Abstract
Background
The World Health Organization (WHO) recommends combinations of an artemisinin derivative plus an anti-malarial drug of longer half-life as treatment options for uncomplicated Plasmodium falciparum infections. In Africa, artesunate–mefloquine (ASMQ) is an infrequently used artemisinin-based combination therapy (ACT) because of perceived poor tolerance to mefloquine. However, the WHO has recommended reconsideration of the use of ASMQ in Africa. In this large clinical study, the pharmacokinetics (PK) of a fixed dose combination of ASMQ was investigated in an African paediatric population to support dosing recommendations used in Southeast Asia and South America.
Methods
Among the 472 paediatric patients aged 6–59 months from six African centres included in the large clinical trial, a subset of 50 Kenyan children underwent intensive sampling to develop AS, its metabolite dihydroartemisinin (DHA) and MQ PK models. The final MQ PK model was validated using sparse data collected in the remaining participants (NONMEM®). The doses were one or two tablets containing 25/55 mg AS/MQ administered once a day for 3 days according to patients’ age. A sensitive LC–MS/MS method was used to quantify AS, DHA and MQ concentrations in plasma. An attempt was made to investigate the relationship between the absence/presence of malaria recrudescence and MQ area under the curve (AUC) using logistic regression.
Results
AS/DHA concentration–time profiles were best described using a one-compartment model for both compounds with irreversible AS conversion into DHA. AS/DHA PK were characterized by a significant degree of variability. Body weight affected DHA PK parameters. MQ PK was characterized by a two-compartment model and a large degree of variability. Allometric scaling of MQ clearances and volumes of distribution was used to depict the relationship between MQ PK and body weight. No association was found between the model predicted AUC and appearance of recrudescence.
Conclusions
The population pharmacokinetic models developed for both AS/DHA and MQ showed a large variability in drug exposure in the investigated African paediatric population. The largest contributor to this variability was body weight, which is accommodated for by the ASMQ fixed dose combination (FDC) dosing recommendation. Besides body weight considerations, there is no indication that the dosage should be modified in children with malaria compared to adults.
Trial registration Pan African Clinical Trials Registry PACTR201202000278282 registration date 2011/02/16
Similar content being viewed by others
Background
The World Health Organization (WHO) estimates a significant 18% reduction in the incidence of malaria along with a considerable 28% decrease in the malaria mortality rate between 2010 and 2017 [1]. Despite this substantial progress, the disease still caused an estimated 435,000 deaths worldwide, mostly in Africa (93%) and in children under 5 years of age (61%) [1]. Artemisinin-based combination therapy (ACT) is the first-line treatment for uncomplicated Plasmodium falciparum infection, the predominant cause of malaria in Africa, recommended by the WHO since 2001 [2]. These combinations involve a rapidly eliminated and fast-acting artemisinin derivative together with a much more slowly eliminated drug that kills the remaining parasites. One of the five WHO recommended artemisinin-based combinations is artesunate (AS) associated with mefloquine (MQ), extensively used in Asia and Latin America for the last 20 years [3]. This combination is less commonly selected in Africa, because of the availability of other affordable and already registered artemisinin-based combinations [4], as well as existing concerns about MQ tolerability [5, 6]. However, the WHO has recommended reconsideration of the use of ASMQ in Africa in order to increase the number of artemisinin-based combinations available, with the consequent reduction of the risk of developing drug resistance [4].
The development of a fixed-dose combination (FDC) of AS and MQ was begun in 2002 by the Drugs for Neglected Diseases initiative (DNDi) with the fixed-dose artesunate-based combination therapy (FACT) Consortium [3]. This combination has been demonstrated to be efficacious and safe in Asia and Latin America [7,8,9], but there is still limited experience with its use in Africa. Therefore, an open-label, prospective, randomized, controlled, multi-centre, non-inferiority clinical trial evaluating the efficacy, safety and pharmacokinetics of the ASMQ FDC versus artemether–lumefantrine (AMLF) in children aged 6–59 months was conducted in Africa by DNDi (Pan African Clinical Trials Registry number PACTR201202000278282). Because MQ dose splitting into three equal daily doses has been shown to optimize treatment compliance and to improve MQ tolerability [10, 11], FDC ASMQ dispersible tablets were administered over three consecutive days based on the patients’ age. The efficacy of ASMQ was found to be non-inferior to the efficacy of AMLF and the safety of the two treatments was found to be similar with low risk of repeated early vomiting, indicating that ASMQ is a valuable treatment option for children younger than 5 years with uncomplicated falciparum malaria in Africa [12]. Within the framework of this previous study, a pharmacokinetic study was conducted to characterize ASMQ FDC pharmacokinetics in the African paediatric patient population, to compare it to data gathered in adult patients and volunteers, to validate the recommended treatment regimen, and to explore the relationships between drug exposure and treatment outcomes.
Methods
Study design and participants
The clinical trial was carried out in six African centres: three in Tanzania, two in Burkina Faso and one in Kenya. Written informed consent from a parent/guardian was required to enrol children younger than 5 years in the trial, who were infected by P. falciparum, as confirmed by microscopy (density between 2000 and 200,000 asexual parasites/µL), and with fever equal to or higher than 37.5 °C. Exclusion criteria were children with body weight less than 5 kg, signs of severe/complicated malaria, febrile conditions caused by diseases other than malaria, a known hypersensitivity to the study drugs, a mixed plasmodium infection, a history of anti-malarial treatment in the 2 weeks preceding the trial or 4 weeks in case of mefloquine and piperaquine, prior participation in a therapeutic trial within 3 months or inability to tolerate oral medication. Patients were followed up to day 63 after start of treatment or to the first recurrence of infection. The study protocol was reviewed and approved by national and independent ethics committees of all participating centres.
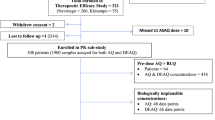
Of the 945 patients enrolled in the trial, 473 were randomized to the ASMQ arm (one of them was never dosed) and 472 were randomized to the AMLF arm. The pharmacokinetic analysis described here was performed on the 472 patients who received ASMQ.
Administered doses for these patients were one or two dispersible tablets containing 25 mg AS and 55 mg MQ once a day for three consecutive days to children aged from 6 to 11 months and from 12 to 59 months, respectively. Clinical and parasitological examinations were scheduled at baseline, i.e. before drug administration, at day 0 (D0), D1, D2, D3, D7, D14, D21, D28, D35, D42, D49, D56 and D63 and on any other day if the patient spontaneously returned and parasitological reassessment was required (as per protocol). A margin of ± 2 days to the assigned day of visit was allowed from D7 onward. In case of recurrence of parasitaemia on D7, D14, D21, D28, D35, D42, D49, and D56 the date was recorded and the type of recurrence was determined by PCR (appearance of new infection, malaria recrudescence, missing PCR information or undetermined type).
According to the study protocol, the first fifty children from Kenya enrolled in the ASMQ arm underwent intensive blood sampling: at baseline, on D0 after drug administration (until 6 h after first dosing), D2 (until 6 h after the third dose), D3 (72 h after first dose), D7 and on one other occasion on day 28, 35, 42, 49, 56 or 63. Two blood samples, at baseline and on D7, were collected for all the other participants. Additionally, for all patients with recurrence of parasitaemia, a blood sample was taken on the day of failure.
Analytical methods
The mass spectrometry assay for AS, DHA and MQ used for the analysis of study samples is an adaptation of a previously published validated multiplex method [13]. The assay has been further improved by the use of stable isotopically labelled internal standards for MQ (mefloquine-d9) and DHA (DHA-13Cd4) to circumvent the potential matrix effect that may affect the accuracy of mass detection.
The mobile phase was delivered at a flow rate of 0.3 mL/min on a 2.1 mm × 75 mm XSelect HSS 3.5 μm column (Waters, Milford, MA, USA), using solvent A (2 mM ammonium acetate + 0.1% FA) and solvent B (MeCN + 0.1% FA) distributed according to the following stepwise gradient program: 98% A: 0 min; 98% A → 15% A: from 0.0 min → 13.0 min followed by a re-equilibration step to the initial solvent proportions. The retention time of mefloquine/mefloquine-d9, DHA/DHA-13Cd4 and artesunate is 7.4 min, 8.2 min and 9.2 min, respectively. The chromatographic system was coupled to a triple stage quadrupole (TSQ) Quantum Ion mass spectrometer (MS) from Thermo Fischer Scientific (Waltham, MA, USA) equipped with an Ion Max electrospray ionization (ESI) interface. The limits of quantification (LOQ) of the method are 2.5 ng/mL for MQ and 2 ng/mL for AS and DHA.
Plasma samples were isolated by centrifugation and stored at − 20 °C until batch analysis. Briefly, 100 μL of plasma sample were mixed with 50 µL internal standard (DHA-13Cd4 at 130 ng/mL; mefloquine-d9 at 43 ng/mL) and extracted with 600 µL of acetonitrile. The supernatant (700 µL) was evaporated under nitrogen at room temperature and was reconstituted in 150 µL of MeOH/ammonium acetate 2 mM (1:1) adjusted with formic acid at 0.1%, vortex-mixed and centrifuged again. The samples were maintained at +5 °C in autosampler racks throughout the analytical series. The injection volume was 20 μL.
The method is precise (with mean inter-day CV % < 10%), and accurate (inter-day deviation from nominal values < 5%). Since its initiation, the laboratory has participated in the Pharmacology Proficiency Testing Programme for anti-malarial drugs (http://www.wwarn.org/toolkit/qaqc) organized by the World Wide Antimalarial Resistance Network WWARN (http://www.wwarn.org/).
Pharmacokinetics analysis
Non-linear mixed effects modelling program (NONMEM®, version 7.3) [14] with the Perl-Speaks NONMEM® (PsN) toolkit (version 3.7.6) [15] was used to estimate average population pharmacokinetic parameters and their associated between-subject variability (BSV) and to identify factors that influence them. MQ and AS/DHA pharmacokinetic models were developed on the data collected from 50 Kenyan patient subjects with extensive sampling. Molar units were used for AS/DHA pharmacokinetic analyses. Because of the very fast rate of AS and DHA elimination and the selection of the trial sampling times, an external model validation could only be performed for MQ on the clinical trial data not used for model-building. Graphical exploration and statistical analyses were performed by means of the R package (version 2.15.1, R Development Core Team, http://www.r-project.org/).
Structural and statistical model
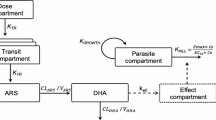
A stepwise modelling approach was undertaken to identify models that best described the MQ and AS/DHA pharmacokinetics. Multi-compartment dispositions with first-order absorption and elimination processes were compared for MQ. Due to the restricted amount of AS and DHA data, drug and metabolite pharmacokinetics were modelled simultaneously and directly described by means of a one compartment model with linear absorption and elimination. Moreover, since AS is rapidly and almost completely hydrolysed in DHA, its elimination was assumed to occur exclusively via irreversible conversion to DHA [16, 17]. An adequate AS absorption rate constant (Ka) estimation could not be made because of the small number of samples collected right after dose intake (one sample at maximum for each enrolled child on the first and third treatment day). Ka was thus fixed to 3.2 h−1, the mean of previously published estimates retrieved from papers using a first-order process to depict AS absorption [17, 18].
Parameterization was performed in terms of clearances (CL for drugs and CLM for metabolite), inter-compartmental clearance (Q), central (VC for drugs and VM for metabolite) and peripheral (VP) volumes of distribution and Ka. The metabolic conversion rate from AS to DHA was estimated by CL/Vc as previously discussed. AS and MQ relative bioavailability (F1, fixed to 100% and with estimated BSV) were also tested for AS/DHA and MQ to account for dose variation with respect to the nominal value due to the administration of water dispersible tablets. Since the ASMQ combination is administered orally, the pharmacokinetic parameter estimates represent apparent values.
Exponential errors were assumed to capture BSV in all the pharmacokinetic parameters. Proportional, additive and combined proportional-additive error models were compared to describe drugs and metabolite intra-patient (residual) variability. Finally, the correlation between AS and DHA concentration measurements was tested using the L2 function in NONMEM®.
Covariate analysis
Available covariates were: body weight (BW), height/length, age, sex, creatinine, total bilirubin (BIL), aspartate (AST) and alanine (ALT) aminotransferases, haemoglobin (Hb), haematocrit (Ht), total parasitaemia and co-medications categorized as CYP3A4 inducers. Visual inspection of the correlation between post hoc individual estimates of the pharmacokinetic parameters and the available patients’ characteristics was initially conducted to identify potential physiologically plausible relationships. Creatinine clearance was not evaluated since MQ elimination occurs mainly through non-renal processes and AS is completely converted into DHA, which is eliminated via glucuronidation [16]. A stepwise forward insertion/backward deletion approach was then undertaken. Potential covariates influencing the kinetic parameters were first incorporated one at a time and tested for significance (univariate analysis). Sequential multivariate combinations of the identified factors were investigated to discard redundancies and to build an intermediate model with all the most important covariates (multivariate analysis). Finally, backward deletion consisted of removing covariates one at a time from the intermediate model, starting from the most insignificant until no further deterioration of the model was observed.
The influence of body weight on all MQ and DHA pharmacokinetic parameters (PAR) was tested using allometric scaling:
with θ PAR population estimate, MBW the median population body weight and PWR the function power fixed to 0.75 for clearances and 1 for volumes of distribution [19]. A linear relationship between the typical value of a parameter and all the other covariates (continuous centered on the population median; dichotomous coded as 0 and 1) was used. Additionally, AST, ALT and BIL were implemented in the model as dichotomous variables, by introducing a boundary condition, i.e. below or exceeding 1.5 times the upper limit of normal (ULN). Children’s age was used to investigate the impact of organ maturation on MQ and DHA clearances, using the following equations, in addition to the simple linear one:
where Hill is the sigmoid power, TM50 the AGE at 50% of maturation, MATmag, the maturation magnitude for age, and Kmat the age maturation rate constant [20, 21]. The population median covariate value was assigned to patients with missing information.
The acute phase of malaria is associated with altered gastrointestinal motility and an increased likelihood of vomiting. In the three-daily dose ASMQ regimen, the second dose is administered when the patient is in an improved state of health, thanks to the first dose of AS, that kills most of the parasites [22]. The potential impact of parasitaemia on AS and MQ F1 was studied using a linear model of log-transformed (base 10) parasite counts measured at baseline of each ASMQ administration day. Missing parasitaemia information was imputed at the median value of the specific study day. Treatment day (0 vs. 1 and 2), considered as a surrogate marker of the rapid improvement in health due to the first AS dose, was also evaluated on AS and MQ F1. Since parasite counts and treatment day are correlated, differences in individual day 0 F1 due to parasitaemia at enrolment were explored, i.e. baseline parasite counts recorded at the first treatment day, by combining these two covariates. Furthermore, it was hypothesized that a patient’s clinical condition affects MQ Ka and this was tested by integrating the effect of the treatment day (0 vs. 1 and 2) on Ka.
Terminal half-lives (t1/2), maximum concentration (Cmax), and time to achieve Cmax (tmax) for all the three drugs, MQ area under the curve to infinite (AUC0–inf) and AS and DHA AUC0–24 after the first and the third ASMQ intake were computed using final pharmacokinetic parameter estimates and classic pharmacokinetic equations or NONMEM integration, as appropriate.
Parameter estimation, model selection and exclusion criteria
MQ and AS/DHA concentrations were fitted using the first-order conditional (FOCE) method with interaction. AS and DHA non-zero concentrations measured more than a week after last drug intake were thought unreliable and thus omitted from the analysis. Other missing variables (unreported concentration measurements, dose intake or sampling times, inconsistent date/time of dose intake and sampling) were also omitted. Data below the quantification limit (BQL) of the assays were handled by setting the first of a series of BQL samples at LOQ/2 and as missing all the others (M6 method) [23].
Diagnostic goodness-of-fit plots, along with differences in the NONMEM® objective function value (ΔOFV), were employed to discriminate between nested models. Since a ΔOFV between any two hierarchical models approximates a χ2 distribution, a change of more than 3.84 (p < 0.05) and 6.63 (p < 0.01) points was considered statistically significant for one additional parameter in model-building or forward insertion and backward-deletion covariate steps, respectively. Akaike’s information criterion (AIC) was used for non-hierarchical models. Shrinkage was also evaluated. Sensitivity analyses removing outlying data with absolute conditional weighted residuals (CWRES) greater than 4 or potentially unreliable covariate values and concentration measurements were finally performed to avoid any potential bias in parameter estimation and covariate exploration.
Model validation and assessment
The stability of the final MQ and AS/DHA models was assessed by means of the bootstrap method implemented in PsN-Toolkit [15]. Median parameter values with their 95% confidence interval (CI95%) were derived from 2000 replicates of the initial datasets and compared with the original estimates. Prediction-corrected visual predictive checks (pcVPC) were also performed using the PsN-Toolkit and the R package Xpose4 by simulations based on the final pharmacokinetic models with variability using 1000 children [15, 24]. Moreover, the final MQ pharmacokinetic model was validated using concentrations collected from participants not used in initial model development. The accuracy and precision of the model were estimated by means of prediction error (MPE) and root mean square error (RMSE), using log-transformed concentrations, for the entire dataset and also for each study site [25].
Comparison between mefloquine exposures in children and adult volunteers and patients
Median and 90% prediction interval (PI90 %) of children and adult concentration–time profiles were obtained through simulations (n = 1000) using the final pharmacokinetic model described above and published MQ pharmacokinetic models including BSV and intra-individual variability, respectively. A literature search allowed the identification of two pharmacokinetic models developed in adults receiving the same fixed dose formulation of ASMQ as the one administered to the children enrolled in this clinical trial [26, 27]. The investigated populations consisted of Indian adult patients and Thai adult patients and volunteers, administered with 400 mg of MQ once per day over three consecutive days. MQ disposition was described by a two compartment model with linear elimination in both analyses. A first-order and a single transit compartment models in Julien et al. [26] and Reuter et al. [27], respectively, characterized the absorption phase. The two models were implemented in NONMEM®, fixing simulated individuals’ body weight to the corresponding median population value. Administered MQ doses were 110 mg and 400 mg over three consecutive days for children and adults, respectively. MQ drug exposure was quantified by computing median and PI95% AUC over the whole study period (AUC0–day63) by NONMEM integration for all the simulated population/model.
Mefloquine pharmacokinetic–pharmacodynamic analysis
This exploratory analysis was carried out on MQ data collected from all children participating in the trial with complete dosing history information that did not drop out in the early days of the study. Model predicted MQ cumulative AUC (AUC0–dayx) on study days 7, 28, 42, and 63 were calculated by concentration integration in NONMEM®. The relationship between recrudescence of infection (response variable, coded as 0/1) and model predicted AUC0–dayx (independent variable) on study days 7, 28, 42, and 63 was inspected by means of logistic regression using STATA (StataCorp. 2013. Stata Statistical Software: Release 13. College Station, TX: StataCorp LP). The independent variable was log-transformed (using base 2) and cantered on its median value. The level of significance was set at 0.05.
Results
Of the 472 children enrolled in the trial and randomized in the ASMQ arm, 21 were removed according to the exclusion criteria of the pharmacokinetic analysis. MQ and AS/DHA pharmacokinetic model development was carried out on 48 patients and MQ model validation on 378 patients, after removal of subjects with unreliable data. The characteristics of the patients used in the MQ and AS/DHA model-building, as well as the final MQ model validation and MQ pharmacokinetic–pharmacodynamic analysis datasets, are listed in Table 1.
Population pharmacokinetic analysis
A total of 216 MQ, 117 AS and 134 DHA (including BQL) concentrations were available for the 48 Kenyan patients selected for the pharmacokinetic model development. Of note, none of the MQ concentrations were quantified as a BQL, while 71% and 57% of AS and DHA samples were BQL data. Median (range) treatment duration per study subject was 3 days (1–3) and the number of available non-BQL samples was 5 (1–7) for MQ, 1 (1–2) for AS and 2 (1–3) for DHA. MQ concentrations ranged between 0.17 ng/mL and 6552.51 ng/mL, AS (> BQL) between 2.1 and 8469.8 ng/mL and DHA (> BQL) between 2.9 and 2400.9 ng/mL.
Artesunate and dihydroartemisinin
Structural and statistical model
As previously described, a two-compartment model was used to simultaneously fit AS and DHA data with first-order absorption, drug exclusive elimination via irreversible conversion to DHA and first-order elimination of metabolite. Initially, BSV was assigned only on CL and a mixed error model was assumed for the intra-patient variability of both drug and metabolite. Model stability was achieved by integrating a correlation between AS and DHA concentration measurements (ΔOFV = − 25, p < 0.001). BSV on VC did not improve data description (ΔOFV = 0, p > 0.05) whilst assignment of BSV to CLM (ΔOFV = − 7.3, p < 0.01) and to VM (ΔOFV = − 8.0, p < 0.01) yielded a better fit of the data. Inclusion of relative F1 (fixed to 100% with estimated BSV) explained all the BSV on AS and DHA clearance and significant decreased the OFV (ΔOFV = − 17.7, p < 0.01). The estimates and variability (CV%) of the pharmacokinetic parameters obtained by the base population model were a relative F1 of 100% (67%), a CL of 180 L/h, a VC of 166 L, a CLM of 12.5 L/h and a VM of 13.8 L (57%).
Covariate analysis
Age, sex and BIL as well as the hepatic liver tests ALT and AST had a significant impact on F1 (ΔOFV < − 9.6, p < 0.01). Because of poor effect estimation (relative standard error, RSE = 155%), BIL was not kept for further covariate analyses. Sensitivity analyses revealed that the effect of ALT and AST on F1 were purely due to a single patient having the highest values for both hepatic enzyme tests. Whether this finding was a true or an incidental effect could not be validated and these covariates were thus not retained in the model. F1 was found to increase with the parasite counts (ΔOFV = − 13.2, p < 0.01), and to be higher at day 0 compared to days 1 and 2 of treatment (ΔOFV = − 13.7, p < 0.01). As shown in Table 1, baseline parasite counts were extremely high before starting the anti-malarial treatment and dropped to 0 before administration of the third ASMQ, a consequence of the important and immediate AS effect. Differences in F1 at day 0 related to parasite counts were investigated but did not improve the fit with respect to the model including only the treatment day or the parasite counts as covariate (ΔOFV < 3.8, p > 0.05). Because of the correlation between the two factors and the absence of fit improvement by combining the parasite information and the treatment day, only the latter was kept in the model. BW allometric scaling on CLM and VM markedly decreased the objective function (AIC difference of − 22 with respect to the basic structural model). Maturation on CLM was adequately described using Eq. 3 and improved the model fit (ΔOFV = − 18.9, p < 0.01). VM was significantly impacted by sex (ΔOFV = − 8.8, p < 0.01). Complete multivariate analyses allowed for the effect of sex on VM and F1 to be discarded, as well as that of maturation on DHA clearance. These results show that F1 is reduced by 68% upon doubling child age with respect to the population median (2.6 years), and is 29% higher in the first day of therapy than in the subsequent treatment days. The effect of BW on CLM and VM was also retained.
Model evaluation and assessment
The final model parameter estimates, together with their bootstrap estimations, are shown in Table 2 and the goodness-of-fit plots in Additional file 1. Model predicted secondary parameters are presented in Table 4. Shrinkage was lower than 30% for BSV and 10% for residual variabilities. The model was considered reliable since the parameter estimates were within the bootstrap CI95% and differed less than 15% from their bootstrap estimations. Prediction corrected VPCs shown in fig. 1 evidence model misspecification. However, the model was judged acceptable because of the paucity of available AS/DHA data.
Prediction corrected visual predictive check of the final model of a artesunate and b dihydroartemisinin. Open circles represent prediction corrected observed plasma concentration; black solid and dashed lines the median and PI90% of the observed data; shaded magenta and grey surfaces the model predicted 90% confidence interval of the simulated median and PI90%, respectively; horizontal black lines are the LOQ of AS (0.005 nmol/mL) and DHA (0.005 nmol/mL). The lower panels show the fraction of observed (open circles) with the PI95% of the simulated (shaded magenta surface) BQL data
Mefloquine
Structural and statistical model
A two-compartment model with first-order absorption and elimination described MQ data better than a one-compartment model (ΔOFV = − 64, p < 0.001). No additional benefit was observed using three compartments (ΔOFV = − 0.9, p > 0.05). BSV on VC (ΔOFV = − 22, p < 0.001) in addition to CL yielded a better fit of the data, which was further enhanced by inclusion of BSV on Ka (ΔOFV = − 19, p < 0.001). No improvement of the model fit was observed associating BSV on Q or VP (ΔOFV = 0, p > 0.05). The inclusion of MQ F1 fixed to 100% with an estimated BSV significantly decreased the OFV whilst explaining all the BSV associated to VC (ΔOFV = − 9.4, p < 0.01). Finally, a proportional model was retained to describe the intra-patient variability. The estimates and variability (CV %) of the pharmacokinetic parameters obtained by the base population model were an F1 of 100% (39%), a CL of 0.48 L/h (40%), a VC of 88 L, a Q of 0.41 L/h, a VP of 69 L, and a Ka of 0.15 h−1 (87%).
Covariate analysis
The univariate analyses showed no association between the covariates tested and MQ bioavailability, clearances and volumes of distribution (ΔOFV ≥ − 3.2, p > 0.05; AIC difference of 2 points with respect to the structural model for BW on all the PK parameters). However, the sensitivity analysis performed while removing the patient with extremely low concentrations after the second and third ASMQ dose revealed that this outlier masked the real impact of BW on clearances and volumes of distribution and of age on F1 (AIC difference of − 5 and ΔOFV = − 5.4, p < 0.05, respectively), without inducing any modification in the MQ basic model. Sex and age were found to significantly influence Ka (ΔOFV ≤ − 7.0, p < 0.05). A decrease of 74% in Ka was observed while doubling the age with respect to the population median (2.6 year) and female children had 55% lower Ka than male children. Multivariate analysis showed that age accounted for the effect of sex on Ka and allowed for the discarding of the impact of age on F1. Finally, significantly different Ka at day 0 and 1/2 of ASMQ treatment were identified due to improvement in patient health following the first intake of AS (ΔOFV = − 39.2, p < 0.001). Multivariate and backward deletion step analyses performed using the reduced dataset, obtained by removal of the outlying patient, showed that the BW effect on clearances and volumes of distribution, as well as age and treatment day effect on Ka, should be retained in the final MQ pharmacokinetic model.
Model evaluation and assessment
The final model parameters, together with their bootstrap estimations, are displayed in Table 3 and the goodness-of-fit plots presented in Additional file 2. Model predicted secondary parameters are shown in Table 4. Shrinkage was 28% for residual variability and lower than 15% for BSV. The model was considered reliable since the parameters were within the bootstrap CI95% and differed less than 5% from the bootstrap estimations. The results of the pcVPC (Fig. 2) support the predictive performance of the model. Moreover, the external validation done using the remaining 538 concentrations from 378 children enrolled in the trial showed a negligible bias of 0% (CI95% − 2 to 1%) with a precision of 16% at an individual level. A small bias of 18% (CI95% 13–24%) with a precision of 81% was calculated for population predictions. Non-significant or small (absolute values ≤ 6%) biases were calculated at each study site on an individual level (Table 5). Furthermore, the precision of drug predictions was close to the estimated residual intra-patient variability, which strongly supports the predictive performance of the model (Table 5).
Prediction corrected visual predictive check of the final model with MQ prediction corrected plasma concentration (open circles) and quartiles (black solid and dashed lines) with model-based percentiles 90% confidence interval (shaded magenta and grey surfaces for the median and low/high percentiles, respectively). Horizontal black line represents the MQ LOQ (2.5 ng/mL)
Comparison between mefloquine exposures in children and adult volunteers and patients
Horizontal black line represents the MQ LOQ (2.5 ng/mL).
Figure 3 compares the model-predicted AUC0–day63 for children and adult volunteers and patients. Median (PI95%) AUC0–day63 of 725 mg/L/h (310–1718) was computed through simulations of the final pharmacokinetic model for children weighting 12.2 kg and taking 110 mg of MQ once per day over three consecutive days. Adult patients had a median (PI95%) AUC0–day63 of 1080 mg/L/h (599–1911) and 936 mg/L/h (570–1413) calculated using the model of Julien et al. and Reuter et al. respectively, while adult volunteers of 865 mg/L/h (555–1211) under the dosage regimen of MQ 400 mg once per day over three consecutive days. Median (PI90%) concentration time profiles for adult and children patient are shown in Fig. 4.
Median and 90% prediction intervals of MQ concentration–time profiles for children and adult patients receiving 110 mg and 400 mg of MQ once per day over three consecutive days obtained with this study (children, magenta solid line and shaded surface), the Julien et al. (adult, light grey line and shaded surface), and Reuter et al. (adult, dark grey line and shaded surface) models, respectively [26, 27]
Mefloquine pharmacokinetic-pharmacodynamic analysis
Treatment failure was reported for 212 (56%) of the children enrolled in the study, of these failures, 81% (n = 171) were due to new infections and 7% (15) to recrudescence during the 63 days of follow-up. In 2% of the enrolled individuals PCR information was missing and in 10% it was not possible to determine the nature of the treatment failure. Median (range) model-predicted AUC0–day7 were estimated to be 281 mg/L/h (70–854 mg/L/h) in children with reported treatment success within the follow-up period, and 286 mg/L/h (167–378 mg/L/h) and 286 mg/L/h (70–579 mg/L/h) for children with or without malaria recrudescence, respectively. No significant associations were found through logistic regression between model-predicted AUC0–day at day 7, 28, 42 or 63 and appearance/absence of recrudescence (p > 0.05) (data not shown for day > 7).
Discussion
The present analysis describes the pharmacokinetics of fixed-dose ASMQ in African children under the age of 5 years, with the aim of identifying the source of the significant variability in drug exposure and validating the recommended weight-for-age dosage regimen. The very short half-lives estimated for AS and DHA are in good agreement with reported values ranging from 22 to 72 min for the drug and from 30 to 186 min for the metabolite [28, 29]. The calculated tmax for AS and DHA agree with reported peak AS and DHA plasma concentrations within the 1st h and 2 h post-dose, also supporting the appropriateness of the value chosen for AS Ka in this work [28, 29].
A two-compartment model was used to describe mefloquine pharmacokinetics as already shown in previous analyses [26, 27]. Drug clearance and central and peripheral volumes of distribution were found to be markedly lower than the values estimated in adult patients of African, Caucasian or Asian origin [26, 27, 30]. However, estimated median terminal half-life and mean absorption times are comparable to those obtained for adults [26, 27, 30,31,32]. In addition, simulations performed using the final model in children and previously published pharmacokinetic models in adult patients and volunteers show that these populations have comparable exposure under the specific recommended dosing regimen.
Considerable between-subject variability characterized the pharmacokinetics of both anti-malarial drugs. Such variability remained largely unexplained by the inclusion of the available covariates. Body weight was associated to all the MQ and DHA pharmacokinetic parameters. The association between this demographic characteristic and AS and DHA dispositions has already been described [17, 33]. Reported discrepancies in MQ disposition and elimination between adult and children may be ascribed to differences in patients’ body weight. These results illustrate the association between body weight and AS/DHA and MQ dispositions after ASMQ FDC administration in African children and thus support the recommended dose adjustments according to weight-for-age, in order to obtain similar exposures in adults and children.
Twenty-one percent of the initial AS relative F1 variability was explained by age and treatment day. It is worth realizing that F1 is intrinsically connected to AS and DHA pharmacokinetic parameters, apparent because of ASMQ oral administration. The decrease in F1 observed with age implies an increase in drug and metabolite eliminations. This effect might thus be related to organ maturation in the study population. F1 was significantly higher at day 0 than days 1 and 2 of treatment. This is a consequence of the rapid and efficacious therapeutic AS effect observed already after the first AS dose intake. Recently, the relationship between malaria disease and AS bioavailability has been described using parasitaemia variation [34, 35]. An increase of AS F1, resulting in augmented drug exposure, was reported with increasing parasite counts. This same trend was found in univariate analysis in the study population but was not retained after multivariate combination with treatment day. These two covariates are indeed correlated. However, it was not possible to identify differences in the first dose F1 due to variations in parasite counts in the study population. This suggests that the general health improvement and not only the disappearance of the parasite after the first ASMQ dose affects AS pharmacokinetics.
Age was found to markedly decrease MQ drug absorption rate. A significantly higher tmax has been reported in healthy fasting volunteers taking an MQ dose compared to those having a high-fat breakfast (36 vs. 17 h), meaning that food would increase MQ Ka [36]. This is consistent with the hypothesis that the younger children in the African paediatric population investigated were breastfed and thus received a more appropriate amount of food compared to the older ones. Under this hypothesis, and according to the results of the previously cited study, younger children are expected to have higher MQ Ka than older ones. Of note, the impact of food on MQ Ka remains controversial [37, 38]. Finally, the rapid and significant therapeutic AS effect, captured in the analysis by treatment day, induced a significant increase in MQ absorption rate after the first ASMQ FDC administration. It is possible that the dramatic decrease of parasite load following the first intake of AS improves patient state of health resulting in the disappearance of gastrointestinal tract disturbances [22]. The PK of the second and third MQ doses thus might benefit from the AS treatment effect with a favourable modification of drug absorption rate.
As already described in studies performed in Tanzania and Cambodia, more than half of the African paediatric participants had a residual concentration of at least one anti-malarial drug above the limit of quantification at baseline (lumefantrine was measured in 64% of the patients, sulphadoxine in 11%, amodiaquine/deshethylamodiaquine in 16%, pyrimethamine in 2% and quinine in 6%) [39, 40]. These findings are worrying since they indicate that parasites have been exposed to sub-therapeutic concentrations of anti-malarials for some time in a population presenting an elevated risk of developing drug resistance [22]. This might contribute to the dangerous spread of anti-malarial drug resistance.
The MQ model developed in Kenyan children using intensive sampling was applied to data collected from children from Burkina Faso, Tanzania and Kenya. Similar non-significant or small biases and precision per study centre were estimated, suggesting comparable drug exposure among different populations. The relationships between therapeutic response and pharmacokinetics of MQ as monotherapy and in combination with AS have been previously compared in a Thai population [41]. Recrudescence of infection in 24% and 2% of patients was reported in cases of MQ administered alone and with AS, respectively, indicating that the addition of the artemisinin derivative improved the cure rates considerably. Furthermore, no significant association could be found between MQ pharmacokinetics and treatment response. In line with these results, only 3% of the African paediatric population studied presented recrudescence of infection, which could not be related to MQ exposure within the study period. The low number of cases of malaria recrudescence might have limited the likelihood of detecting such an association.
Conclusions
The study described provides the pharmacokinetic parameters for MQ and AS, administered as a FDC of AS/MQ, in African children under the age of 5 years with acute P. falciparum malaria. The considerable variability characterizing the pharmacokinetics of these two anti-malarial drugs can be partly explained by children’s body weight, justifying the current dosing recommendations based on weight-for-age considerations, to ensure similar exposure in children and adults.
Abbreviations
- ACT:
-
artemisinin-based combination therapy
- AIC:
-
Akaike’s information criterion
- ALT:
-
alanine aminotransferases
- AMLF:
-
artemether–lumefantrine
- ASMQ:
-
artesunate–mefloquine
- AST:
-
aspartate
- AUC:
-
area under the curve
- BIL:
-
total bilirubin
- BQL:
-
below the quantification limit
- BSV:
-
between-subject variability
- BW:
-
body weight
- CL:
-
clearance (CL for drugs and CLM for metabolite)
- Cmax :
-
maximum concentration
- CWRES:
-
conditional weighted residuals
- DHA:
-
dihydroartemisinin
- DNDi :
-
Drugs for Neglected Diseases initiative
- ESI:
-
electrospray ionization
- F1:
-
relative bioavailability
- FACT:
-
fixed-dose artesunate-based combination therapy consortium
- FDC:
-
fixed dose combination
- FOCE:
-
first-order conditional method
- Hb:
-
haemoglobin
- Ht:
-
haematocrit
- Ka :
-
absorption rate constant
- Kmat :
-
the age maturation rate constant
- LOQ:
-
limits of quantification
- MATmag :
-
maturation magnitude for age
- MPE:
-
means of prediction error
- OFV:
-
objective function value
- PAR:
-
pharmacokinetic parameters
- PcVPc:
-
prediction-corrected visual predictive checks
- PI90 % :
-
90% prediction interval
- PK:
-
pharmacokinetics
- Q:
-
inter-compartmental clearance
- RMSE:
-
root mean square error
- t1/2 :
-
terminal half-life
- TM50 :
-
age at 50% of maturation
- tmax :
-
time to achieve Cmax
- TSQ:
-
a triple stage quadrupole
- ULN:
-
upper limit of normal
- V:
-
central volume of distribution (VC for drugs and VM for metabolite)
- VP :
-
peripheral volume of distribution
- WHO:
-
World Health Organization
- WWARN:
-
World Wide Antimalarial Resistance Network
References
WHO. World Malaria Report 2018. Geneva: World Health Organization; 2018.
WHO. Antimalarial drug combination therapy. Report of a WHO technical Consultation. Geneva: Roll Back Malaria/World Health Organization; 2018.
Wells S, Diap G, Kiechel JR. The story of artesunate–mefloquine (ASMQ), innovative partnerships in drug development: case study. Malar J. 2013;12:68.
WHO. Guidelines for the treatment of malaria. Geneva: World Health Organization; 2015.
Luxemburger C, Price RN, Nosten F, Ter Kuile FO, Chongsuphajaisiddhi T, White NJ. Mefloquine in infants and young children. Ann Trop Paediatr. 1996;16:281–6.
ter Kuile FO, Nosten F, Luxemburger C, Kyle D, Teja-Isavatharm P, Phaipun L, et al. Mefloquine treatment of acute falciparum malaria: a prospective study of non-serious adverse effects in 3673 patients. Bull World Health Organ. 1995;73:631–42.
Leang R, Ros S, Duong S, Navaratnam V, Lim P, Ariey F, et al. Therapeutic efficacy of fixed dose artesunate–mefloquine for the treatment of acute, uncomplicated Plasmodium falciparum malaria in Kampong Speu, Cambodia. Malar J. 2013;2:343.
Valecha N, Srivastava B, Dubhashi NG, Rao BHK, Kumar A, Ghosh SK, et al. Safety, efficacy and population pharmacokinetics of fixed-dose combination of artesunate–mefloquine in the treatment of acute uncomplicated Plasmodium falciparum malaria in India. J Vector Borne Dis. 2013;50:258–64.
Santelli AC, Ribeiro I, Daher A, Boulos M, Marchesini PB, Santos R, et al. Effect of artesunate–mefloquine fixed-dose combination in malaria transmission in Amazon basin communities. Malar J. 2012;11:286.
Ashley EA, Stepniewska K, Lindegårdh N, McGready R, Hutagalung R, Hae R, et al. Population pharmacokinetic assessment of a new regimen of mefloquine used in combination treatment of uncomplicated falciparum malaria. Antimicrob Agents Chemother. 2006;50:2281–5.
Krudsood S, Looareesuwan S, Silachamroon U, Chalermrut K, Pittrow D, Cambon N, et al. Artesunate and mefloquine given simultaneously for three days via a prepacked blister is equally effective and tolerated as a standard sequential treatment of uncomplicated acute Plasmodium falciparum malaria: randomized, double-blind study in Thailand. Am J Trop Med Hyg. 2002;67:465–72.
Sirima SB, Ogutu B, Lusingu J, Mtoro A, Mrango Z, Ouedraogo A, et al. Comparison of artesunate–mefloquine and artemether–lumefantrine fixed-dose combinations for treatment of uncomplicated Plasmodium falciparum malaria in children younger than 5 years in sub-Saharan Africa: a randomised, multicentre, phase 4 trial. Lancet Infect Dis. 2016;16:1123–33.
Hodel EM, Zanolari B, Mercier T, Biollaz J, Keiser J, Olliaro P, et al. A single LC-tandem mass spectrometry method for the simultaneous determination of 14 antimalarial drugs and their metabolites in human plasma. J Chromatogr B Analyt Technol Biomed Life Sci. 2009;877:867–86.
Beal, SL. Boeckmann A, Sheiner L. NONMEM User’s Guides (1989–2009). Icon Development Solutions, Ellicot City, MD, USA.
Lindbom L, Pihlgren P, Jonsson EN. PsN-Toolkit–a collection of computer intensive statistical methods for non-linear mixed effect modeling using NONMEM. Comput Meth Progr Bio. 2005;79:241–57.
Ilett BF, Ethell RT, Maggs JL, Davis TME, Batty KT, Burchell B, et al. Glucuronidation of dihydroartemisinin in vivo and by human liver microsomes and expressed UDP-glucuronosyltransferases. Drug Metab Dispos. 2002;30:1005–12.
Tan B, Naik H, Jang IJ, Yu KS, Kirsch LE, Shin CS, et al. Population pharmacokinetics of artesunate and dihydroartemisinin following single- and multiple-dosing of oral artesunate in healthy subjects. Malar J. 2009;8:304.
Morris CA, Tan B, Duparc S, Borghini-Fuhrer I, Jung D, Shin CS, et al. Effects of body size and gender on the population pharmacokinetics of artesunate and its active metabolite dihydroartemisinin in pediatric malaria patients. Antimicrob Agents Chemother. 2013;57:5889–900.
Holford NH. A size standard for pharmacokinetics. Clin Pharmacokinet. 1996;30:329–32.
Holford N, Heo YA, Anderson B. A pharmacokinetic standard for babies and adults. J Pharm Sci. 2013;102:2941–52.
Savic RM, Cowan MJ, Dvorak CC, Pai SY, Pereira L, Bartelink IH, et al. Effect of weight and maturation on busulfan clearance in infants and small children undergoing hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2013;19:1608–14.
White NJ. Assessment of the pharmacodynamic properties of antimalarial drugs in vivo. Antimicrob Agents Chemother. 1997;41:1413–22.
Ahn JE, Karlsson MO, Dunne A, Ludden TM. Likelihood based approaches to handling data below the quantification limit using NONMEM VI. J Pharmacokinet Pharmacodyn. 2008;35:401–21.
Jonsson EN, Karlsson MO. Xpose–an S-PLUS based population pharmacokinetic/pharmacodynamic model building aid for NONMEM. Comput Methods Programs Biomed. 1999;58:51–64.
Sheiner LB, Beal SL. Some suggestions for measuring predictive performance. J Pharmacokinet Pharmacodyn. 1981;9:503–12.
Jullien V, Valecha N, Srivastava B, Sharma B, Kiechel JR. Population pharmacokinetics of mefloquine, administered as a fixed-dose combination of artesunate–mefloquine in Indian patients for the treatment of acute uncomplicated Plasmodium falciparum malaria. Malar J. 2014;13:187.
Reuter SE, Upton RN, Evans AM, Navaratnam V, Olliaro PL. Population pharmacokinetics of orally administered mefloquine in healthy volunteers and patients with uncomplicated Plasmodium falciparum malaria. J Antimicrob Chemother. 2015;70:868–76.
Benakis A, Paris M, Loutan L, Plessas CT, Plessas ST. Pharmacokinetics of artemisinin and artesunate after oral administration in healthy volunteers. Am J Trop Med Hyg. 1997;56:17–23.
Morris CA, Duparc S, Borghini-Fuhrer I, Jung D, Shin C-S, Fleckenstein Morris L. Review of the clinical pharmacokinetics of artesunate and its active metabolite dihydroartemisinin following intravenous, intramuscular, oral or rectal administration. Malar J. 2011;10:263.
Staehli Hodel EM, Guidi M, Zanolari B, Mercier T, Duong S, Kabanywanyi AM, et al. Population pharmacokinetics of mefloquine, piperaquine and artemether–lumefantrine in Cambodian and Tanzanian malaria patients. Malar J. 2013;12:235.
Gutman J, Green M, Durand S, Rojas OV, Ganguly B, Quezada WM, et al. Mefloquine pharmacokinetics and mefloquine-artesunate effectiveness in Peruvian patients with uncomplicated Plasmodium falciparum malaria. Malar J. 2009;8:58.
Krudsood S, Looareesuwan S, Tangpukdee N, Wilairatana P, Phumratanaprapin W, Leowattana W, et al. New fixed-dose artesunate–mefloquine formulation against multidrug-resistant Plasmodium falciparum in adults: a comparative phase IIb safety and pharmacokinetic study with standard-dose nonfixed artesunate plus mefloquine. Antimicrob Agents Chemother. 2010;54:3730–7.
Stepniewska K, Taylor W, Sirima SB, Ouedraogo EB, Ouedraogo A, Gansané A, et al. Population pharmacokinetics of artesunate and amodiaquine in African children. Malar J. 2009;8:200.
Lohy Das J, Dondorp AM, Nosten F, Phyo AP, Hanpithakpong W, Ringwald P, et al. Population pharmacokinetic and pharmacodynamic modeling of artemisinin resistance in Southeast Asia. AAPS J. 2017;19:1842–54.
Lohy Das JP, Kyaw MP, Nyunt MH, Chit K, Aye KH, Moe M, et al. Population pharmacokinetic and pharmacodynamic properties of artesunate in patients with artemisinin sensitive and resistant infections in Southern Myanmar. Malar J. 2018;17:126.
Crevoisier C, Handschin J, Barré J, Roumenov D, Kleinbloesem C. Food increases the bioavailability of mefloquine. Eur J Clin Pharmacol. 1997;53:135–9.
Dao NVH, Quoc NP, Ngoa ND, Thuy LT, The ND, Dai B, et al. Fatty food does not alter blood mefloquine concentrations in the treatment of falciparum malaria. Trans R Soc Trop Med Hyg. 2005;99:927–31.
Price R, Simpson JA, Teja-Isavatharm P, Than MM, Luxemburger C, Heppner DG, et al. Pharmacokinetics of mefloquine combined with artesunate in children with acute falciparum malaria. Antimicrob Agents Chemother. 1999;43:341–6.
Hodel EM, Genton B, Zanolari B, Mercier T, Duong S, Becket H-P, et al. Residual antimalarial concentrations before treatment in patients with malaria from Cambodia: indication of drug pressure. J Infect Dis. 2010;202:1088–94.
Hodel EM, Kabanywanyi AM, Malila A, Zanolari B, Mercier T, Beck HP, et al. Residual antimalarials in malaria patients from Tanzania–implications on drug efficacy assessment and spread of parasite resistance. PLoS ONE. 2009;4:e8184.
Simpson JA, Price R, Kuile F, Teja-Isavatharm P, Nosten F, Chongsuphajaisiddhi T, et al. Population pharmacokinetics of mefloquine in patients with acute falciparum malaria. Clin Pharmacol Ther. 1999;66:472–84.
Authors’ contributions
JRK was the originator of the study proposal, coordinated the study, and reviewed the manuscript; MG performed the mathematical evaluation and drafted the manuscript; TM performed the bioanalytical data analysis; LD managed the work at CHUV, analysed and interpreted the data; CC designed the methodology and supervised the population PK work; BO performed and supervised the clinical work; GC coordinated all aspects related to the clinical study; MA contributed to the mathematical evaluation and preparation of the manuscript. All authors read and approved the final manuscript.
Acknowledgements
We would like to thank Joelle Vanraes for her continuous and thorough managerial support of the study and Louise Burrows for help with editing the manuscript. We would also like to thank the authors of the large clinical therapeutic study: Sirima BS, Ogutu B, Lusingu JPA, Mtoro A, Mrango Z, Ouedraogo A, Yaro JB, Onyango KO, Gesase S, Mnkande E, Ngocho JS, Ackermann I, Aubin F, Vanraes J, Strub N, and Carn G.
Competing interests
The authors declare that they have no competing interests.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author, according to DNDi’s institutional guidelines.
Consent for publication
Not applicable.
Ethics approval and consent to participate
The study protocol was reviewed and approved by national and independent ethics committees of all participating centers: Comité d’éthique pour la Recherche en Santé, Burkina Faso; KEMRI/National Ethics Review Committee, Kenya; Ministry of Health and Social Welfare, Tanzania. Written informed consent from a parent/guardian was obtained to enroll children younger than 5 years in the trial.
Funding
Agence Française de Développement, France; Department for International Development, UK; Dutch Ministry of Foreign Affairs, Netherlands; European and Developing Countries Clinical Trials Partnership; Fondation Arpe, Switzerland; Médecins Sans Frontières; Swiss Agency for Development and Cooperation, Switzerland.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Author information
Authors and Affiliations
Corresponding author
Additional files
Additional file 1.
Artesunate (upper panel) and dihydroartemisinin (lower panel) goodness-of-fit plots of observed vs. individual and population predicted concentrations, and conditional weighted residuals (CWRES) vs. population predicted concentrations and time after dose.
Additional file 2.
Mefloquine goodness-of-fit plots of observed vs. individual and population predicted concentrations, and conditional weighted residuals (CWRES) vs. population predicted concentrations and time after dose.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Guidi, M., Mercier, T., Aouri, M. et al. Population pharmacokinetics and pharmacodynamics of the artesunate–mefloquine fixed dose combination for the treatment of uncomplicated falciparum malaria in African children. Malar J 18, 139 (2019). https://doi.org/10.1186/s12936-019-2754-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12936-019-2754-6