Abstract
Background
In Loreto Department, Peru, a successful 2005–2010 malaria control programme (known as PAMAFRO) included massive distribution of long-lasting insecticidal nets (LLINs). Additional local distribution of LLINs occurred in individual villages, but not between 2012 and 2015. A 2011–2012 study of the primary regional malaria vector Anopheles darlingi detected a trend of increased exophagy compared with pre-PAMAFRO behaviour. For the present study, An. darlingi were collected in three villages in Loreto in 2013–2015 to test two hypotheses: (1) that between LLIN distributions, An. darlingi reverted to pre-intervention biting behaviour; and, (2) that there are separate sub-populations of An. darlingi in Loreto with distinct biting behaviour.
Results
In 2013–2015 An. darlingi were collected by human landing catch during the rainy and dry seasons in the villages of Lupuna and Cahuide. The abundance of An. darlingi varied substantially across years, villages and time periods, and there was a twofold decrease in the ratio of exophagic:endophagic An. darlingi over the study period. Unexpectedly, there was evidence of a rainy season population decline in An. darlingi. Plasmodium-infected An. darlingi were detected indoors and outdoors throughout the night, and the monthly An. darlingi human biting rate was correlated with the number of malaria cases. Using nextRAD genotyping-by-sequencing, 162 exophagic and endophagic An. darlingi collected at different times during the night were genotyped at 1021 loci. Based on model-based and non-model-based analyses, all genotyped An. darlingi belonged to a homogeneous population, with no evidence for genetic differentiation by biting location or time.
Conclusions
This study identified a decreasing proportion of exophagic An. darlingi in two villages in the years between LLIN distributions. As there was no evidence for genetic differentiation between endophagic and exophagic An. darlingi, this shift in biting behaviour may be the result of behavioural plasticity in An. darlingi, which shifted towards increased exophagy due to repellence by insecticides used to impregnate LLINs and subsequently reverted to increased endophagy as the nets aged. This study highlights the need to target vector control interventions to the biting behaviour of local vectors, which, like malaria risk, shows high temporal and spatial heterogeneity.
Similar content being viewed by others
Background
The main driver of early behavioural resistance in many malaria vectors globally was the extensive reliance on DDT-based indoor residual spraying (IRS). Widespread use of DDT led to modification of behaviour of several vector species that had previously taken blood meals indoors and rested indoors during egg development (endophagy and endophily, respectively), to mostly indoor feeding/outdoor resting (endophagy, exophily), to avoid insecticide exposure [1, 2]. Over the past ~ 10 years, the most effective vector intervention has been long-lasting insecticidal nets (LLINs), which, alone or in combination with IRS, and together with rapid diagnosis and treatment and combination drug therapy, have reduced malaria such that elimination is being considered feasible [3,4,5]. Despite these advances, primary reliance on LLINs and IRS for vector control has driven physiological resistance to insecticides [6, 7], and behavioural resistance or resilience (e.g., increased exophagy and early evening or daytime biting). Such behavioural modifications have enhanced residual transmission, or transmission that persists despite the reduction of vector populations through control activities [8], including outdoor and non-night-time transmission, in several malaria-endemic areas [9,10,11,12,13,14,15,16], although there are counter-examples [17,18,19].
It is unclear whether shifting biting behaviour after exposure to LLINs and/or IRS is the result of genetically differentiated populations of anophelines with different feeding behaviours, or of behavioural plasticity, the ability of individuals of the same genotype to adopt different behaviour in response to different environments. Insecticides commonly used for LLINs and IRS have been reported to exert spatial repellent as well as insecticidal effects on anophelines [20]. If anophelines are deterred by the presence of insecticide from feeding successfully at their preferred time or location, they may continue to quest for a blood meal, shifting the overall biting behaviour of the population [13]. If shifts in biting behaviour are instead caused by replacement by genetically different anophelines, these interventions may no longer be as effective against vector populations that have become behaviourally resistant [12]. Evidence for genetic differentiation of anophelines by biting and resting behaviour has been inconclusive: studies have found chromosomal inversion frequency differences between exophagic and endophagic Anopheles gambiae s.l. [21, 22] and exophilic and endophilic Anopheles funestus [23]; yet studies comparing single nucleotide polymorphisms (SNPs) in putative circadian clock genes between exophagic, endophagic, early and late feeding Anopheles arabiensis [24], and whole genome SNPs between exophilic and endophilic An. arabiensis [25], detected no genetic differentiation. Understanding whether distinct sub-populations of malaria vectors with different biting behaviour exist could help predict responses to vector control measures and advise vector control programmes as to the most efficient use of their resources.
Except for Venezuela, which in 2015 accounted for an estimated 30% of malaria cases in the Americas [26], Latin American countries have reduced the malaria case load substantially during the last 10 years [26], mainly through rapid case detection and treatment. Generally in this region there is lower coverage of LLINs and IRS compared with endemic areas in Africa and Asia [27,28,29]. In Peru, the northeastern Loreto Department reports most of the total malaria cases, with an estimated 80% of malaria cases caused by Plasmodium vivax [26, 30]. Transmission is seasonal (mainly rainy season, January to June), linked to river levels and mosquito abundance [31,32,33,34]. Between 2005 and 2010 in Loreto, the Global Fund’s PAMAFRO (Spanish acronym) initiative strengthened malaria diagnosis and case management, and distributed LLINs, achieving high local coverage, estimated at 98.7% 1 year after distribution in a sub-set of targeted communities [35] and a remarkable monthly malaria case incidence rate below 1/1000 in Peru in 2010–2011, compared to 6–7/1000 in 2005–2006 [36]. However, between 2010 and 2015 (post-PAMAFRO period), there were no widespread LLIN distributions in Loreto, and overall case numbers and the proportion of Plasmodium falciparum cases increased [26, 29, 36]. Furthermore, due to the reduced malaria incidence rate, the Peruvian Ministry of Health (MOH) shifted its focus on vector control intervention towards new arboviral outbreaks following the PAMAFRO initiative [36].
The main malaria vector in Latin America, Anopheles darlingi, dominates several regions of the Amazon Basin, accounting for > 85% of the anopheline fauna feeding on humans, and much of the malaria transmission, particularly in frontier zones [33, 37,38,39]. It has successfully invaded human-modified habitats, such as fish ponds, agricultural settlements, highways, mining sites and urban areas [40,41,42,43,44]. This species is behaviourally very plastic, displaying mainly exophily with some reports of endophily (reviewed in [45]), and both endophagy and exophagy (reviewed in [46, 47]), depending on region, season and local environmental variables such as bed-net coverage, house type, and host number and availability [48, 49]. In Amazonian Peru, there are regional records of both endophagic and exophagic behaviour in this species [31, 33, 50]. A previous investigation in peri-Iquitos from 2011 to 2012 found many more exophagic than endophagic An. darlingi, although rigorous longitudinal assessment of biting behaviour was not the main focus of that study [34]. To investigate whether there was a modification in An. darlingi’s feeding behaviour following the end of the PAMAFRO initiative, the present study was designed to quantify the abundance of exophagic versus endophagic An. darlingi from 2013 to 2015, especially during the 6-month transmission season (~ January–June). Additionally, to test the hypothesis that there are distinct sub-populations of An. darlingi with different biting behaviour in peri-Iquitos, a sub-set of collected An. darlingi were genotyped using nextRAD (nextera-tagmented, reductively amplified DNA) genotyping-by-sequencing to compare individual exophagic and endophagic mosquitoes biting at different times.
Methods
Study area and collection methods
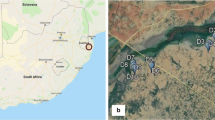
Adult female An. darlingi were collected from three villages in Loreto: San Jose de Lupuna (LUP) (03°44′35.45″S, 73°19′36.91″W) and Cahuide (CAH) (04°13′49.26″S, 73°29′16.20″W) in the peri-Iquitos area, and Santa Emilia (SEM) (04°11′58.99″S, 74°12′20.12″W), a remote site ~ 150 km by river from Iquitos (Fig. 1). Details of these villages are in Moreno et al. [34] and Lainhart et al. [52]. All three sites were part of the PAMAFRO project, which funded comprehensive control activities, particularly LLIN distribution, from 2005 to 2010 [36]. LLINs were distributed twice in LUP and CAH during the PAMAFRO initiative, with the last distributions in October 2010 (CAH) and November 2010 (LUP). In SEM, LLINs were distributed once, in February 2010. Additionally, LLINs were distributed in CAH twice by the International Federation of Red Cross and Red Crescent Societies (IFRC), once in 2012 and once in 2015. Exact dates and coverage for IFRC LLIN distributions are not available. A survey of bed-net coverage in 2012 found that 45% of households in CAH and 88% of households in LUP owned an LLIN [34]. IRS (5% deltamethrin) was conducted sporadically in all three villages between 2012 and 2014 (LUP: August 2012, April 2013, October 2013, December 2014; CAH: May 2012, June 2012, March 2013, November 2014; SEM: March 2012, March 2013, October 2014).
In LUP and CAH, paired collections were conducted indoors and outdoors monthly from January to June (rainy/malaria transmission season) in 2013-2015, and in August, October, and December (dry season) in 2013–2014. In SEM, collections were conducted in January, February and April 2014, and monthly from May–September in 2015 (Additional file 1: Fig. S1). Access to SEM on the Rio Nahuapa (Fig. 1) is difficult, requiring 2 days of travel by boat, and some trips were cancelled due to logistics or flooding issues: in January and February 2014 only outdoor collections were possible, in April 2014 only indoor; but paired outdoor and indoor collections were done monthly from May to September in 2015.
For all collections in LUP and CAH, and 2015 collections in SEM, specimens were collected from a different house each night for two nights/month by human landing catch (HLC) for 12 h (18.00–06.00), using an identical protocol outdoors (peridomestic, within ~ 10 m of each house) and indoors, as previously described in [34]. There were a total of 12 collection hours each indoors and outdoors per night. Individual collectors worked 3 h, then rested 3 h, rotating indoors and outdoors. In SEM in 2014, two collectors worked outside (January and February) or inside (April) at a time for the 12-h collections, for a total of 24 collection hours per night.
Human landing was assumed to result from seeking of blood meals; therefore, the human landing rate was considered equivalent to the human biting rate (HBR), calculated with data obtained from the 12-h collections. Mosquitoes were stored by date, location (village, exophagic/endophagic) and hour of collection. Specimens (nearly exclusively An. darlingi) were identified morphologically using entomological keys [53,54,55]. Mosquitoes were labelled and stored individually with silica gel at room temperature until subsequent analysis.
Laboratory procedures
DNA extraction and Plasmodium testing
Genomic DNA from An. darlingi specimens collected from LUP, CAH and SEM in 2014–2015 was extracted using the Qiagen DNeasy Blood & Tissue kit (Qiagen, Hilden, Germany), and DNA concentrations were measured using a Qubit Fluorometer (ThermoFisher Scientific, Waltham, MA, USA). Specimens from 2013 were not tested for Plasmodium infection due to budgetary constraints. Plasmodium infection was detected using real-time PCR of the small sub-unit of the 18S rRNA, with monoplex and triplex TaqMan assays (Life Technologies, Carlsbad, CA, USA), as described in [56]. Pools of heads/thoraces of up to five mosquitoes were analysed for detection of P. vivax and P. falciparum, and each individual from a positive pool was tested to calculate infection rate (IR = # An. darlingi infected with Plasmodium/# An. darlingi tested) and entomological inoculation rate (EIR = HBR * IR).
nextRAD DNA sample preparation
Anophles darlingi specimens for nextRAD analysis were selected from among specimens collected from LUP and CAH in March–May 2014 and 2015. For sample selection, the 12-h collection period was split into 4 3-h periods (18.00–21.00, 21.00–00.00, 00.00–03.00, 03.00–06.00). Ten individuals with DNA concentration ≥ 0.5 ng/µL as measured by Qubit were selected from each of the 32 village/year/biting location (exophagic vs endophagic)/time period combinations, for a total of 320 mosquitoes. The genomic DNA was sent to SNPsaurus (Institute of Molecular Biology, Eugene, OR, USA), where the samples were genotyped using standard nextRAD genotyping-by-sequencing methods, as in [57]. Briefly, the genomic DNA was fragmented using a Nextera reaction to ligate adapter sequences to the fragments. The fragments were amplified with Nextera primers, and the library was pooled and purified, then size selected to 350–500 bp. The resulting library was then sequenced on an Illumina HiSeq 4000, generating 150 bp reads.
Statistical analysis
Negative binomial regression of Anopheles darlingi counts
Analyses of An. darlingi counts were conducted on data from the 2013–2015 rainy season (January–June) collections in LUP and CAH (Additional file 2). To account for overdispersion, the count data were analysed by negative binomial regression in R 3.4.1 [58] using the MASS package [59] glm.nb() function. The following independent variables were included: year, site, biting location (exophagic/endophagic), time period (18.00–21.00, 21.00–00.00, 00.00–03.00, 03.00–06.00), and their two-, three- and four-way interactions. Forward selection was used to select variables and interactions for inclusion in the model. The irregular collection schedule for SEM precluded statistical analysis of these data, and LUP and CAH dry season collections were excluded from the analysis because of low collection numbers.
Correlation of monthly HBR and human malaria cases
For CAH and LUP in the rainy season in 2013–2015, HBR was aggregated monthly to compare with monthly malaria cases (P. falciparum and P. vivax combined) reported by the Peruvian MOH. As neither the HBR nor the malaria case distributions were normally distributed, non-parametric Spearman rank correlation was used to assess the relationship between them.
nextRAD data analysis
Raw sequence reads were analysed using STACKS v1.75 [60, 61]. Low-quality reads were dropped using the STACKS process_radtags program, and retained reads were aligned to the An. darlingi genome scaffolds [62] using gsnap [63]. The STACKS ref_map pipeline was used to assign genotypes, with the minimum number of reads required to create a stack set at 5, and the STACKS correction module rxstacks was used to correct genotype assignments. To increase the quantity of loci and confidence in genotype calls, nextRAD sequencing reads from 57 Brazilian An. darlingi previously described in [57] were included to build the catalogue, but excluded in creation of the final SNP database and for subsequent population genetic analysis. The STACKS populations program was used to select a single SNP from each locus found in at least 75% of individuals in the dataset. A bash script showing all STACKS parameters used is included as Additional file 3, and the final STRUCTURE dataset used for subsequent analysis is included as Additional file 4.
STRUCTURE analysis [64] was run using the Python program StrAuto, which allows for parallel computation [65]. The STRUCTURE admixture model was run assuming correlated allele frequencies for 20 replicates each of K = 1 to 10, with a burn in of 100,000 generations and an MCMC chain of 1,000,000 generations. The Evanno method [66] as implemented in STRUCTURE Harvester [67] was used to determine the optimal value of K. CLUMPP v.1.1.2, [68] using the greedy algorithm with random input orders, was used to average the files for each value of K shown at the individual level, and distruct v.1.1 [69] was used to create STRUCTURE plots.
Principal components analysis (PCA) was performed using the R ade4 package [70] dudi.pca() function, and PCA plots were created using the R factoextra package [71] fviz_pca_ind() function. Discriminant analysis of principal components (DAPC) [72] was performed using the R package adegenet [73]. In addition, ARLEQUIN v. 3.5.2.2 [74] was used to compute pairwise F ST values.
Results
Heterogeneity of Anopheles darlingi biting behaviour
The overall number of An. darlingi collected from the three localities was 4423 from LUP and 4796 from CAH in 2013–2015 (Additional file 1: Table S1A), 581 from SEM in 2015 (Additional file 1: Table S1B), and 836 from SEM in 2014 (Additional file 1: Table S2). As expected, over 90% of exophagic and endophagic An. darlingi were collected in the rainy season, between January and June (Additional file 1: Tables S1, S2, Fig. 2). In all villages and years, more exophagic and endophagic An. darlingi were collected before midnight than after (Fig. 3). Most years and villages showed a second peak around 02.00; this is absent in LUP in 2013 in exophagic An. darlingi, and very minor in the LUP 2013 endophagic population (Fig. 3).
Summary of Anopheles darlingi collected monthly, biting outside (exophagic) and inside (endophagic) from 2013 to 2015 in the Peruvian villages of Lupuna (LUP) and Cahuide (CAH), and in 2015 in Santa Emilia (SEM). The month of collection of each Plasmodium-infected An. darlingi is represented by an arrow, with the colour of the arrow indicating whether the mosquito was exophagic or endophagic and the texture indicating the species of Plasmodium. Specimens were not tested for Plasmodium in 2013. The months during which IRS was conducted in each village are indicated by black bars
Average proportion of Anopheles darlingi collected per hour, biting outside (exophagic) and inside (endophagic), in Lupuna (LUP) and Cahuide (CAH) in 2013–2015 and Santa Emilia (SEM) in 2015. Confidence intervals not shown (for clarity). The hour of collection of each Plasmodium-infected An. darlingi is represented by an arrow, with the colour of the arrow indicating whether the mosquito was exophagic or endophagic and the texture indicating the species of Plasmodium. Specimens were not tested for Plasmodium in 2013
Of 4561 An. darlingi tested for Plasmodium, 30 (0.7%) were infected (Table 1). In the three villages, only mosquitoes collected during the rainy season were infected (Fig. 2). More An. darlingi were infected with P. vivax (n = 23), which is more prevalent in Peru [75, 76], than with P. falciparum (n = 5). The Plasmodium in two infected An. darlingi could not be identified to species (Additional file 1: Table S3). Infected An. darlingi were detected biting across the whole 12-h collecting period (Fig. 3). For each locality, the monthly endophagic and exophagic HBR, and the monthly endophagic and exophagic IR and EIR for 2014–2015 collections, are shown in Additional file 1: Tables S1, S2.
Negative binomial regression of 8894 An. darlingi collected in CAH and LUP during the rainy season in 2013–2015 shows significant differences in counts across years, villages and time periods, and between endophagic and exophagic An. darlingi (Table 2). Significant interactions (year X exophagic/endophagic, year X village and year X time period) indicate that these relationships showed considerable context dependence: e.g., CAH counts were much higher than LUP in 2013, but were lower in 2014 and 2015. In both villages, An. darlingi counts were higher in 2013 than in 2014–2015 (Fig. 2). More exophagic than endophagic An. darlingi were collected in all years in both villages, but the ratio of exophagic to endophagic An. darlingi decreased over the study period in both villages, from 2.9 (CAH) and 3.0 (LUP) in 2013 to 1.4 (CAH) and 1.6 (LUP) in 2015 (Fig. 4). As a comparison, in SEM, the exophagic:endophagic ratio in 2015 was 2.0 (Additional file 1: Table S1B), although the months of collection were different from those in CAH and LUP. Significant differences in An. darlingi counts were also found among 3-h collection time periods, with overall higher biting from 18.00–21.00 to 21.00–00.00 in 2013, and from 18.00–21.00 in 2014–2015, than during the other time periods. Non-parametric Kruskal–Wallis analysis of the count dataset produced comparable results to the negative binomial regression (Additional file 1: Table S4).
Correlation between monthly HBR and human malaria cases
The number of yearly malaria cases of P. vivax and P. falciparum from 2010 to 2016 in LUP (population = 432; 2014 human census), CAH (population = 910; 2014 human census), and SEM (population = 212; 2014 human census) is depicted in Additional file 1: Fig. S2, demonstrating substantial fluctuation in cases in all three villages, and a distinct temporal pattern in each village, with increasing P. falciparum in LUP. In LUP and CAH, the monthly HBR of exo- and endophagic An. darlingi combined was moderately but significantly correlated with the number of malaria cases in the same locality in the current month (Additional file 1: Fig. S3, ρ = 0.55, p = 0.0005) and the previous month (ρ = 0.45, p = 0.004). The exophagic HBR was more highly correlated with malaria cases in the current month (ρ = 0.548, p = 0.0005) than the endophagic HBR (ρ = 0.344, p = 0.040).
No evidence of population genetic structure of Anopheles darlingi by biting behaviour
From the 320 nextRAD-genotyped An. darlingi, an average of 1,912,124 (range: 2712–17,342,438) reads per sample passed quality filtering, and an average of 1,007,380 (range 701–8,124,687) reads per sample were aligned to An. darlingi reference genome scaffolds. To increase the number of loci in the final analysis, only samples with at least 200,000 aligned reads (n = 162) were included for genotyping. Genotypes were called at an average of 58,480 (SD 65,548) loci per sample. Within individuals, 12.92% (SD 6.11%) loci were polymorphic (Additional file 5). The final SNP dataset includes one biallelic SNP from each locus genotyped in at least 75% of the 162 individual mosquitoes, a total of 1021 loci (Additional file 4). The average sequencing depth across all 1021 loci and 162 individuals was 96X.
STRUCTURE and STRUCTURE Harvester analyses of the SNP dataset supported 3 genetic clusters (optimal K = 3); however, there was not a clear peak for the value of ΔK for K = 2–9 (ΔK = 10 for K = 3), and the estimated natural log probability of the data (lnPr(X|K)) was very similar for K = 1 through 8 (Additional file 1: Fig. S4A, B). The Evanno method is not able to find the optimal K if K = 1 [66]. In the STRUCTURE plot for K = 3, all individuals are admixed among the three clusters, regardless of biting location (Fig. 5a), collection village/year (Additional file 1: Fig. S5D), or biting time (Additional file 1: Fig. S5E), indicating that all 162 individuals belong to a single population. The STRUCTURE plots for K = 2 also support this lack of population genetic structure (Additional file 1: Fig. S5A–C).
Results of STRUCTURE and PCA of 1021-locus SNP dataset, comparing endophagic and exophagic Anopheles darlingi. a STRUCTURE results depicting three inferred genetic clusters. Although the proportion of membership in each cluster varies across individual An. darlingi, all individuals have non-zero membership in all three clusters, indicating admixture and no significant structuring. b PCA, with colours reflecting endophagic vs exophagic individuals
PCA of the SNP dataset was consistent with a single homogeneous population, with no separation by biting location (Fig. 5b), collection village/year (Additional file 1: Fig. S6A), or biting time (Additional file 1: Fig. S6B). Similarly, the Bayesian information criterion (BIC) of the k-means clustering algorithm implemented in preparation for DAPC indicated that the optimal number of clusters was 1 (Additional file 1: Fig. S4C). Pairwise F ST values indicated low genetic differentiation of exophagic and endophagic An. darlingi (F ST = 0.0016, p = 0.62); An. darlingi at different biting times (all pairwise F ST values < 0.005 with p > 0.40); and An. darlingi from different collection villages/years (CAH 2014 vs CAH 2015 F ST = 0.0149, p < 0.05; all other pairwise F ST values < 0.001 with p > 0.88) (Additional file 1: Table S5A–C).
Discussion
This study detected, across 3 years, a shift in An. darlingi in LUP and CAH towards decreased exophagy. This shift occurred between distributions of LLINs in these villages; the most recently distributed LLINs in CAH were a year old and in LUP were over 2 years old by the start of this study, and LLINs in both villages were at least 3 years old (the expected lifetime of LLINs [51]) by the end of the study. It is therefore possible that this shift represents a return to baseline biting behaviour in these villages following a previous shift towards increased exophagy driven by LLIN exposure.
IRS was conducted in CAH and LUP sporadically during this study. However, An. darlingi is known to rest mainly outdoors following blood feeding [47], so it is unlikely that IRS is effective against this vector. In this study, there was not a consistent effect of IRS on the An. darlingi abundance or exophagic:endophagic ratio in either the month IRS was conducted or the following month in these two villages (Fig. 2).
This study further confirms the heterogeneous biting behaviour of An. darlingi [34, 43, 47, 77,78,79]. A range of local environmental or ecological changes can influence the ratio of exo/endophagic An. darlingi. For example, in a gold mining area in Venezuela, An. darlingi was found to be significantly more exo- than endophagic, attributed mainly to the location of villages within forested areas, and to houses with incomplete walls [42]. A similar pattern of high exophagy/low endophagy in An. darlingi was detected along a corridor of a highway deforested for power line construction in Porto Velho, Rondonia state, Brazil [80].
Shifts in behaviour in vector anophelines towards increased exophagy following LLIN distribution are relatively common [9,10,11, 14]. In these studies, such shifts were seen within 1 year after LLIN distribution, or during periods of high LLIN usage. Shifts in biting behaviour can result from physiological (insecticide-induced) or behavioural resistance, which can be difficult to distinguish [12]. Because there is little documented physiological resistance in An. darlingi, including populations sampled for this study [81], the documented exophagic/endophagic shift may be evidence for behavioural resistance that emerged as a result of LLIN usage. However, some previously reported shifts may be the result of changes in species composition, or plasticity in feeding responses [2, 13]. For example, using mark-release-recapture, individual Anopheles farauti were found to feed both outdoors and indoors [19].
In the current study, using 1021 genome-wide SNPs, there was no evidence of genetic differentiation between exophagic and endophagic An. darlingi, or among An. darlingi biting at different times during the night. These results were consistent across both model-based (STRUCTURE) and non-model-based analyses (PCA/DAPC). In addition, thorough exploration of the parameter space, by changing the exclusion criteria for low coverage samples and the STACKS settings for filtering of loci, consistently returned evidence that all samples belonged to a single homogeneous population. The lack of detectable population structural differences among the An. darlingi from this study suggests that the reported shifts in biting behaviour are due to behavioural plasticity resulting from reduced spatial repellence of aging LLINs, rather than genetically differentiated populations of exophagic and endophagic An. darlingi. However, the lack of population genetic structure does not preclude a genetic basis for changes in An. darlingi biting behaviour. It is possible that the methods used were unable to detect smaller-scale genetic differences between exophagic and endophagic An. darlingi that do not influence the overall genetic structure.
Previous studies have found microgeographic genetic differentiation between An. darlingi by habitat [52] and season [77]. In addition, a recent study using whole-genome SNPs found genetic differentiation between An. darlingi collected in two rural Brazilian villages ~ 60 km apart, which had experienced different levels of deforestation [82]. Although these Brazilian villages were approximately the same distance apart as the villages in the current study, it is not surprising that there was no evidence of genetic structure between An. darlingi collected in LUP and CAH, because both are riverine villages with similar ecological characteristics [34, 52].
Across its broad distribution, An. darlingi populations exhibit a wide range of peak biting times and patterns (unimodal, bimodal, trimodal, no peak) [47, 83, 84]. Furthermore, in a study in Amapá state, northern Amazonian Brazil, Voorham [85] found intra-population variation of biting activity in An. darlingi to be as high as inter-population variation. Some variation is attributed to seasonality [50, 77, 86], and some is assumed to be the result of interaction between local ecological and endogenous factors [45]. The current study determined that in the peri-Iquitos area, more An. darlingi were biting before midnight than afterwards, especially early in the evening, in agreement with observations in previous studies in Peru [32,33,34] and some regions of Brazil [43, 78]; although another Brazilian study found An. darlingi biting throughout the night [84]. A preponderance of early evening biting is likely related to the availability of humans as hosts while they are engaged in various activities prior to retiring under bed nets.
There was a second, smaller biting peak around 02.00 in all years and villages except 2013 in the exophagic individuals from LUP (Fig. 3). Although there were no genetic structural differences by biting time, it is possible that there are individual genes determining biting time in the population that do not influence the overall genetic structure (as investigated in [24]). Alternatively, this additional peak may be the result of phenotypic plasticity in biting behaviour within the population.
This study shows similar overall patterns in abundance, HBR, and biting behaviour of An. darlingi for LUP and CAH between 2013 and 2015. That the two communities are ~ 60 km apart and located on different rivers suggests that these populations may respond to some types of regional environmental or anthropogenic change as a single metapopulation. In 2012 [34], peak monthly HBRs of exophagic populations of An. darlingi were similar in LUP and CAH, and by 2015, they had declined similarly. In addition, the changing ratio of exophagic:endophagic An. darlingi from year-to-year is quite congruent (Fig. 4). However, as the negative binomial regression results demonstrate, local context strongly influences the patterns of abundance in these populations of An. darlingi. In summary, there is evidence for both metapopulation and local population behavioural patterns in An. darlingi in Loreto, but the mechanisms involved have not yet been identified.
The aggregated monthly HBR of An. darlingi (exophagic, endophagic and both together) was significantly correlated with monthly malaria case numbers in LUP and CAH. This is particularly interesting because cases of P. falciparum have increased since 2015, especially in LUP, representative of a wider regional trend in Loreto [29, 87], whereas P. vivax cases peaked in LUP in 2014, and CAH experienced a major P. vivax outbreak in 2012–2013 after which cases have fluctuated considerably (Additional file 1: Fig. S2). These data confirm that the HBR and malaria incidence are highly related, though it is clear that other parameters used in the calculation of vectorial capacity, such as vector survival rates [88], are also valuable for predicting malaria risk.
Although in the present study the overall numbers of infected An. darlingi (n = 30) were insufficient for statistical analysis, there were endophagic and exophagic An. darlingi infected with both P. vivax and P. falciparum throughout the rainy season (January–June), before and after midnight. Thus, during the rainy season, villagers are at risk of malaria infection both inside their houses and in the peridomestic area throughout the night.
An explanation for the decline in the rainy season An. darlingi population sizes in LUP and CAH over time is elusive. A massive flood in Loreto in April 2012 [89], attributed mainly to an early La Niña event [90], may have influenced survival or population dynamics, perhaps by destruction of breeding sites. Less massive flooding in Suriname, combined with several vector interventions and malaria case management, reduced malaria incidence to near zero, in a region where An. darlingi was the principal vector [91]. In the village of LUP, there was no discernible immediate effect of the flood on the peak HBR in April–May between 2011 (before the flood) and 2012 (immediately after the flood [34]), although longer term effects cannot be ruled out.
Modelling has demonstrated that P. falciparum is likely more sensitive than P. vivax to changes in malaria vector survival rates due to longer sporogonic cycle duration [88]. If An. darlingi survival rates had been measured over the 3 years of this study, the relationship between survival and the increase in P. falciparum cases could have been investigated. In addition, as this study analysed only An. darlingi collected by HLC, it is possible that a sub-population of An. darlingi, not sampled in this study because it feeds mainly on animals, also contributes to malaria transmission. Although a previous analysis of blood meal sources in resting An. darlingi in LUP, CAH and SEM found that the majority of mosquitoes tested had fed on humans (human blood index (HBI): 0.58–0.87), with a similar infection rate to that found in the current study (0.42%) [49], a substantial proportion of An. darlingi feed on non-human hosts in this region. It is unknown whether An. darlingi feeding on different hosts are genetically distinct, as has been demonstrated in An. arabiensis in Tanzania [25]. Another limitation of this study was the sporadic collections at SEM, which prohibited statistical and genetic comparisons with LUP and CAH. Finally, this study did not determine whether LLINs were used or IRS was conducted in the individual houses in which collections were conducted. However, collections rotated between different houses on different nights throughout the study in an effort to obtain an unbiased, representative sampling of An. darlingi biting behaviour in the villages during the study period.
Conclusions
This study identified a decreasing proportion of outdoor biting among An. darlingi in the years following LLIN distributions in two villages in Amazonian Peru. The results strongly suggest that LLINs (to be replaced every ~ 3 years [51]) would reduce endophagic malaria transmission risk even where An. darlingi is also exophagic. Controlling exophagic malaria transmission, on the other hand, is extremely challenging [92]. Potential solutions include use of genetically modified mosquitoes, personal and/or spatial repellents, insecticide treatment of livestock, insecticidal sugar baits and perhaps, under certain circumstances, treatment of asymptomatic persons. Lastly, given the tremendous temporal and spatial heterogeneity of An. darlingi, the context of each village needs to be considered in planning malaria control programmes, highlighting the need for continuous monitoring of vector abundance and behaviour in malaria-endemic areas, particularly after changes in vector control interventions.
Abbreviations
- BIC:
-
Bayesian information criterion
- CAH:
-
Cahuide
- DAPC:
-
discriminant analysis of principal components
- EIR:
-
entomological inoculation rate
- HBI:
-
human blood index
- HBR:
-
human biting rate
- HLC:
-
human landing catch
- IR:
-
infection rate
- IRS:
-
indoor residual spray
- LLINs:
-
long-lasting insecticidal nets
- LUP:
-
Lupuna
- MOH:
-
Peruvian Ministry of Health
- nextRAD:
-
nextera-tagmented, reductively-amplified DNA
- PAMAFRO:
-
Global Fund programme to control malaria in Andean country border areas (Spanish acronym)
- PCA:
-
principal components analysis
- SEM:
-
Santa Emilia
- SNP:
-
single nucleotide polymorphism
References
Elliott R. The influence of vector behavior on malaria transmission. Am J Trop Med Hyg. 1972;21:755–63.
Russell TL, Beebe NW, Cooper RD, Lobo NF, Burkot TR. Successful malaria elimination strategies require interventions that target changing vector behaviours. Malar J. 2013;12:56.
Aultman K, Burkot TR, Chandre F, Coetzee M, Collins FH, Corbel V, et al. A research agenda for malaria eradication: vector control. PLoS Med. 2011;8:e1000401.
Mnzava AP, Macdonald MB, Knox TB, Temu EA, Shiff CJ. Malaria vector control at a crossroads: public health entomology and the drive to elimination. Trans R Soc Trop Med Hyg. 2014;108:550–4.
Bhatt S, Weiss DJ, Cameron E, Bisanzio D, Mappin B, Dalrymple U, et al. The effect of malaria control on Plasmodium falciparum in Africa between 2000 and 2015. Nature. 2015;526:207–11.
Glunt KD, Abilio AP, Bassat Q, Bulo H, Gilbert AE, Huijben S, et al. Long-lasting insecticidal nets no longer effectively kill the highly resistant Anopheles funestus of southern Mozambique. Malar J. 2015;14:298.
Ranson H, Lissenden N. Insecticide resistance in African Anopheles mosquitoes: a worsening situation that needs urgent action to maintain malaria control. Trends Parasitol. 2016;32:187–96.
Killeen GF. Characterizing, controlling and eliminating residual malaria transmission. Malar J. 2014;13:330.
Reddy MR, Overgaard HJ, Abaga S, Reddy VP, Caccone A, Kiszewski AE, et al. Outdoor host seeking behaviour of Anopheles gambiae mosquitoes following initiation of malaria vector control on Bioko Island, Equatorial Guinea. Malar J. 2011;10:184.
Russell TL, Govella NJ, Azizi S, Drakeley CJ, Kachur SP, Killeen GF. Increased proportions of outdoor feeding among residual malaria vector populations following increased use of insecticide-treated nets in rural Tanzania. Malar J. 2011;10:80.
Moiroux N, Gomez MB, Pennetier C, Elanga E, Djenontin A, Chandre F, et al. Changes in Anopheles funestus biting behavior following universal coverage of long-lasting insecticidal nets in Benin. J Infect Dis. 2012;206:1622–9.
Gatton ML, Chitnis N, Churcher T, Donnelly MJ, Ghani AC, Godfray HC, et al. The importance of mosquito behavioural adaptations to malaria control in Africa. Evolution. 2013;67:1218–30.
Govella NJ, Chaki PP, Killeen GF. Entomological surveillance of behavioural resilience and resistance in residual malaria vector populations. Malar J. 2013;12:124.
Meyers JI, Pathikonda S, Popkin-Hall ZR, Medeiros MC, Fuseini G, Matias A, et al. Increasing outdoor host-seeking in Anopheles gambiae over 6 years of vector control on Bioko Island. Malar J. 2016;15:239.
Reimer LJ, Thomsen EK, Koimbu G, Keven JB, Mueller I, Siba PM, et al. Malaria transmission dynamics surrounding the first nationwide long-lasting insecticidal net distribution in Papua New Guinea. Malar J. 2016;15:25.
Thomsen EK, Koimbu G, Pulford J, Jamea-Maiasa S, Ura Y, Keven JB, et al. Mosquito behavior change after distribution of bednets results in decreased protection against malaria exposure. J Infect Dis. 2017;215:790–7.
Bayoh MN, Walker ED, Kosgei J, Ombok M, Olang GB, Githeko AK, et al. Persistently high estimates of late night, indoor exposure to malaria vectors despite high coverage of insecticide treated nets. Parasit Vectors. 2014;7:380.
Helinski ME, Nuwa A, Protopopoff N, Feldman M, Ojuka P, Oguttu DW, et al. Entomological surveillance following a long-lasting insecticidal net universal coverage campaign in Midwestern Uganda. Parasit Vectors. 2015;8:458.
Russell TL, Beebe NW, Bugoro H, Apairamo A, Collins FH, Cooper RD, et al. Anopheles farauti is a homogeneous population that blood feeds early and outdoors in the Solomon Islands. Malar J. 2016;15:151.
Killeen GF, Chitnis N, Moore SJ, Okumu FO. Target product profile choices for intra-domiciliary malaria vector control pesticide products: repel or kill? Malar J. 2011;10:207.
Coluzzi M, Sabatini A, Petrarca V, Angela Di Deco M. Behavioural divergences between mosquitoes with different inversion karyotypes in polymorphic populations of the Anopheles gambiae complex. Nature. 1977;266:832–3.
Coluzzi M, Sabatini A, Petrarca V, Di Deco MA. Chromosomal differentiation and adaptation to human environments in the Anopheles gambiae complex. Trans R Soc Trop Med Hyg. 1979;73:483–97.
Guelbeogo WM, Sagnon NF, Liu F, Besansky NJ, Costantini C. Behavioural divergence of sympatric Anopheles funestus populations in Burkina Faso. Malar J. 2014;13:65.
Maliti DV, Marsden CD, Main BJ, Govella NJ, Yamasaki Y, Collier TC, et al. Investigating associations between biting time in the malaria vector Anopheles arabiensis Patton and single nucleotide polymorphisms in circadian clock genes: support for sub-structure among An. arabiensis in the Kilombero valley of Tanzania. Parasit Vectors. 2016;9:1–15.
Main BJ, Lee Y, Ferguson HM, Kreppel KS, Kihonda A, Govella NJ, et al. The genetic basis of host preference and resting behavior in the major African malaria vector, Anopheles arabiensis. PLoS Genet. 2016;12:e1006303.
WHO. World malaria report 2016. Geneva: World Health Organization; 2016.
Flores W, Chang J, Barillas E. Rapid assessment of the performance of malaria control strategies implemented by countries in the Amazon subregion using adequacy criteria: case study. Malar J. 2011;10:379.
Ferreira MU, Castro MC. Challenges for malaria elimination in Brazil. Malar J. 2016;15:284.
Rosas-Aguirre A, Gamboa D, Manrique P, Conn JE, Moreno M, Lescano AG, et al. Epidemiology of Plasmodium vivax malaria in Peru. Am J Trop Med Hyg. 2016;95:133–44.
MINSA. Ministerio de Salud del Perú: Sala de Situación de Salud: Epidemiológica N° 52–2016. 2016.
Aramburu Guarda J, Ramal Asayag C, Witzig R. Malaria reemergence in the Peruvian Amazon region. Emerg Infect Dis. 1999;5:209–15.
Turell MJ, Sardelis MR, Jones JW, Watts DM, Fernandez R, Carbajal F, et al. Seasonal distribution, biology, and human attraction patterns of mosquitoes (Diptera: Culicidae) in a rural village and adjacent forested site near Iquitos, Peru. J Med Entomol. 2008;45:1165–72.
Reinbold-Wasson DD, Sardelis MR, Jones JW, Watts DM, Fernandez R, Carbajal F, et al. Determinants of Anopheles seasonal distribution patterns across a forest to periurban gradient near Iquitos, Peru. Am J Trop Med Hyg. 2012;86:459–63.
Moreno M, Saavedra MP, Bickersmith SA, Lainhart W, Tong C, Alava F, et al. Implications for changes in Anopheles darlingi biting behaviour in three communities in the peri-Iquitos region of Amazonian Peru. Malar J. 2015;14:290.
Rosas-Aguirre A, Guzman-Guzman M, Moreno-Gutierrez D, Rodriguez-Ferrucci H, Vargas-Pacherrez D, Acuna-Gonzalez Y. Long-lasting insecticide—treated bednet ownership, retention and usage 1 year after their distribution in Loreto, Peru. Rev Peru Med Exp Salud Publica. 2011;28:228–36 (in Spanish).
Soto-Calle V, Rosas-Aguirre A, Llanos-Cuentas A, Abatih E, DeDeken R, Rodriguez H, et al. Spatio-temporal analysis of malaria incidence in the Peruvian Amazon Region between 2002 and 2013. Sci Rep. 2017;7:40350.
da Silva-Vasconcelos A, Kato MY, Mourao EN, de Souza RT, Lacerda RN, Sibajev A, et al. Biting indices, host-seeking activity and natural infection rates of anopheline species in Boa Vista, Roraima, Brazil from 1996 to 1998. Mem Inst Oswaldo Cruz. 2002;97:151–61.
Jiménez IP, Conn J, Brochero H. Preliminary biological studies on larvae and adult Anopheles mosquitoes (Diptera: Culicidae) in Miraflores, a malaria endemic locality in Guaviare Department, Amazonian Colombia. J Med Entomol. 2014;51:1002–9.
de Barros FS, Honorio NA. Deforestation and malaria on the Amazon frontier: larval clustering of Anopheles darlingi (Diptera: Culicidae) determines focal distribution of malaria. Am J Trop Med Hyg. 2015;93:939–53.
Vittor AY, Gilman RH, Tielsch J, Glass G, Shields T, Lozano WS, et al. The effect of deforestation on the human-biting rate of Anopheles darlingi, the primary vector of falciparum malaria in the Peruvian Amazon. Am J Trop Med Hyg. 2006;74:3–11.
Gil LH, Tada MS, Katsuragawa TH, Ribolla PE, da Silva LH. Urban and suburban malaria in Rondonia (Brazilian Western Amazon) II. Perennial transmissions with high anopheline densities are associated with human environmental changes. Mem Inst Oswaldo Cruz. 2007;102:271–6.
Moreno JE, Rubio-Palis Y, Paez E, Perez E, Sanchez V. Abundance, biting behaviour and parous rate of anopheline mosquito species in relation to malaria incidence in gold-mining areas of southern Venezuela. Med Vet Entomol. 2007;21:339–49.
Moutinho PR, Gil LH, Cruz RB, Ribolla PE. Population dynamics, structure and behavior of Anopheles darlingi in a rural settlement in the Amazon rainforest of Acre, Brazil. Malar J. 2011;10:174.
Pommier de Santi V, Girod R, Mura M, Dia A, Briolant S, Djossou F, et al. Epidemiological and entomological studies of a malaria outbreak among French armed forces deployed at illegal gold mining sites reveal new aspects of the disease’s transmission in French Guiana. Malar J. 2016;15:35.
Charlwood JD. Biological variation in Anopheles darlingi Root. Mem Inst Oswaldo Cruz. 1996;91:391–8.
Sinka ME, Bangs MJ, Manguin S, Coetzee M, Mbogo CM, Hemingway J, et al. The dominant Anopheles vectors of human malaria in Africa, Europe and the Middle East: occurrence data, distribution maps and bionomic precis. Parasit Vectors. 2010;3:117.
Hiwat H, Bretas G. Ecology of Anopheles darlingi Root with respect to vector importance: a review. Parasit Vectors. 2011;4:177.
Zimmerman RH, Galardo AK, Lounibos LP, Arruda M, Wirtz R. Bloodmeal hosts of Anopheles species (Diptera: Culicidae) in a malaria-endemic area of the Brazilian Amazon. J Med Entomol. 2006;43:947–56.
Moreno M, Saavedra MP, Bickersmith SA, Prussing C, Michalski A, Tong Rios C, et al. Intensive trapping of blood-fed Anopheles darlingi in Amazonian Peru reveals unexpectedly high proportions of avian blood-meals. PLoS Negl Trop Dis. 2017;11:e0005337.
León W, Valle J, Naupay R, Tineo E, Rosas A, Palomino M. Comportamiento estacional del Anopheles (Nyssorhynchus) darlingi Root 1926 en localidades de Loreto y Madre de Dios, Peru 1999–2000. Rev Peru Med Exp Salud Publica. 2003;20:22–7.
Tan KR, Coleman J, Smith B, Hamainza B, Katebe-Sakala C, Kean C, et al. A longitudinal study of the durability of long-lasting insecticidal nets in Zambia. Malar J. 2016;15:106.
Lainhart W, Bickersmith S, Nadler K, Moreno M, Saavedra M, Chu VM, et al. Evidence for temporal population replacement and the signature of ecological adaptation in a major Neotropical malaria vector in Amazonian Peru. Malar J. 2015;14:375.
Forattini OP. Entomologia Medica, vol. 1. São Paulo: Faculdade de Higiene e Sáude Publica; 1962.
Faran ME, Linthicum KJ. A handbook of the Amazonian species of Anopheles (Nyssorhynchus) (Diptera: Culicidae). Mosq Syst. 1981;13:1–81.
Consoli RA, Lourenco-de-Oliveira R. Principais mosquitos de importância sanitária no Brasil. Rio de Janeiro: Editora Fiocruz: Fundação Oswaldo Cruz; 1994.
Bickersmith SA, Lainhart W, Moreno M, Chu VM, Vinetz JM, Conn JE. A sensitive, specific and reproducible real-time PCR method for detection of Plasmodium vivax and P. falciparum infection in field-collected anophelines. Mem Inst Oswaldo Cruz. 2015;110:573–6.
Emerson KJ, Conn JE, Bergo ES, Randel MA, Sallum MAM. Brazilian Anopheles darlingi (Diptera: Culicidae) clusters by major biogeographical region. PLoS ONE. 2015;10:e0130773.
R Core Team. R: A language and environment for statistical computing. Vienna: R Foundation for Statistical Computing; 2017. https://www.R-project.org/.
Venables WN, Ripley BD. Modern applied statistics with S. New York: Springer; 2002. p. 498.
Catchen JM, Amores A, Hohenlohe P, Cresko W, Postlethwait JH. Stacks: building and genotyping loci de novo from short-read sequences. G3 (Bethesda). 2011;1:171–82.
Catchen J, Hohenlohe PA, Bassham S, Amores A, Cresko WA. Stacks: an analysis tool set for population genomics. Mol Ecol. 2013;22:3124–40.
Marinotti O, Cerqueira GC, De Almeida LGP, Ferro MIT, Da Silva Loreto EL, Zaha A, et al. The genome of Anopheles darlingi, the main neotropical malaria vector. Nucleic Acids Res. 2013;41:7387–400.
Wu TD, Nacu S. Fast and SNP-tolerant detection of complex variants and splicing in short reads. Bioinformatics. 2010;26:873–81.
Pritchard JK, Stephens M, Donnelly P. Inference of population structure using multilocus genotype data. Genetics. 2000;155:945–59.
Chhatre VE, Emerson KJ. StrAuto: automation and parallelization of STRUCTURE analysis. BMC Bioinformatics. 2017;18:192.
Evanno G, Regnaut S, Goudet J. Detecting the number of clusters of individuals using the software structure: a simulation study. Mol Ecol. 2005;14:2611–20.
Earl DA, von Holdt BM. STRUCTURE HARVESTER: a website and program for visualizing STRUCTURE output and implementing the Evanno method. Conserv Genet Resour. 2012;4:359–61.
Jakobsson M, Rosenberg NA. CLUMPP: a cluster matching and permutation program for dealing with label switching and multimodality in analysis of population structure. Bioinformatics. 2007;23:1801–6.
Rosenberg NA. Distruct: a program for the graphical display of population structure. Mol Ecol Resour. 2004;4:137–8.
Dray S, Dufour A-B. The ade4 package: implementing the duality diagram for ecologists. J Stat Software. 2007;22:20.
Kassambara A, Mundt F. Factoextra: extract and visualize the results of multivariate data analyses. R package version 1.0.4. 2017. https://CRAN.R-project.org/package=factoextra. Accessed 3 Aug 2017.
Jombart T, Devillard S, Balloux F. Discriminant analysis of principal components: a new method for the analysis of genetically structured populations. BMC Genet. 2010;11:1–15.
Jombart T. adegenet: a R package for the multivariate analysis of genetic markers. Bioinformatics. 2008;24:1403–5.
Excoffier L, Lischer HEL. Arlequin suite ver 3.5: a new series of programs to perform population genetics analyses under Linux and Windows. Mol Ecol Resour. 2010;10:564–7.
Kobayashi T, Gamboa D, Ndiaye D, Cui L, Sutton PL, Vinetz JM. Malaria diagnosis across the International Centers of Excellence for Malaria Research: platforms, performance, and standardization. Am J Trop Med Hyg. 2015;93:99–109.
Pizzitutti F, Pan W, Barbieri A, Miranda JJ, Feingold B, Guedes GR, et al. A validated agent-based model to study the spatial and temporal heterogeneities of malaria incidence in the rainforest environment. Malar J. 2015;14:514.
Angella AF, Salgueiro P, Gil LH, Vicente JL, Pinto J, Ribolla PE. Seasonal genetic partitioning in the neotropical malaria vector, Anopheles darlingi. Malar J. 2014;13:203.
Barbosa LM, Souto RN, Dos Anjos Ferreira RM, Scarpassa VM. Behavioral patterns, parity rate and natural infection analysis in anopheline species involved in the transmission of malaria in the northeastern Brazilian Amazon region. Acta Trop. 2016;164:216–25.
Vezenegho SB, Adde A, Pommier de Santi V, Issaly J, Carinci R, Gaborit P, et al. High malaria transmission in a forested malaria focus in French Guiana: how can exophagic Anopheles darlingi thwart vector control and prevention measures? Mem Inst Oswaldo Cruz. 2016;111:561–9.
Gil LH, Rodrigues Mde S, de Lima AA, Katsuragawa TH. Seasonal distribution of malaria vectors (Diptera: Culicidae) in rural localities of Porto Velho, Rondonia, Brazilian Amazon. Rev Inst Med Trop Sao Paulo. 2015;57:263–7.
Quinones ML, Norris DE, Conn JE, Moreno M, Burkot TR, Bugoro H, et al. Insecticide resistance in areas under investigation by the International Centers of Excellence for Malaria Research: a challenge for malaria control and elimination. Am J Trop Med Hyg. 2015;93:69–78.
Campos M, Conn JE, Alonso DP, Vinetz JM, Emerson KJ, Ribolla PE. Microgeographical structure in the major Neotropical malaria vector Anopheles darlingi using microsatellites and SNP markers. Parasit Vectors. 2017;10:76.
Rosa-Freitas MG, Broomfield G, Priestman A, Milligan PJ, Momen H, Molyneux DH. Cuticular hydrocarbons, isoenzymes and behavior of three populations of Anopheles darlingi from Brazil. J Am Mosq Control Assoc. 1992;8:357–66.
Zimmerman RH, Lounibos LP, Nishimura N, Galardo AK, Galardo CD, Arruda ME. Nightly biting cycles of malaria vectors in a heterogeneous transmission area of eastern Amazonian Brazil. Malar J. 2013;12:262.
Voorham J. Intra-population plasticity of Anopheles darlingi’s (Diptera, Culicidae) biting activity patterns in the state of Amapa, Brazil. Rev Saude Publica. 2002;36:75–80.
Jiménez IP, Conn JE, Brochero H. Malaria vectors in San José del Guaviare, Orinoquia, Colombia. J Am Mosq Control Assoc. 2014;30:91–8.
Quispe AM, Llanos-Cuentas A, Rodriguez H, Clendenes M, Cabezas C, Leon LM, et al. Accelerating to zero: strategies to eliminate malaria in the Peruvian Amazon. Am J Trop Med Hyg. 2016;94:1200–7.
de Barros FS, Honorio NA, Arruda ME. Survivorship of Anopheles darlingi (Diptera: Culicidae) in relation with malaria incidence in the Brazilian Amazon. PLoS ONE. 2011;6:e22388.
Espinoza JC, Ronchail J, Frappart F, Lavado W, Santini W, Guyot JL. The major floods in the Amazonas river and tributaries (Western Amazon Basin) during the 1970–2012 period: a focus on the 2012 flood. J Hydrometeorol. 2013;14:1000–8.
Marengo JA, Espinoza JC. Extreme seasonal droughts and floods in Amazonia: causes, trends and impacts. Int J Climatol. 2016;36:1033–50.
Hiwat H, Mitro S, Samjhawan A, Sardjoe P, Soekhoe T, Takken W. Collapse of Anopheles darlingi populations in Suriname after introduction of insecticide-treated nets (ITNs); malaria down to near elimination level. Am J Trop Med Hyg. 2012;86:649–55.
Killeen GF, Marshall JM, Kiware SS, South AB, Tusting LS, Chaki PP, et al. Measuring, manipulating and exploiting behaviours of adult mosquitoes to optimise malaria vector control impact. BMJ Global Health. 2017;2:e000212.
Authors’ contributions
CP, MM and JEC wrote the manuscript with contributions from CDS and JMV; MM, MPS and FA collected field data; SAB and CP conducted molecular work; DG helped oversee field collections; CP, CDS and KJE conducted data analysis. All authors read and approved the final manuscript.
Acknowledgements
We thank Eliseo Ramirez, José Manuel Reyna, Victor Pacaya, David Arimuya, and Hercules Maytahuari for field assistance, and Carlos Tong for assistance in mosquito identification. We appreciate the enthusiastic support of the communities of Cahuide, Lupuna and Santa Emilia (Loreto Department). We are grateful to Dirección Regional de Salud (DIRESA, Iquitos, Loreto) for collaboration and facilitating logistics in Loreto Department.
Competing interests
The authors declare that they have no competing interests.
Availability of data and materials
Raw Illumina sequences were uploaded to the NCBI SRA database (Study Number SRP118830; Run Accession Numbers SRR6073706–SRR6074025). All other data supporting the conclusions of this article are included within the article and its additional files.
Consent for publication
Not applicable.
Ethics approval and consent to participate
This study was approved by the Human Subjects Protection Program of the University of California San Diego, La Jolla, and by the Ethical Boards of Universidad Peruana Cayetano Heredia and Asociación Benéfica PRISMA, Lima, Peru.
Funding
The research leading to these results was funded by NIH-NIAID (U19AI089681) to JMV and funded in part by NIH-NIAID (R01AI110112) to JEC. The Biodefense and Emerging Infectious Disease training fellowship grant T32AI05532901 provided partial support for CP.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Author information
Authors and Affiliations
Corresponding author
Additional files
Additional file 1: Table S1A.
Monthly abundance, HBR, IR, EIR of Anopheles darlingi from Cahuide and Lupuna, 2013–2015. Table S1B. Monthly abundance, HBR, IR, EIR of Anopheles darlingi from Santa Emilia, 2015. Table S2. Monthly abundance, HBR, IR, EIR of Anopheles darlingi from Santa Emilia, 2014. Table S3. Infection of Anopheles darlingi by year, month, time, exophagic versus endophagic, village and Plasmodium species. Table S4. Kruskal-Wallis analysis on ranked abundance of Anopheles darlingi, Cahuide and Lupuna, 2013–2015 rainy season (January-June). Table S5. Pairwise F ST values comparing Anopheles darlingi by (A) year/locality, (B) exophagic/endophagic, and (C) collection time. Figure S1. Schedule of Anopheles darlingi human landing catch collections in Lupuna, Cahuide, and Santa Emilia, 2013–2015. Figure S2. Number of reported human cases of Plasmodium vivax and Plasmodium falciparum in Cahuide, Lupuna, and Santa Emilia, 2010–2016. Figure S3. Monthly human biting rate plotted against the monthly number of malaria cases in Cahuide and Lupuna, rainy season, 2013–2015. Figure S4. Estimation of number of clusters in SNP dataset. Figure S5. Results of STRUCTURE analysis of SNP dataset for K=2 and K=3, with individual Anopheles darlingi ordered by locality/year, exophagic/endophagic, and collection time period. Figure S6. Results of PCA of SNP dataset, with individual Anopheles darlingi coloured by locality/year and collection time period.
Additional file 2.
Dataset of Anopheles darlingi counts by collection, month, year, site, exophagic/endophagic, and time period. Collections in Cahuide and Lupuna from 2013-2015 and in Santa Emilia from 2015 are included.
Additional file 3.
Bash scripts with commands used to align the sequencing reads to the Anopheles darlingi genome scaffolds and to run the Stacks pipeline.
Additional file 4.
STRUCTURE file used for STRUCTURE, PCA and DAPC analyses, with genotypes for 162 individuals at 1,021 loci.
Additional file 5.
nextRAD sample collection information, number of sequence reads, and unique stacks genotyped. Includes collection and sequencing details for all 320 individuals sequenced, and Stacks details for 162 individuals included in Stacks and downstream analyses.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Prussing, C., Moreno, M., Saavedra, M.P. et al. Decreasing proportion of Anopheles darlingi biting outdoors between long-lasting insecticidal net distributions in peri-Iquitos, Amazonian Peru. Malar J 17, 86 (2018). https://doi.org/10.1186/s12936-018-2234-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12936-018-2234-4