we received a collective of 15 matched pairs (30 patients in total).
Statistics were performed using the software SPSS 11.0.0 (IBM Germany, Munich, Germany) and Microsoft Excel 2003/2007, Microsoft Corp. Washington, USA. By Pretrail-Power analysis of the first three patients we calculated that 15 matched pairs would be necessary to achieve statistical significance. To show comparisons over the time within one group, we first performed Friedman’s test. As post-hoc test we then performed Wilcoxon-test. We performed Wilcoxon’s rank-sum test in addition to compare both groups at one point in time. P ≤ 0.05 was considered to be significant.
Results
Between March 2007 and February 2009 54 patients over 50 years of age who had suffered a metaphyseal fracture of the proximal humerus, the distal radius or the proximal femur were included in our study. In total 15 matched pairs were constructed according to the presented matching criteria (Table 1) and their base line demographic data (Table 2). There were 7 pairs with fractures of the distal radius, 3 pairs with proximal humeral fractures and 6 pairs with proximal femoral fractures (including femoral neck and intertrochanteric fractures).
The results of laboratory analyses of 25(OH)D3 and PTH are presented graphically in Figures 1 and 2.
Serum values of 25(OH)D3 during fracture healing of low BMD level patients and normal BMD level patients show no significant differences.
The time course of PTH also showed no significant changes in both groups. In the first phase of fracture healing PTH of the low BMD level group stayed below the level of the normal BMD level group.
Discussion
Vitamin D is transformed into its active hormonal conditions through hydroxylation of hormone precursors, e.g. into 25-hydroxyvitamin D in the liver and then into 1α, 25-dihydroxyvitamin D [1.25-(OH)2D] in the kidneys. This active hormonal state of vitamin D plays an essential role in bone homeostasis. Upon reaching the bones as one of its target tissue, several functions are discussed. The leading effect of the vitamin D hormone on bones is indirect and mediated through its endocrine function on mineral homeostasis [23]. However, also binding its receptor (vitamin D receptor or VDR) a receptor-regulated pathway acts regulating transcription of target genes responsible for conducting the physiological process of 1.25-(OH)2D [24, 25].
The physiological level of 25(OH)D3 still remains unclear and ranges from 12 ng/ml to 40 ng/ml mean [3, 22]. The observed mean level of 25(OH)D3 in our study ranged from 16.3 ± 5.2 ng/ml to 18.3 ± 5.1 ng/ml and is similar to mean values found with other authors, describing the mean 14.7 ± 6.4 ng/ml [26].
Concerning fracture repair, the time course of 25(OH)D3 was examined in chicks over periods of maximal 21 days. Increasing levels of 25(OH)D3 were observed in the chicks due to an increase in CYP24A1 activity, whereas serum calcium levels revealed the same. Exposure of kidney cells in cultures to serum obtained from chicks with fractures showed an increase in CYP24A1 activity compared to control serum. This suggests that 25(OH)D3 is involved in the early stages of fracture repair and that there is some form of physiological communication between the fractured bone and the kidneys leading to an increase of renal 24-hydroxylase and the circulating concentration of this metabolite [9].
In mice CYP24a1 mRNA levels were significantly elevated in the fracture callus compared to the callus of the undamaged contralateral bone [23] and in CYP24a1-/- mice a delay in the mineralization of the cartilagous matrix of the soft callus and an altered expression of differentiation marker genes were found [23].
The presence of a nonnuclear membrane receptor for 25(OH)D3 in the fracture healing callus has been suggested [27, 28] as well as a receptor/binding protein for 25(OH)D3 in the fracture healing callus membrane fraction from 25(OH)D3 depleted chicks [28].
Serum 25(OH)D3 levels after sustaining a fracture have only been measured in few studies with humans [10–12]. Significantly reduced levels were found 28 hours after sustaining the fracture.
There are two interesting studies from Meller et al. in humans in 1982 and 1984 (11,12). In the first study, regarding 25(OH)D3, 13 young adults who had sustained fractures of a long bone, pelvis and vertebral bodies were included. Blood samples were taken within 12 hours after sustaining the fracture and also after six to eight weeks (with the sign of primary callus). During the healing period of the fractures no significant changes could be detected in the selected two pints of time. In the second study he investigated a population of 41 geriatric patients with 43 fractures of the proximal femur. 31 fractures were treated operatively while 12 non-operatively. Again blood samples were taken at two points in time, i.e. 48 hours after administration and after 8 weeks. Concerning the time course of 25(OH)D3 again no significant changes could be demonstrated.
Our study is the first study to examen the time course of 25(OH)D3 during human fracture healing in normal BMD level bone and low BMD level bone in a time course with blood samples taken at four different points in time. We could not confirm the increase of 25(OH)D3 during the healing period, which we had expected, but rather a slight decrease. This heterogeneity may be explained by the different study designs, meaning results of animal studies may be different than human situation. Comparing our results with the studies of Meller, it only remains for us to show these first results as a basis for further investigations.
In addition, no significant differences could be detected between the normal and low BMD level groups in our study with regard to levels of 25(OH)D3. This contradicts reports of low levels of 25(OH)D3 in osteoporotic patients in the literature so far [1, 29]. In fact, there are other authors, who failed to show any significant correlation between 25(OH)D3 and BMD, e.g. Garnero et al. in the OFELY (Os de Femmes de Lyon) study [30] and others [11, 12, 31, 32] after adjusting for age.
The observed mean level of PTH ranged in our study from 34.6 ± 10.9 pg/ml to 40.8 ± 23.3 pg/ml and is similar to the mean values found of other authors, describing the mean as 47.9 ± 30.4 ng/ml [26].
Potentially, PTH can exert anabolic and catabolic effects on osteoblasts by initiating different signalling cascades [33]. To date, three receptors for PTH/PTHrP are known on osteoblasts (PTH1R-PTH3R) [34] inducing formation of cyclic 3’.5’-adenosine monophosphatase (cAMP) by activating the adenylate cyclase through stimulatory G-alpha proteins coupled to the receptor [33]. Studies in vitro and in vivo have shown that the N-terminal 1-34 synthetic fragment of the 84 amino acids of PTH mediates full PTH activity [35], eliciting a cAMP response and stimulation of bone formation [36]. Thus we propose activation of the bone anabolic effects of PTH especially in the first period after fracture, when most important stabilization processes occur.
In recent literature significant negative correlations between 25(OH)D3 and PTH have been described [22]. These findings support concur with the results of our study, where an increase of PTH in first week after fracture healing is accompanied by a slight decrease of 25(OH)D3.
One lack of our study is of course a missing control group with matching demographics without fractures, since especially 25(OH)D3 levels are dependent on multiple effects like oral supply or exposure to the sun. We had to accept this problem in our study design, as we rarely see patients without fractures in our trauma department.
Conclusions
Herewith the time course of 25(OH)D3 and PTH during fracture healing of normal and low BMD level bone have been examined in humans in this setting for the first time providing biological insights into fracture healing in metaphyseal bones on a molecular. There were no significant differences between normal and low BMD level patients. Further studies are necessary to obtain a more detailed insight into fracture healing in order to provide reliable decision criteria for therapy and monitoring of fracture healing.
Integrity of research and reporting
The study is approved by the local Ethical Committee of Heidelberg with the approval number 1572002. All patients gave their informed consent prior to their inclusion in the study.
References
Bischoff-Farrari HA, Dietrich T, Orav EJ, Dawson-Hughes B: Positive association between 25-hydroxy vitamin D levels and bone mineral density: a population-based study on younger and older adults. Am J Med. 2004, 1: 634-639.
Kuchuk NO, Pluijm SM, van Schoor NM, Looman CW, Smit JH, Lips P: Relationships of serum 25-hydroxyvitamin D to bone mineral density and serum parathyroid hormone and markers of bone turnover in older persons. J Clin Endocrinol Metab. 2009, 94: 1244-1250. 10.1210/jc.2008-1832.
Lips P, Hosking D, Lippuner K, Norquist JM, Wehren L, Maalouf G: The prevalence of vitamin D inadequacy amongst women with osteoporosis: an international epidemiological investigation. J Int Med. 2006, 260: 245-254. 10.1111/j.1365-2796.2006.01685.x.
Lips P: Vitamin D, deficiency and secondary hyperparathyroidism in the elderly: consequences for bone loss and fractures and therapeutic implications. Endocr Rev. 2001, 22: 477-501. 10.1210/edrv.22.4.0437.
Looker AC, Dawson-Hughes B, Calvo MS, Gunter EW, Sahyoun NR: Serum 25-hydroxyvitamin D status of adolescents and adults in two seasonal subpopulations from NHANES III. Bone. 2002, 30: 771-777. 10.1016/S8756-3282(02)00692-0.
Janssen HC, Samson MM, Verhaar HJ: Vitamin D deficiency, muscle function, and falls in elderly people. Am J Clin Nutr. 2002, 75: 611-615.
Sambrook PN, Seeman E, Phillips SR, Ebeling PR: Preventing osteoporosis: outcomes of the Australian Fracture Prevention Summit. The Medical Journal of Australia. 2002, 176: 1-16.
Party Writing Group. Osteoporosis: Clinical guidelines for Prevention and Treatment. Update on Pharmacological Interventions and an Algorithm for Management. 2001, London: Royal College of Physicians, 1-14.
Seo EG, Norman AW: Three-fold induction of renal 25-hydroxyvitamin D3-24-hydroxylase activity and increased serum 24,25-dihydroxyvitamin D3 levels are correlated with the healing process after chick tibial fracture. J Bone Miner Res. 1997, 12: 598-606. 10.1359/jbmr.1997.12.4.598.
Alkalay D, Shany S, Dekel S: Serum and bone vitamin D metabolites in elective patients and patients after fracture. J Bone Joint Surg Br. 1989, 71: 85-87.
Meller Y, Kestenbaum RS, Shany S, Galinsky D, Zuili I: Parathyroid hormone, calcitonin and vitamin D metabolites during normal fracture healing in humans a preliminary report. Clinical Orthopedics and Related Research. 1984, 183: 238-245.
Meller Y, Kestenbaum RS, Shany S, Galinsky D, Zuili I: Parathhormone, calcitonin and vitamin D metabolites during normal fracture healing in geriatric patients. Clinical Orthopedics and Related Research. 1985, 199: 272-274.
Partridge NC, Li X, Qin L: Understanding parathyroid hormone action. Annals of the New York Acededmy of Science. 2006, 1068: 187-193. 10.1196/annals.1346.024.
Gagnon C, Li V, Eberling PR: Osteoporosis in men: its pathophysiology and the role of teriparatide in its treatment. Clin Interv Aging. 2008, 3 (4): 635-645.
Strewler GJ, Stern PH, Jacobs JW, Eveloff L, Klein RF, Leung SC: Parathyroid hormonelike protein from human renal carcinoma cells. Structural and functional homology with parathyroid hormone. J Clin Invest. 1987, 80: 1803-1807. 10.1172/JCI113275.
Teitelbaum SL: Bone resorption by osteoclasts. Science. 2000, 289: 1504-1508. 10.1126/science.289.5484.1504.
Samadfam R, Xia Q, Miao D, Hendy GN, Goltzman D: Exogenous PTH and endogenous 1,25-dihydroxyvitamin D Are complementary in inducing an anabolic effect on bone. J Bone Miner Res. 2008, 23: 1257-1266. 10.1359/jbmr.080318.
Tam CS, Heersche JN, Murray TM, Parsons JA: Parathyroid hormone stimulates the bone apposition rate independently of ist resorptive action: differential effects of intermittent and continuous administration. Endocrinology. 1982, 110: 506-512. 10.1210/endo-110-2-506.
Goltzman D: Studies on the mechanisms of the skeletal anabolic action of endogenous and exogenous parathyroid hormone. Arch Biochem Biophys. 2008, 473: 218-224. 10.1016/j.abb.2008.03.003.
Sone T, Fukunaga M, Ono S, Nishiyama T: A small dose of human parathyroid hormone(1-34) increased bone mass in the lumbar vertebrae in patients with senile osteoporosis. Miner Electrolyte Metab. 1995, 21: 232-235.
Hodsman AB, Bauer DC, Dempster D, Dian L, Hanley DA, Harris ST: Parathyroid hormone and teriparatide fort he treatment of osteoporosis: a review of the evidence and suggested guidelines for its use. Endocr Rev. 2005, 26: 688-703. 10.1210/er.2004-0006.
Komrakova M, Stuermer EK, Werner C, Wicke M, Kolios L, Sehmisch S: Effect of human parathyroid hormone hPTH (1-34) applied at different regimes on fracture healing and muscle in ovariectomized and healthy rats. Bone. 2010, 47: 480-492. 10.1016/j.bone.2010.05.013.
St-Arnaud R, Naja RP: Vitamin D metabolism, cartilage and bone fracture repair. Mol Cel Endocr. 2011, 347: 48-54. 10.1016/j.mce.2011.05.018.
Haussler MR, Whitfield GK, Haussler CA, Hsieh JC, Thompson PD, Selznick SH: The nuclear vitamin D receptor: biological and molecular regulatory properties revealed. J Bone Miner Res. 1998, 13: 325-349. 10.1359/jbmr.1998.13.3.325.
Norman AW: Minireview: vitamin D receptor: new assignments for an already busy receptor. Endocrinology. 2006, 147: 5542-5548. 10.1210/en.2006-0946.
Arabi A, Baddoura R, El-Rassi R, El-Hajj Fuleihan G: PTH level but not 25 (OH) vitamin D level predicts bone loss rates in the elderly. Osteoporos Int. 2011, 10.1007/s00198-011-1659-1. published online 08 June
Seo EG, Kato A, Norman AW: Evidence for a 24R, 25(OH)2-vitamin D3 receptor/binding protein in a membrane fraction isolated from a chick tibial fracture-healing callus. Biochem Biophys Res Commun. 1196, 225: 203-208.
Kato A, Seo EG, Einhorn TA, Bishop JE, Norman AW: Studies on 24R, 25-dihydroxyvitamin D3: evidence for a nonnuclear membrane receptor in the chick tibial fracture-healing callus. Bone. 1998, 23: 141-146. 10.1016/S8756-3282(98)00085-4.
Sadat-Ali M, Al Elq AH, Al-Turki HA, AL-Mulhim FA, Al-Ali AK: Influence of vitamin D levels on bone mineral density and osteoporosis. Ann Saudi Med. 2011, 31: 602-608. 10.4103/0256-4947.87097.
GArnero P, Munoz F, Sornay-Rendu E, Delmas PD: Associations of vitamin D status with bone mineral density, bone turnover, bone loss and fracture risk in healthy postmenopausal women. The OFELY study. Bone. 2007, 40: 716-722. 10.1016/j.bone.2006.09.026.
Hosseinpanah F, Rambod M, Hossein-nejad A, Larijani B, Azizi F: Association between vitamin D and bone mineral density in Iranian postmenopausal women. J Bone Miner Metab. 2008, 26: 86-92. 10.1007/s00774-007-0791-7.
Akhter N, Sinnott B, Mahmood K, Rao S, Kukreja S, Barengolts E: Effects of vitamin D insufficiency on bone mineral density in African American men. Osteoporos Int. 2009, 20: 745-750. 10.1007/s00198-008-0746-4.
Swarthout JT, D’Alonzo RC, Selvamuruga N, Partridge NC: Parathyroid hormone-dependent signaling pathways regulating genes in bone cells. Gene. 2002, 282: 1-17. 10.1016/S0378-1119(01)00798-3.
Abou-Samra AB, Juppner H, Force T, Freeman MW, Kong XF, Schipani E: Espression cloning of s common receptor for parathyroid hormone and parathyroid hormone-related peptide from rat osteoblast-like cells; a single receptor stimulates intracellular accumulation of both cAMP and inositol triphosphates and increases intracellular free calcium. Proc Natl Acad Sci USA. 1992, 89: 2732-2736. 10.1073/pnas.89.7.2732.
Habener JF, Rosenblatt M, Potts JT: Parathyroid hormone: biochemical aspects of biosynthesis, secretion, action, and metabolism. Physiol Rev. 1984, 64: 985-1053.
Mosekilde L, Sogaard CH, Danielsen CC, Torring O: The anabolic effects of human parathyroid hormone (hPTH) on rat vertebral body mass are also reflected in the quality of bone, assessed by biomechanical testing: a comparison study between hPTH-(1-34) and hPTH-(1-84). Endocrinology. 1991, 129: 421-428. 10.1210/endo-129-1-421.
Pre-publication history
The pre-publication history for this paper can be accessed here:http://www.biomedcentral.com/1471-2474/14/6/prepub
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
CW planned the study, participated in the evaluation of patients, data, statistics and wrote the paper, he has seen and approved the final version as the corresponding author. SE participated in planning of the study, evaluation of patients and data and has seen and approved the final version. AM participated in planning of the study, evaluation of patients, data and statistics and has seen and approved the final version. GZ participated in planning of the study, evaluation of patients, data and statistics and has seen and approved the final version, HS-G participated essentially in establishment of laboratory analyses, their evaluation and statistics, but unfortunately died before submission of this study. BH participated substantially in planning of the study and performing of statistics and has seen and approved the final version. AH participated in the evaluation of patients, revision of the written manuscript and has seen and approved the final version. PAG participated in planning of the study, supervision of the study, evaluation of patients, data and statistics and has seen and approved the final version. LK participated substantially in planning of the study, supervision of the study, evaluation of patients, data and statistics and has seen and approved the final version. All authors read and approved the final manuscript.
An erratum to this article is available at http://dx.doi.org/10.1186/1471-2474-15-70.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
This article is published under license to BioMed Central Ltd. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Wöfl, C., Englert, S., Moghaddam, A.A. et al. Time course of 25(OH)D3 vitamin D3 as well as PTH (parathyroid hormone) during fracture healing of patients with normal and low bone mineral density (BMD). BMC Musculoskelet Disord 14, 6 (2013). https://doi.org/10.1186/1471-2474-14-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1471-2474-14-6