Abstract
Background
After 2.83% genome reduction in Lactococcus lactis NZ9000, a good candidate host for proteins production was obtained in our previous work. However, the gene deletion process was time consuming and laborious. Here, we proposed a convenient gene deletion method suitable for large-scale genome reduction in L. lactis NZ9000.
Results
Plasmid pNZ5417 containing a visually selectable marker PnisZ-lacZ was constructed, which allowed more efficient and convenient screening of gene deletion mutants. Using this plasmid, two large nonessential DNA regions, L-4A and L-5A, accounting for 1.25% of the chromosome were deleted stepwise in L. lactis 9k-3. When compared with the parent strain, the mutant L. lactis 9k-5A showed better growth characteristics, transformability, carbon metabolic capacity, and amino acids biosynthesis.
Conclusions
Thus, this study provides a convenient and efficient system for large-scale genome deletion in L. lactis through application of visually selectable marker, which could be helpful for rapid genome streamlining and generation of restructured L. lactis strains that can be used as cell factories.
Similar content being viewed by others
Introduction
With a crucial role in dairy and health industries, Lactococcus lactis is a GRAS (generally regarded as safe) microorganism [1]. Features such as lack of immunogenic lipopolysaccharides and secretion of only one major protein [2] make L. lactis the commonly used microorganism in traditional food fermentation. Nowadays, with the development of whole genome sequencing and functional genomics technology, abundant data, including whole genome sequence and metabolic pathway of L. lactis, are available, which allow using L. lactis as an “efficient cell factory” for recombinant protein production and secretion [2]. However, an efficient genetic engineering system for L. lactis is still missing.
In recent decades, synthetic biology approaches have been used to improve the growth rate and other characteristics of bacteria, including Escherichia coli [3,4,5,6,7], Pseudomonas putida [8, 9], and Bacillus subtilis [10, 11], for industrial applications [12, 13]. In our previous study, 2.83% genome reduction was accomplished in L. lactis NZ9000 [14]. Various genome engineering tools have been constructed and used for genome editing in L. lactis, such as site-specific integration based on homologous recombination [15], marker-free method for chromosomal mutations/deletions using ssDNA oligo’s Cre/loxp recombination system [16, 17], and CRISPR–Cas9/CRISPR-based genome editing system [17, 18]. The most recently reported tool that allows multiple genes and large-scale genome deletion in L. lactis is two-plasmid (pNZ5319 and pNZTS-Cre) and Cre/loxp based site-specific recombination system [14, 19]. Plasmid pNZ5319 [20] was used to construct knock-out vectors, which was subsequently transformed into L. lactis and replaced the target gene with cassette lox66-P32-cat-lox71 through double-crossover recombination. Then, temperature-sensitive plasmid pNZTS-Cre [19] for Cre recombinase expression was transformed into the mutants, which integrated with the lox66-P32-cat-lox71 cassette. The Cre recombinase excised the cat gene through the two lox sites (lox66 and lox71) and generated a double-mutant loxP site (lox72), which displayed strongly reduced recognition by Cre recombinase [20], finally, pNZTS-Cre was cured through the shift of temperature [19]. However, screening of the second-crossover recombination through replica plating method is laborious and time-consuming.
In the present study, a convenient gene deletion system was established by replacing plasmid pNZ5319 with a new plasmid containing a selectable marker PnisZ-lacZ. This enabled identification of deletions through visual screening, which facilitated quicker and easier detection of deletion of genes in L. lactis. With this system, two large nonessential DNA regions (L-4A and L-5A) accounting for 1.25% of the genome were selected and deleted stepwise in L. lactis 9K-3 [14] with high efficiency, and ultimately, five large nonessential DNA regions deletion mutant L. lactis 9K-5A with 3.24% genome reduction was constructed. To explore the genetic potential of mutants, the whole genome of L. lactis 9K-5A was sequenced. Comparison of physiological traits and transcriptome analysis revealed that L. lactis 9k-5A outperformed the wild strain in several physiological traits assessed and exhibited much higher expression of genes involved in routine metabolism.
Results
Construction of vector pNZ5417
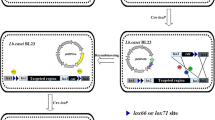
Plasmid pNZ5417, based on pNZ5319, was constructed by replacing ery with lacZ gene under the control of nisin-inducible promoter PnisZ, which produced blue colony on LB plate containing chloramphenicol and X-gal LB-CX (Fig. 1d).
Schematic representation of deletion plasmid pNZ5417 construction. a Scheme of pNZ5319; b color selectable marker gene cassettes (PnisZ-lacZ); c scheme of pNZ5417; d chromogenic reaction of pNZ5417 in E. coli on LB medium containing chloramphenicol and X-gal; e chromogenic reaction of L. lactis NZ9000 harboring pNZ5417Δ L4A in M17 medium containing chloramphenicol, X-gal, and gradient nisin
Evaluation of the new gene deletion system in L. lactis NZ9000
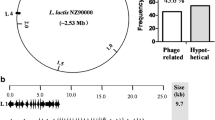
To evaluate the feasibility of pNZ5417 for large-scale gene deletion in Lactococcus strains, two large nonessential DNA regions, L4A and L5A, were successfully deleted stepwise in L. lactis 9k-3. The distribution of L4A and L5A throughout the genome is indicated in Fig. 2a. The genetic organization of the two deleted DNA regions is shown in Fig. 2b. A detailed description of genes included in the L4A and L5A regions is provided in Additional file 1: Table S1. These two DNA regions formed approximately 1.25% of the L. lactis 9k genome, as shown in Table 1.
To prove the higher deletion efficiency of plasmid pNZ5417, L4A was also deleted with plasmid pNZ5319 (Fig. 3). The deletion mutants were generated with both the deletion plasmids through double-crossover recombination and the following three steps were executed to obtain genes deletion mutants: (i) the deletion vector was constructed and the first-crossover recombination was accomplished; (ii) the cells were cultured and second-crossover recombination was achieved; and (iii) chloramphenicol resistance marker was deleted, and after it, plasmid pNZTS-Cre was cured. When compared with pNZ5319, 65% of the time was saved at the screening of second-crossover recombination, pNZ5417 gene deletion system allowed mutants screening based on color change, which is convenient and time-saving compared to the replica plating method.
Overall scheme for L4A deletion using two systems in L. lactis 9k. (1) Plasmid pNZ5319ΔL4A or pNZ5417ΔL4A was first loaded into L. lactis 9k-3 cells and the recombinants were selected on M17 plates supplemented with 5 μg/mL chloramphenicol (Cm); for pNZ5417ΔL4A, 40 μg/mL X-gal and 10 IU/mL nisin were also added; (2) recombinants harboring plasmid pNZ5319ΔL4A or pNZ5417ΔL4A were cultured in M17 medium supplemented with 5 μg/mL chloramphenicol for generations, and positive mutants with successful L4A deletion were selected by replica plating method (for “pNZ5319/pNZTS-Cre gene deletion system”) or color change (for “new gene deletion system”); (3) deletion of the chloromycetin resistance marker and elimination of temperature-sensitive plasmid pNZTS-Cre [19]
Assessment of growth profiles and transformability of mutants
The growth profiles of Lactococcus strains (L. lactis 9k, L. lactis 9k-4A, L. lactis 9k-5A) were monitored by recording the OD600 in GM17, respectively. The growth curves are shown in Fig. 4a (GM17). The results revealed that both the mutants grew much faster in the exponential phase, and reached the lag growth phase 1 h earlier than the parent strain. The doubling time of the two mutants proved to be much shorter than that of L. lactis 9k in the exponential growth phase (Table 1). As reported before, genome evolution should promote an enlarged genome size by incorporating non-essential accessory genes [21, 22], which might be disadvantageous for the growth fitness, given the additional cost for the replication and expression of the newly acquired sequences. After genome reduction, accumulative loss of dispensable genomic sequences like pseudogenes, phage/IS and unknown function genes contributes to bacterial growth in a dose-dependent manner [23]. Thus, we suppose the losing of unknown nonessential genes cased faster growth of mutants.
The electroporation efficiency of all the strains was measured by electroporating a small supercoiled plasmid, pNZ8048, into the cells. As shown in Fig. 4b, both mutants L. lactis 9k-4A (1.70-fold, P < 0.001) and L. lactis 9k-5A (2.45-fold, P < 0.001) exhibited higher electroporation efficiency than the parent strain (1.10 × 106/μg plasmid DNA). GyöRgy et al. [7] reported that removal of external structures and unknown deoxyribonuclease or restriction system or activation of an unknown DNA uptake factor could affect the recovery of transformants. Considering the results of transcriptome analysis (Additional file 2: Fig. S1), we suppose deletion of unknown genes affected the cellular component including membrane composition, and altered the ability of mutants to receive exogenous DNA.
Assessment of mutants’ phenotype
Extensive fermentation phenotype analyses of L. lactis 9k, L. lactis 9k-4A, and L. lactis 9k-5A were conducted using the phenotype microarrays to explore the physiological difference between the wild and mutant Lactococcus strains. All of the substrates that the mutants consumed were significantly different from those of L. lactis 9k. The result shown in Fig. 4c revealed that L. lactis 9k-4A can efficiently metabolize 12 carbon sources, particularly, α-cyclodextrin, β-cyclodextrin, and maltose; while L. lactis 9k-5A effectively metabolized 19 carbon sources, among which metabolism of α-cyclodextrin, β-cyclodextrin, maltose, maltotriose, and adenosine was 4.9-, 10.8-, 5.1-, 3.2-, and 4.6-fold higher than that of wild strain, respectively. We suppose this make it possible for mutants to utilize more carbon sources as the sole carbon source, especially α-cyclodextrin and β-cyclodextrin, as a member of oligosaccharide, they are much easier to get and cheaper carbon source than glucose. In contrast, both the mutants showed poor capacity to metabolize d-galacturonic acid and 3-methyl-d-glucose, with L. lactis 9k-5A losing its ability to metabolize d-galacturonic acid.
Genome sequencing and analysis of L. lactis 9k-5A
The genome of mutant L. lactis 9k-5A was sequenced by Shanghai Majorbio Bio-pharm Technology Co. (Shanghai, China) using the Illumina MiSeq platform. As shown in Additional file 3: Data S1A, the final assemble consisted of 91 scaffolds with a total size of 23,33,697 bp and 35.61% G+C content, including 66 large scaffolds with the largest scaffold comprising 396,482 bp. Furthermore, 2390 genes with a total length of 1,985,466 bp were predicted and annotated. The putative replication origins of L. lactis 9k-5A were localized by GC skew (Additional file 4: Data S2A). The 66 large scaffolds of L. lactis 9k-5A were arranged (Additional file 5: Data S1B) in the order of genome sequence of L. lactis NZ9000, and then subjected to BLAST (National Center for Biotechnology Information; https://blast.ncbi.nlm.nih.gov/Blast.cgi?PROGRAM=blastn&PAGE_TYPE=BlastSearch&LINK_LOC=MultiSensor). Sequence alignment indicated that the five nonessential DNA regions L1, L2, L3, L4A, and L5A were successfully deleted (Additional file 6: Data S2B).
Transcriptome assessment of mutants
Gene expression of L. lactis 9k, L. lactis 9k-4A, and L. lactis 9k-5A was analyzed using transcriptome analysis, and differentially expressed genes (DEGs; false discovery rate ≤ 0.001 and |log2| ≥ 1) were identified and subjected to Gene Ontology enrichment analysis (Additional file 7: Fig. S2A–C). When compared with L. lactis 9k, 117 genes were upregulated and 75 genes were downregulated in L. lactis 9k-4A (Additional file 7: Fig. S2D), and the expression of genes located in the four nonessential DNA regions L1, L2, L3, and L4A was significantly downregulated (Additional file 8: Table S2). In L. lactis 9k-5A, 245 genes were upregulated and 93 genes were downregulated (Additional file 7: Fig. S2D), and the expression of genes in the five nonessential DNA regions L1, L2, L3, L4A, and L5A was significantly downregulated, when compared with those in L. lactis 9k (Additional file 8: Table S2).
With the exception of genes with low expression level or located in the deleted nonessential DNA regions, 93 DEGs (FDR < 0.05) in the mutants were selected and analyzed in this study (Additional file 9: Fig. S3 and Additional file 10: Table S3). When compared with L. lactis 9k, biosynthesis of amino acids, purine metabolism, starch and sucrose metabolism, and some other pathways were significantly enriched in L. lactis 9k-5A (Fig. 5). As shown in Additional file 11: Data S3A, malL and malP genes involved in the metabolism of maltose, and malD, mdxF, and malX genes that participate in the transformation of maltotriose were significantly upregulated in L. lactis 9k-5A, which were in agreement with the GP2 MicroPlate results (Fig. 4c). Besides, the expression levels of genes involved in the synthesis of valine and isoleucine (ilvA, ilvB, ilvC, ilvD, ilvH), histidine (hisA, hisB, hisC, hisD, hisF, hisG, hisH, hisI, hisK, hisZ), and genes involved in glutamine metabolism (gltA, gltB, purC, purD, purE, purF, purK, purL, purM, purN, purQ, purS) were also upregulated in L. lactis 9k-5A (Additional file 11: Data S3A, Additional file 12: Data S3B and Additional file 13: Data S3C).
Subsequently, four genes with higher expression levels were examined by RT-qPCR to check the transcriptome data. When compared with L. lactis 9k, the expression levels of genes malD, purF, galE, and galK were only slightly changed in L. lactis 9k-4A (about 2.2-, 0.8-, 0.5-, and 0.5-fold, respectively), but were upregulated in L. lactis 9k-5A (up to 27.2-, 12.7-, 3.5-, and 7.9-fold, respectively) (Additional file 14: Fig. S4), which were in agreement with the results of transcriptome assay.
Discussion
With the rapid development of metabolic engineering, genome editing in bacteria, including industrial microorganisms such as E. coli [4, 24] and B. subtilis [25], has provided significant benefits. Xin et al. [26, 27] reported a single-plasmid genome editing system in lactic acid bacteria, which offered convenient and easy-to-use genome-editing tool for metabolic engineering in Lactobacillus casei. Guo et al. [17] established a rapid tool for genomic engineering by combining ssDNA recombinants with improved CRISPR/Cas9 counter selection, and achieved seamless genomic DNA deletions (50/100 bp) in L. lactis. However, sequential deletion of multiple genes and large-scale genome in L. lactis is still a time-consuming and laborious process. In the present study, we proposed a convenient system for sequential generation of combinatorial genome deletions in L. lactis.
Counter selection method based on homologous recombination is a convenient and efficient technique for L. lactis genome streaming [28, 29]. Nevertheless, the percentage of revertant mutations (60–92%) is much higher in double-crossover mutants [28], which makes the whole process of gene deletion much laborious. In our previous study, a two-plasmid (pNZ5319 and pNZTS-Cre) based gene deletion system was established with 100% correct deletion efficiency [14, 19]. However, laborious procedures were still needed to screen the second-crossover recombination through replica plating method (Fig. 3, Additional file 15: Table S4).
In the present study, the screening time decreased by 38% with the developed gene deletion system (Additional file 15: Table S4). Our proposed system comprised a visually selectable marker lacZ, which was under the control of inducible promoter PnisZ [30]. While the chromogenic reaction became more significant with the induction of nisin, the presence of original constitutive promoter P32 in “NZ9000 and MG1363 harboring pNZ5417ΔL4A” turned the cells blue in GM17 medium containing chloramphenicol and X-gal without nisin induction (Fig. 1e, Additional file 16: Fig. S5). Therefore, the use of this plasmid is not limited to nisin-controlled gene expression (NICE) system.
Two large nonessential DNA regions (L4A and L5A) of the L. lactis NZ9000 genome were deleted sequentially with our developed gene deletion system, and subsequently, a five large nonessential DNA regions (3.24% of the genome) deletion mutant was constructed. When compared with the parent strain, the two mutants, L. lactis 9k-4A and L. lactis 9K-5A, showed some good phenotypic changes, including better growth characteristics and transformability. The capability of the strains in metabolizing 95 carbon sources was compared using GP2 MicroPlate, which revealed that L. lactis 9k-4A and L. lactis 9k-5A had better capacity to metabolize 12 and 19 carbon sources, respectively. The results of transcriptome analysis indicated that 245 genes were upregulated and 93 genes were downregulated in L. lactis 9k-5A. The selected 93 DEGs showed significant enrichment of KEGG pathway, which indicated a much higher expression of malL, malP, malD, mdxF, and malX genes involved in the metabolism of maltose and transformation of maltotriose, suggesting that L. lactis 9k-5A had better ability to metabolize maltose and maltotriose, similar to the results of GP2 MicroPlate. Besides, genes involved in the pathway of histidine, valine, and isoleucine biosynthesis and some other pathways were significantly upregulated in L. lactis 9k-5A, implying that this mutant could be employed as a possible industrial cell factory for the production of these three amino acids.
Conclusion
To the best of our knowledge, this study is the first to introduce inducible visually selectable marker PnisZ-lacZ into L. lactis NZ9000 gene deletion system with improved efficiencies of 38% in achieving gene deletion mutants, which will save much more time in genome reduction. By using this system, two nonessential DNA regions were deleted sequentially in L. lactis. Our main contributions, in addition to the improved gene deletion system, was the final genome-streamlined mutant L. lactis 9k-5A exhibited good phenotypic changes, including better growth characteristics, transformability, carbon metabolic capacity, and biosynthesis of amino acids. The results of this study indicated that further genome refinements and reductions in L. lactis could eventually generate a significantly simplified strain that could contribute to broadening the use of this bacterium.
Methods
Bacterial strains, plasmids, and culture conditions
The strains and plasmids used in this study are listed in Table 2. L. lactis was grown at 30 °C under static condition in GM17 medium supplemented with 0.5% (w/v) glucose. E. coli DH5α cells were used as cloning host and grown aerobically at 37 °C in LB medium (1% tryptone, 0.5% yeast extract, and 1% NaCl; the solid medium contained 1.5% agar). Antibiotic selection was used when appropriate: for E. coli (per mL), 150 μg of erythromycin and 15 μg of chloramphenicol were employed and for L. lactis (per mL), 5 μg of erythromycin and 5 μg of chloramphenicol were applied. X-gal was used at a concentration of 80 μg/mL and nisin was utilized at a concentration of 10 IU/mL.
DNA manipulations and chemicals
DNA marker, T4 DNA ligase, restriction enzymes, and DNA gel extraction kit were purchased from Takara (Dalian, China). The PCR product purification kit, first-strand cDNA synthesis kit, and SYBR Green RT-qPCR kit were obtained from Thermo Fisher Scientific (Waltham, USA). The commercial X-gal and nisin were bought from Sigma-Aldrich (St. Louis, USA). L. lactis plasmid DNA, chromosomal DNA, and total RNA were isolated by using Qiaprep spin kit (small scale) following manufacturer’s instructions. PCR was performed with Phusion enzyme (Thermo Fisher Scientific, Waltham, USA). Primers were synthesized by BGI (Beijing, China) and the corresponding sequences are listed in Additional file 17: Table S5. PCR products and plasmids were sequenced by GENEWIZ service (Hangzhou, China). The competent E. coli DH5α cells were purchased from Takara (Dalian, China) and transformed by CaCl2 procedure [31]. Recombinant plasmids were introduced into L. lactis by electroporation according to the method described earlier [32].
Construction of vector pNZ5417
The plasmid pNZ5417 (Fig. 1c), containing lacZ gene under the control of nisin-inducible promoter PnisZ [30], was constructed from pNZ5319. Promoter PnisZ and lacZ gene were obtained from L. lactis N8 with primer pairs PnisZ-F/R and LacZ-F/R, combined by overlap PCR (Fig. 1b), and digested with BglI–SphI, and replaced the ery gene of pNZ5319 to generate pNZ5417.
Feasibility of new gene deletion system in L. lactis NZ9000
To evaluate the new gene deletion system, we deleted the large nonessential DNA region L4A in L. lactis 9k-3 [14] by using pNZ5319/pNZTS-Cre [19] and pNZ5417/pNZTS-Cre gene deletion system, respectively, and successfully constructed L. lactis 9k-4A. Gene knock-out vectors pNZ5319Δ L4A and pNZ5417Δ L4A were generated with the primer pairs L4A-UP-F/R and L4A DP-F/R. Vector pNZ5319Δ L4A was transformed into L. lactis 9k-3, and the deletion of L4A mutant with pNZ5319/pNZTS-Cre system was achieved as described earlier [19]. pNZ5417Δ L4A was transformed into L. lactis 9k-3, and single cross-over recombinant was selected at 30 °C on GM17-CXN solid medium containing chloramphenicol, X-gal, and nisin. The single cross-over recombinants were sub-cultured at 30 °C in GM17-CXN liquid medium several times, and the overnight cultures were diluted and plated on GM17-CXN medium at 37 °C until most colonies turned blue. The white colonies were selected and identified by primer pairs L4A Int-F/R and L4A Out-F/R. After single colony isolation, the cat selectable marker was excised as described previously, and the deletion mutants were tested by PCR with appropriate primers. The gene knock-out vector pNZ5417Δ L5A was constructed with primer pairs L5A-UP-F/R and L5A-DP-F/R, and the nonessential DNA region L5A was deleted in L. lactis 9k-4A with pNZ5417 gene deletion system to obtain five large nonessential DNA regions deletion mutant L. lactis 9k-5A.
Analysis of growth profiles
Lactococcus lactis 9k, L. lactis 9k-4A, and L. lactis 9k-5A were cultured to OD600 of 0.8 in GM17 medium and diluted to OD600 of 0.4. Then, 2 µL of the diluted cultures were reinoculated into 200 µL of GM17 medium in shake-flasks. The growth profiles were monitored by measuring OD600 for 10 h at 30 °C by using a Bioscreen machine (Lab-systems, Helsinki, Finland) [33]. The experiment was repeated three times.
Measurement of electroporation efficiency
Electrocompetent cells of all the strains were prepared by the method of Holo [32], and 2.5 µg of plasmid pNZ8048 DNA were added to 0.1 mL of competent cells. After electroporation, the cells were cultured in plates containing 15 µg/mL chloramphenicol for the selection of chloramphenicol-resistant transformants. The transformants were enumerated after 2 days of incubation at 30 °C, and the experiment was repeated three times.
Microarray analysis of mutants’ phenotype
The metabolism of the wild strain and mutants was examined with GP2 MicroPlate™ using phenotype microarrays system (Biolog, California, USA). Sample preparation and assays were conducted according to the manufacturer’s instructions. In brief, Lactococcus cells on the surface of solid medium were collected using cotton swab and suspended in inoculating fluid (0.40% NaCl, 0.03% Pluronics F-68, and 0.02% Gellan Gum) (Biolog, California, USA). The cell density was equalized, and 150 µL of the cells suspension were pipetted into GP2 plates with various substrates, respectively. Then, the plates were incubated in OmniLog® instrument (Biolog, California, USA) at 30 °C for 24 h. The data were automatically recorded every 30 min, and were analyzed by OL-OM software (version 3.0) (Biolog, California, USA).
Sequencing and analysis of L. lactis 9k-5A genome
The genomic DNA of L. lactis 9k-5A was extracted and purified, and then quantified using Nanodrop 2000 spectrophotometer (Thermo Scientific, USA). The L. lactis 9k-5A genome was sequenced by Shanghai Majorbio Bio-pharm Technology Co. (Shanghai, China) using Illumina MiSeq platform with a paired-end library. Following trimming and merging, the reads were assembled de novo using SOAP denovo V2.04 [34]. Open reading frames (ORFs) were predicted using Glimmer 3.02 program [35], and annotated by comparison with NCBI-NR and KEGG databases using BLASTp (BLAST 2/2/28+). Furthermore, tRNA and rRNA were predicted using tRNA scan-SE v. 1.3.1 and Barr nap 0.4.2 (www.vicbioinformatics.com/software.barrnap.shtml) programs, respectively [36].
Transcriptome analyses of mutants
The total RNA of L. lactis 9k, L. lactis 9k-4A, and L. lactis 9k-5A strains cultured in GM17 to an OD600 of 0.8 was extracted and purified by TRIzol kit (Promega USA), sequenced on Illumina sequencing platform, and analyzed by Genedenovo Biotechnology Co., Ltd (Guangzhou, China). Each sample was prepared in triplicate. The transcription of genes malD, purF, galE, and galK was measured through quantitative real-time PCR (RT-qPCR) to recheck the transcriptomic data. All RT-qPCR reactions were repeated independently three times. Data analysis was conducted by using comparative CT (2−∆∆CT) method with the housekeeping gene rpoB [37] as control. Transcription with more than twofold changes was regarded as significant difference [38].
Statistical analysis
The data obtained are reported as mean ± standard deviation (SD). The difference between two groups was compared by t-test with P < 0.05 considered as significant.
Availability of data and materials
All data generated or analyzed during this study are included in this published article [and its additional files].
Abbreviations
- L-4A, L-5A:
-
two large nonessential DNA regions
- LB-CX:
-
LB medium containing chloramphenicol and X-gal
- GM17-CXN:
-
GM17 medium containing chloramphenicol, X-gal, and nisin
- DEGs:
-
differentially expressed genes
References
Leroy F, De Vuyst L. Lactic acid bacteria as functional starter cultures for the food fermentation industry. Trends Food Sci Technol. 2004;15:67–78.
Morello E, Bermudez-Humaran L, Llull D, Sole V, Miraglio N, Langella P, Poquet I. Lactococcus lactis, an efficient cell factory for recombinant protein production and secretion. J Mol Microbiol Biotechnol. 2008;14:48–58.
Doerks T, Copley RR, Schultz J, Ponting CP, Bork P. Systematic identification of novel protein domain families associated with nuclear functions. Genome Res. 2002;12:47–56.
Hiroshi M, Hideo M, Tatsuro F. Escherichia coli minimum genome factory. Biotechnol Appl Biochem. 2011;46:157–67.
Mizoguchi H, Sawano Y, Kato J, Mori H. Superpositioning of deletions promotes growth of Escherichia coli with a reduced genome. DNA Res. 2008;15:277–84.
Hirokawa Y, Kawano H, Tanaka-Masuda K, Nakamura N, Nakagawa A, Ito M, Mori H, Oshima T, Ogasawara N. Genetic manipulations restored the growth fitness of reduced-genome Escherichia coli. J Biosci Bioeng. 2013;116:52–8.
GyöRgy P, Guy P, Tamás F, David F, Keil GM, Kinga U, Vitaliy K, Buffy S, Sharma SS, Monika DA. Emergent properties of reduced-genome Escherichia coli. Science. 2006;312:1044–6.
Lieder S, Nikel PI, Lorenzo VD, Takors R. Genome reduction boosts heterologous gene expression in Pseudomonas putida. Microb Cell Fact. 2015;14:23.
Martínez-García E, Jatsenko T, Kivisaar M, de Lorenzo V. Freeing P. seudomonas putida KT 2440 of its proviral load strengthens endurance to environmental stresses. Environ Microbiol. 2015;17:76–90.
Morimoto T, Kadoya R, Endo K, Tohata M, Sawada K, Liu S, Ozawa T, Kodama T, Kakeshita H, Kageyama Y, Manabe K. Enhanced recombinant protein productivity by genome reduction in Bacillus subtilis. DNA Res. 2008;15:73–81.
Ara K, Ozaki K, Nakamura K, Yamane K, Sekiguchi J, Ogasawara N. Bacillus minimum genome factory: effective utilization of microbial genome information. Biotechnol Appl Biochem. 2007;46:169–78.
Hal A, Joel M, Elke N, Fink GR, Gregory S. Engineering yeast transcription machinery for improved ethanol tolerance and production. Science. 2006;314:1565–8.
van Tilburg AY, Cao H, van der Meulen SB, Solopova A, Kuipers OP. Metabolic engineering and synthetic biology employing Lactococcus lactis and Bacillus subtilis cell factories. Curr Opin Biotechnol. 2019;59:1–7.
Zhu D, Fu Y, Liu F, Xu H, Saris PEJ, Qiao M. Enhanced heterologous protein productivity by genome reduction in Lactococcus lactis NZ9000. Microb Cell Fact. 2017;16:1.
Pinto JP, Zeyniyev A, Karsens H, Trip H, Lolkema JS, Kuipers OP, Kok J. pSEUDO, a genetic integration standard for Lactococcus lactis. Appl Environ Microbiol. 2011;77:6687–90.
van Pijkeren J-P, Britton RA. High efficiency recombineering in lactic acid bacteria. Nucleic Acids Res. 2012;40:e76.
Guo T, Xin Y, Zhang Y, Gu X, Kong J. A rapid and versatile tool for genomic engineering in Lactococcus lactis. Microb Cell Fact. 2019;18:22.
Berlec A, Škrlec K, Kocjan J, Olenic M, Štrukelj B. Single plasmid systems for inducible dual protein expression and for CRISPR–Cas9/CRISPRi gene regulation in lactic acid bacterium Lactococcus lactis. Sci Rep. 2018;8:1009.
Zhu D, Zhao K, Xu H, Zhang X, Bai Y, Saris PE, Qiao M. Construction of thyA deficient Lactococcus lactis using the Cre-loxP recombination system. Ann Microbiol. 2015;65:1659–65.
Lambert JM, Bongers RS, Kleerebezem M. Cre-lox-based system for multiple gene deletions and selectable-marker removal in Lactobacillus plantarum. Appl Environ Microbiol. 2007;73:1126–35.
Koonin EV, Wolf YI. Genomics of bacteria and archaea: the emerging dynamic view of the prokaryotic world. Nucleic Acids Res. 2008;36:6688–719.
Kurland CG, Canback B, Berg OG. Horizontal gene transfer: a critical view. Proc Natl Acad Sci. 2003;100:9658–62.
Kurokawa M, Seno S, Matsuda H, Ying B-W. Correlation between genome reduction and bacterial growth. DNA Res. 2016;23:517–25.
Umenhoffer K, Draskovits GB, Nyerges AK, Karcagi I, Bogos BZ, Tímár E, Csörgő BL, Herczeg RB, Nagy IN, Fehér TS. Genome-wide abolishment of mobile genetic elements using genome shuffling and CRISPR/Cas-assisted MAGE allows the efficient stabilization of a bacterial chassis. ACS Synth Biol. 2017;6:1471–83.
So Y, Park S-Y, Park E-H, Park S-H, Kim E-J, Pan J-G, Choi S-K. A highly efficient CRISPR–Cas9-mediated large genomic deletion in Bacillus subtilis. Front Microbiol. 2017;8:1167.
Xin Y, Guo T, Mu Y, Kong J. Coupling the recombineering to Cre-lox system enables simplified large-scale genome deletion in Lactobacillus casei. Microb Cell Fact. 2018;17:21.
Xin Y, Guo T, Mu Y, Kong J. A single-plasmid genome editing system for metabolic engineering of Lactobacillus casei. Front Microbiol. 2018;9:3024.
Wan X, Usvalampi AM, Saris PEJ, Takala TM. A counterselection method for Lactococcus lactis genome editing based on class IIa bacteriocin sensitivity. Appl Microbiol Biotechnol. 2016;100:1–9.
Christian S, Els D, Peter Ruhdal J, Jan M. Plasmid pCS1966, a new selection/counterselection tool for lactic acid bacterium strain construction based on the oroP gene, encoding an orotate transporter from Lactococcus lactis. Appl Environ Microbiol. 2008;74:4772–5.
Li R, Takala TM, Qiao M, Xu H, Saris PEJ. Nisin-selectable food-grade secretion vector for Lactococcus lactis. Biotechnol Lett. 2011;33:797–803.
Tang X, Nakata Y, Li HO, Zhang M, Gao H, Fujita A, Sakatsume O, Ohta T, Yokoyama K. The optimization of preparations of competent cells for transformation of E. coli. Nucleic Acids Res. 1994;22:2857–8.
Holo H, Nes IF. High-frequency transformation, by electroporation, of Lactococcus lactis subsp. cremoris grown with glycine in osmotically stabilized media. Appl Environ Microbiol. 1989;55:3119–23.
Wan X, Li R, Saris PE, Takala TM. Genetic characterisation and heterologous expression of leucocin C, a class IIa bacteriocin from Leuconostoc carnosum 4010. Appl Microbiol Biotechnol. 2013;97:3509–18.
Li R, Zhu HJ, Qian W, Fang X, Shi Z, Li Y, Li S, Shan G, Kristiansen K, Li S. De novo assembly of human genomes with massively parallel short read sequencing. Genome Res. 2010;20:265–72.
Delcher AL, Harmon D, Kasif S, White O, Salzberg SL. Improved microbial gene identification with GLIMMER. Nucleic Acids Res. 1999;27:4636–41.
Lowe TM, Eddy SR. tRNAscan-SE: a program for improved detection of transfer RNA genes in genomic sequence. Nucleic Acids Res. 1997;25:955–64.
Yi-Sheng C, Chi-Huan C, Shwu-Fen P, Li-Ting W, Yu-Chung C, Hui-Chung W, Fujitoshi Y. Lactococcus taiwanensis sp. nov., a lactic acid bacterium isolated from fresh cummingcordia. Int J Syst Evol Microbiol. 2013;63:2405–9.
Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods. 2001;25:402–8.
Woodcock DM, Crowther PJ, Doherty J, Jefferson S, Decruz E, Noyerweidner M, Smith SS, Michael MZ, Graham MW. Quantitative evaluation of Escherichia coli host strains for tolerance to cytosine methylation in plasmid and phage recombinants. Nucleic Acids Res. 1989;17:3469–78.
Qiao M, Immonen T, Koponen O, Saris PEJ. The cellular location and effect on nisin immunity of the NisI protein from Lactococcus lactis N8 expressed in Escherichia coli and L. lactis. FEMS Microbiol Lett. 1995;131:75–80.
Gasson MJ. Plasmid complements of Streptococcus lactis NCDO 712 and other lactic streptococci after protoplast-induced curing. J Bacteriol. 1983;154:1–9.
Kuipers OP, de Ruyter PG, Kleerebezem M, de Vos WM. Quorum sensing-controlled gene expression in lactic acid bacteria. J Biotechnol. 1998;64:15–21.
Kanehisa M, Goto S. KEGG: kyoto encyclopedia of genes and genomes. Nucleic acids Res. 2000;28:27–30.
Acknowledgements
We thank International Science Editing (http://www.internationalscienceediting.com) for editing this manuscript.
Funding
This work was financially supported by the National Nature Science Foundation of China (31770102) and the Tianjin Key research Program of Application Foundation and Advanced Technology (17JCZDJC32200). Fulu Liu is supported by the Chinese Scholarship Council (CSC).
Author information
Authors and Affiliations
Contributions
FL designed the study, analyzed the primary data and drafted the manuscript. FL, YZ and WQ carried out the experiments. DZ, HX, PS and MQ supervised the whole research work and revised the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Additional file 1: Table S1.
Gene content of deleted DNA regions.
Additional file 2: Figure S1.
Go terms analysis for all the DEGs of all mutants. (A) Go terms of L. lactis NZ9000-VS-L. lactis 9k-4A, (B) L. lactis NZ9000-VS-L. lactis 9k-5A, (C) L. lactis 9k-4A-VS-L. lactis 9k-5A.
Additional file 3: Data S1A.
Genome sequencing and analysis of L. lactis 9k-5A. (A) Genome sequencing of L. lactis 9k-5A.
Additional file 4: Data S2A.
Circular graph of L. lactis 9K-5A (A) and alignment with parent strain L. lactis NZ9000 (B). (A) Starting from the outside: genes encoded on the top and bottom strand (first and fourth ring), tRNA and rRNA on the bottom and top strand (second and third ring). Genes are colored according to the corresponding functional categories shown on the right side. The fifth ring shows GC content deviations from the genomic average. The innermost ring shows GC skew; positive skew is shown in green, and negative skew is shown in purple.
Additional file 5: Data S1B.
Genome sequencing and analysis of L. lactis 9k-5A. (B) Arrangement of 66 large scaffolds in L. lactis 9k-5A.
Additional file 6: Data S2B.
Circular graph of L. lactis 9K-5A (A) and alignment with parent strain L. lactis NZ9000 (B). (B) Horizontal axis is the genome sequence of L. lactis NZ9000 and vertical axis is the genome sequence of L. lactis 9K-5A.
Additional file 7: Figure S2.
Results of transcriptome analyses. (A–C) Volcano plot of different strains; green (downregulated) and red (upregulated) colors denote genes with significant changes in expression (DEGs), black color indicates no difference in gene expression. (D) Comparison of whole genome expression among all strains by RNA-Seq.
Additional file 8: Table S2.
Results of transcriptome analysis.
Additional file 9: Figure S3.
Heatmap profile and hierarchical cluster analysis of selected 93 genes expression in all the strains.
Additional file 10: Table S3.
List of 93 DEGs in mutants.
Additional file 11: Data S3A.
Results of significant enrichment of KEGG pathway in L. lactis 9k-5A. (A) Transcriptome analysis of 93 DEGs in mutants.
Additional file 12: Data S3B.
Results of significant enrichment of KEGG pathway in L. lactis 9k-5A. Enrichment pathway map of genes involved in (B) glutamine metabolism, (C) and biosynthesis of valine and isoleucine. Map was downloaded from the KEGG server with our data mapping to the pathway (http://www.kegg.jp/kegg). Significant changes in expression are color-coded: red, up-regulated; green, down-regulated [43]. Glutamine, valine and isoleucine histidine are marked with red arrows.
Additional file 13: Data S3C.
Results of significant enrichment of KEGG pathway in L. lactis 9k-5A. Enrichment pathway map of genes involved in (B) glutamine metabolism, (C) and biosynthesis of valine and isoleucine. Map was downloaded from the KEGG server with our data mapping to the pathway (http://www.kegg.jp/kegg). Significant changes in expression are color-coded: red, up-regulated; green, down-regulated [43]. Glutamine, valine and isoleucine histidine are marked with red arrows.
Additional file 14: Figure S4.
RT-qPCR analysis of genes with higher expression level. malD: sugar ABC transporter permease; purF: phosphoribosylpyrophosphate amidotransferase; galE: UDP-glucose 4-epimerase; galk: galactokinase.
Additional file 15: Table S4.
Comparison of the deletion efficiency of the two systems.
Additional file 16: Figure S5.
Chromogenic reaction of (A) L. lactis MG1363 harboring pNZ5417Δ L4A on M17 medium containing chloramphenicol and gradient X-gal, (B) L. lactis MG1363 on M17 medium containing X-gal.
Additional file 17: Table S5.
Primers utilized in this study.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Liu, F., Zhang, Y., Qiao, W. et al. Restructured Lactococcus lactis strains with emergent properties constructed by a novel highly efficient screening system. Microb Cell Fact 18, 198 (2019). https://doi.org/10.1186/s12934-019-1249-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12934-019-1249-z