Abstract
Background
Acid stress is often encountered during industrial fermentation as a result of the accumulation of acidic metabolites. Acid stress increases the intracellular acidity and can cause DNA damage and denaturation of essential enzymes, thus leading to a decrease of growth and fermentation yields. Although acid stress can be relieved by addition of a base to the medium, fermentations with acid-tolerant strains are generally considered much more efficient and cost-effective.
Results
In this study, the global regulator H-NS was found to have significant influence on the acid tolerance of E. coli. The final OD600 of strains overexpressing H-NS increased by 24% compared to control, when cultured for 24 h at pH 4.5 using HCl as an acid agent. To further improve the acid tolerance, a library of H-NS was constructed by error-prone PCR and subjected to selection. Five mutants that conferred a significant growth advantage compared to the control strain were obtained. The final OD600 of strains harboring the five H-NS mutants was enhanced by 26–53%, and their survival rate was increased by 10- to 100-fold at pH 2.5. Further investigation showed that the improved acid tolerance of H-NS mutants coincides with the activation of multiple acid resistance mechanisms, in particular the glutamate- and glutamine-dependent acid resistance system (AR2). The improved acid tolerance of H-NS mutants was also demonstrated in media acidified by acetic acid and succinic acid, which are common acidic fermentation by-products or products.
Conclusions
The results obtained in this work demonstrate that the engineering of H-NS can enhance the acid tolerance of E. coli. More in general, this study shows the potential of the engineering of global regulators acting as repressors, such as H-NS, as a promising method to obtain phenotypes of interest. This approach could expand the spectrum of application of global transcription machinery engineering.
Similar content being viewed by others
Background
During industrial bioprocessing microorganisms often encounter multiple stresses (e.g., high and low temperatures, extreme pH, osmotic stress and toxic compounds) [1], among which acid stress is one of the most concerned. The undissociated weak acids that accumulate during fermentation in acid-hydrolyzed lignocellulose liquor [2], or as metabolic products [3,4,5,6] and by-products [7] can permeate freely through cell membranes and then dissociate once entering into the cell cytoplasm releasing protons and conjugate bases [8,9,10]. The released protons increase the intracellular acidity causing DNA damage [11] and denaturation of essential enzymes [12], leading to impaired growth and fermentation yields [9]. The accumulated conjugate bases can perturb the anion balance of cells and affect their metabolism [8]. Although bases can be used to neutralize the acidic medium, thus preventing the diffusion of acids into the cells, fermentations are considered much more efficient and cost-effective with acid-tolerant strains [13,14,15].
Several approaches have been taken to increase the acid tolerance of bacterial strains. Mutagenesis induced by the chemical mutagen N-methyl-N′-nitro-N-nitrosoguanidine (NTG) has been applied to improve the acid tolerance of Zymomonas mobilis ZM4 [16], and adaptive laboratory evolution has been applied to improve the acid tolerance of E. coli [17,18,19]. These methods are, however, considered laborious and time-intensive since most mutations are neutral or detrimental to the desired phenotype [14, 17, 20, 21]. Other approaches, including overexpression of GadY [22] or ArrS [23], and overexpression of the arginine deaminase and glutamate decarboxylase systems by metabolic engineering [6] have also been applied. However, these approaches are not optimal since the acid tolerant phenotype of microorganisms, which includes both physiological and metabolic changes, is a complex multigenic trait [14, 24,25,26]. In recent years, transcriptional regulator engineering has emerged as an alternative to enhance the acid tolerance of microorganisms [1]. By this method, the expression of hundreds of genes can be perturbed simultaneously at the transcriptional level thus the tolerance can be enhanced without detailed knowledge about genotype–phenotype relationships and metabolic information [25, 27,28,29]. For example, the engineering of the exogenous global regulator, IrrE from Deinococcus radiodurans, was reported to improve the acid tolerance of E. coli DH5α [30]. The obtained mutant A15 had obvious growth advantage both in lysogeny broth (LB) medium supplemented with 0.05% acetate and in LB medium acidified by HCl. More recently, the endogenous global regulator CRP was engineered by error-prone PCR to improve the performance of E. coli DH5α at low pH [15]. The best mutant AcM1 showed almost double the growth rate as that of the control (0.113 and 0.062 h−1, respectively) at pH 4.24. Besides, the acid tolerance of E. coli DH5α was also improved by engineering the global regulator Sigma D factor (RpoD) by a random insertional–deletional strand exchange mutagenesis (RAISE) method [14]. The identified best strain Mutant VII exhibited a growth rate of 0.22 h−1 compared with 0.15 h−1 of the control.
In this work, we focused on the engineering of the histone-like nucleoid structuring factor (H-NS) to improve the acid tolerance of E. coli. H-NS, an abundant protein of 15.5 kDa, is one of the seven global regulators (i.e. CRP, IHF, FNR, FIS, ArcA, Lrp and H-NS) [31] and it is able to influence the expression of 5% of the genes in E. coli [32]. H-NS can preferentially silence the expression of horizontally transferred genes, which plays an important role in acquiring competitive fitness at minimal cost [33,34,35]. It can also interact with the transcription initiation and elongation complex, thereby exerting a dramatic negative effect on gene expression due to its ability to bind the AT-rich region commonly found in promoters [36,37,38]. H-NS regulates many genes involved in the response to environmental changes [39,40,41], and in particular the genes related to acid tolerance [32]. It has been reported that the knockout of hns can improve the survival of E. coli under extreme acid stress and increases the transcription of RpoS [42], which is one of the key regulators of the acid stress response [43, 44]. Further study showed that H-NS can influence the stability of RpoS by inhibiting the expression of IraD and IraM, which are anti-adaptor proteins that stabilize RpoS [45,46,47]. H-NS can repress almost all the structural genes and regulators involved in glutamate- and glutamine-dependent acid resistance (AR2), arginine-dependent acid resistance (AR3) and lysine-dependent acid resistance (AR4) systems [41, 48]. Besides, H-NS can repress the expression of the chaperone HdeA/B [49, 50] which are involved in the acid tolerance response of E. coli [26]. Taken together, these findings suggest that the engineering of H-NS might improve the acid tolerance of E. coli.
In this study, we first confirmed the link between H-NS and acid tolerance by investigating the effect of the knockout and overexpression of hns on cell growth in media acidified by HCl. Subsequently, a random mutagenesis library of hns was constructed by error-prone PCR and subjected to selection under acid stress. To shed some light on the mechanism behind the increase of acid tolerance, the transcriptome analysis of the strains showing the highest acid tolerance was performed. Glutamate decarboxylase (GAD) activity and the release of γ-amino butyric acid (GABA) and ammonia were further measured to test if the AR2 system was implicated. The acid tolerance of the engineered mutants was also evaluated in media supplemented with acetic or succinic acid, which are common acidic fermentation by-products or products.
Methods
Materials
Restriction enzymes, Q5 DNA polymerase and T4 DNA ligase were purchased from New England Biolabs (Beverly, MA, USA) and LA Taq DNA polymerase was purchased from TaKaRa Biotechnology (Dalian, China). Oligonucleotides were synthesized by Taihe Biotechnology Co., Ltd. (Beijing, China). Sequence analysis was performed either by Invitrogen (Carlsbad, CA, USA) or by Taihe Biotechnology Co., Ltd. (Beijing, China). The kits for DNA purification, gel recovery and plasmid mini-prep were purchased from Tiangen (Beijing, China).
Strains and plasmids
The strains and plasmids used in this study are listed in Table 1. The hns knockout strain E. coli MG1655 ∆hns was constructed by a scarless method based on the CRISPR-Cas9 system employing pCas and pTargetT-∆hns [51]. To construct plasmid pBR322-hns (WT), the open reading frame (ORF) of hns gene (414 bp) and the native promoter (604 bp) were amplified together from the genome of E. coli MG1655 with primers AatII-hns-for (CCCGGACGTCCAGCCACAGGCCCTCAATG) and XhoI-hns-rev (CCGCTCGAGTATTGCTTGATCAGGAAATCGTC) and inserted into plasmid pBR322 followed by the insertion of the rrnBT terminator from plasmid pEAS-1b [52].
Library construction and selection
The random mutagenesis of hns was performed by standard error-prone PCR [53]. The ORF of hns and the native promoter were amplified from plasmid pBR322-hns (WT) with primers AatII-hns-for and XhoI-hns-rev. After purification and digestion by AatII and XhoI, the error-prone PCR product was inserted into plasmid pBR322-hns (WT) to replace the wild type hns gene and the native promoter. The ligation product was then transformed into E. coli MG1655 competent cells by electroporation. Cells were incubated overnight at 37 °C on LB plates containing 50 μg/mL ampicillin, and were then scraped off to create a liquid library. The library size was 106 with a mutation rate controlled at 5 mutations/gene by adjusting the concentration of Mn2+.
The mutants were selected considering growth under moderate acid stress (pH 4.5) and survival under extreme acid stress (pH 2.5). For each round, 200 μL aliquots from 10 cultures of the library with initial OD600 0.05 were separately inoculated into 10 mL LBG medium (LB medium supplemented with 2% glucose) acidified by HCl to pH 4.5 and cultivated at 37 °C, 250 rpm for 16 h. After reaching stationary phase, the cultures were diluted to OD600 0.05 with fresh LBG medium of pH 4.5 and cultured to OD600 0.5–0.6, then were incubated for 1 h in LBG medium acidified by HCl to pH 2.5 and then were plated on LB plates containing 50 μg/mL ampicillin. The survived cells were scraped off for the next round. This procedure was repeated for 6 rounds, then 500 clones were selected to monitor the growth curves by an automated turbidimeter (Bioscreen C, Oy Growth Curves Ab Ltd., Helsinki, Finland). The plasmids harboring H-NS mutants showing the highest acid tolerance were extracted and re-transformed into fresh E. coli MG1655 competent cells for confirmation.
Growth assay
MG, Δhns and strains harboring pBR322-derived plasmids were grown overnight (about 16 h) in LBG medium of pH 7.0 at 37 °C. The cultures were then diluted to initial OD600 0.05 in 300 μL LBG medium of pH 7.0, LBG medium acidified by acetic acid to pH 5.35 or LBG medium acidified by HCl or succinic acid to pH 4.5. Then the cultures were incubated at 37 °C in 100-well Honeycomb microplates using an automated turbidimeter (Bioscreen C, Oy Growth Curves Ab Ltd., Helsinki, Finland) for online monitoring of OD600 for 24 h.
Acid shock assay
MG, ∆hns and strains harboring pBR322-derived plasmids were grown overnight in LBG medium of pH 7.0 at 37 °C. The cultures were then diluted to initial OD600 0.05 in LBG medium acidified by HCl to pH 4.5 and grown at 37 °C to exponential phase with OD600 0.5–0.6. The cultures were diluted with fresh LBG medium of pH 4.5 to OD600 0.5, then diluted 1:10 with LBG medium acidified by HCl to pH 2.5 in 96-well microplates and incubated for 1 h at 37 °C. The cultures before and after acid shock were serially diluted, plated on LB plates, incubated at 37 °C overnight and then photographed.
GAD activity assay
Cells grown in LBG medium acidified by HCl to initial pH 4.5 were harvested in exponential phase with OD600 0.5–0.6 and in stationary phase after being cultured for 16 h, by centrifugation. The pellet was washed once with phosphate buffer (20 mM, pH 7.4), pelleted again, and resuspended to 10 OD600/mL with potassium phosphate buffer (20 mM, pH 7.4). After sonication, the cell debris was removed by centrifugation, and the crude extracts were used for GAD activity assay. The colorimetric assay for measuring GAD activity was performed in a 96-well microplate format as previously described [54]. The standard reaction medium in each well consisted of 200 μL acetate buffer (20 mM, pH 4.8) containing 70 μM bromocresol green (BCG), 10 mM pyridoxal-5′-phosphate (PLP), 25 mM l-glutamate and 20 μL of crude extract. Blank medium without l-glutamate was used as background. The absorbance at 620 nm was monitored at 37 °C and data were collected every 1 min for 1 h using an Infinite M200 Pro plate reader (Tecan Group Ltd., Männedorf, Switzerland).
GABA measurement
Cells grown in LBG medium acidified by HCl to initial pH 4.5 were harvested in exponential phase with OD600 0.5–0.6 and in stationary phase after being cultured for 16 h, by centrifugation. The pellet was washed once with citrate buffer (25 mM, pH 4.5), pelleted again, and resuspended to 4 OD600/mL with 1 mL citrate buffer (25 mM, pH 4.5) supplemented with 10 mM l-glutamate. After 1 h of incubation, the culture was centrifuged and the supernatant was filtered for the analysis of GABA concentration. The concentration of GABA was measured as previously described [55, 56]. Briefly, 100 μL of reaction buffer containing sodium phosphate (750 mM, pH 9.0), 0.5 mM NADP+, 0.5 mM α-ketoglutarate, 0.5 mM dithiothreitol (DTT), 10 μg GABase (Sigma-Aldrich, Shanghai, PRC) and 10 μL of filtered supernatant were incubated for 1 h at 37 °C, and the absorbance at 340 nm was measured using an Infinite M200 Pro plate reader (Tecan Group Ltd., Männedorf, Switzerland). The concentration of GABA was determined by comparison of the observed absorbance versus a calibration curve obtained with standard solutions of GABA analyzed in the same conditions.
Ammonia release assay
The ammonia release assay was performed as previously described [57]. Cells grown in LBG medium acidified by HCl to initial pH 4.5 were harvested in exponential phase with OD600 0.5–0.6 and in stationary phase after being cultured for 16 h, by centrifugation. The pellet was washed once with citrate buffer (25 mM, pH 4.5), pelleted again, and resuspended to 1 OD600/mL with 10 mL citrate buffer (25 mM, pH 4.5) supplemented with 10 mM l-glutamine. The concentration of ammonium ions was continuously measured at 25 °C using a SevenCompac™ S220 electrochemical analytical meter (Mettler-Toledo AG, Schwerzenbach, Switzerland) equipped with an ammonia ion selective electrode (model DX218-NH4+, Mettler-Toledo GmbH, Schwerzenbach, Switzerland).
Transcriptome analysis by RNA-seq
Escherichia coli MG1655 and strain 3-36 were cultured overnight in LBG medium of pH 7.0. A 100 μL aliquot of the cultures was inoculated into 10 mL of LBG medium acidified by HCl to pH 4.5 and the cells were grown to exponential phase up to OD600 0.5–0.6. RNA extraction and RNA-seq were carried out by CapitalBio Corporation (Beijing, China). Total RNA was extracted using the TRIzol reagent kit (Invitrogen Life Technologies, Carlsbad, CA, USA) and RNeasy MinElute cleanup kit (Qiagen, Valencia, CA, USA) following the indications of the respective manufacturers. RNA sequencing was carried out with an Illumina HiSeq 2500 system (Illumina, San Diego, CA). The gene expression difference was analyzed by Cuffdiff algorithm [58]. Genes with |log2(fold change)| ≥ 1 and p-value < 0.05 were assigned as differentially expressed genes. Three biological replicates of each strain were analyzed to allow for statistical analysis.
Results
Influence of H-NS on acid tolerance of E. coli
A previous study showed that the overexpression of hns initiated by the strong promoter λPL on the high copy number plasmid pTZ18R is lethal and incompatible with cell survival [59]. Based on this information, we chose to test the effect of H-NS on acid tolerance by using the native promoter and a low copy number plasmid. The hns gene containing the native promoter (1018 bp) was ligated into the low copy number plasmid pBR322 (15–20 copy numbers) [60] to construct strain H-NS(WT) or deleted from the genome via scarless CRISPR-Cas9 genome editing [51] to construct strain Δhns. A growth assay under moderate acid stress (i.e., 24 h of incubation in LBG medium acidified to pH 4.5 by HCl) and an acid shock assay under extreme acid stress (i.e., 1 h of incubation in LGB medium acidified to pH 2.5 by HCl) were performed to investigate the effect of overexpression and knockout of hns on acid tolerance. Under moderate acid stress, only the final OD600 value of strain H-NS(WT) (i.e. the strain overexpressing hns) was 24% higher than that of the wild type strain (MG) (Fig. 1a). However, both modified strains showed growth disadvantages in exponential phase. It is worth to note that the pH value remained constant at pH 4.5 during the whole fermentation period (data not shown). Overexpression of hns had no effect on the survival under extreme acid stress, while the knockout of hns showed an increase of the survived cell number of about 100-fold, compared to the wild type strain MG (Fig. 2).
Growth of strains harboring H-NS mutants under acid stress. The change of OD600 of strains MG, Δhns, H-NS(WT) and strains harboring H-NS mutants cultured for 24 h in LBG medium with different initial pH values obtained by the addition of HCl: a pH 4.5 acidified by HCl, b pH 7.0 (not acidified), c pH 5.35 acidified by acetic acid and d pH 4.5 acidified by succinic acid. Error bars indicate the standard error of at least three biological replicates
Survival of strains harboring H-NS mutants after acid shock. Comparison of the viability of strains MG, H-NS(WT) and strains harboring H-NS mutants incubated for 1 h in LBG medium (left panel) and LBG medium acidified by HCl to pH 2.5 (right panel) The images represent serial dilutions of the cultures in 10-fold steps (from left to right: 1:1 to 1:10,000)
Selection and characterization of acid tolerant mutants
After we confirmed that H-NS has a significant influence on acid tolerance and that the overexpression of hns can improve the growth under moderate acid stress, a library of H-NS mutants was constructed by a standard error-prone PCR protocol. The native promoter of hns was mutated together with the gene considering the dramatic effect of expression level on the growth [59]. The library was subjected to selection considering both growth under moderate acid stress (pH 4.5) and survival rate after acid shock (pH 2.5) as described in “Methods” section. After selection, the five mutants 3-36, 5-30, 9-1, 9-36 and 10-21 were obtained.
As shown in Fig. 1a, the final OD600 values of strains 3-36, 5-30, 9-1, 9-36 and 10-21 after culturing for 24 h under moderate acid stress were enhanced by 53, 33, 31, 49 and 26% compared with strain MG, respectively. The maximum growth rate of strains harboring H-NS mutants in exponential phase was higher than that of strain H-NS(WT), but lower than that of strain MG (Additional file 1: Table S1). The growth in LBG medium of pH 7.0 was also assayed (Fig. 1b). Under this condition, all of the strains harboring H-NS mutants showed a growth advantage compared with strain MG and after 24 h the OD600 value of the best strain, strain 3-36, was 29% higher that of strain MG.
An acid shock assay was subsequently performed to test the acid tolerance of mutants towards extreme acid stress. All the strains harboring H-NS mutants showed a higher survival rate than strain MG (Fig. 2). In particular, compared to strain MG, the number of cells that survived the acid shock was nearly 100-fold higher for mutants 3-36, 9-1, 10-21, and 10-fold higher for mutants 5-30 and 9-36. These results indicated that mutations of H-NS can result in an increase of growth under moderate acid stress and of survival rate under extreme acid stress.
We have also checked the morphology of cells harboring H-NS mutants. Cells of strain H-NS(WT) were much longer in size than those of strain MG, but the morphology of cells harboring H-NS mutants was similar to those of strain MG (Additional file 2: Figure S1).
Growth under moderate acid stress induced by other acids commonly encountered in fermentations
The accumulation of acidic metabolites, in particular acetic acid and succinic acid, is commonly encountered during fermentations. Acetic acid is a well-known growth inhibitor found in lignocellulosic hydrolysates [2] and a typical by-product during fermentation [7]. Succinic acid is a valuable chemical useful for biorefining applications [9]. To explore whether the mutation of H-NS could confer tolerance in acid environments commonly encountered during fermentations, growth assays were performed in LBG medium acidified by the two common acidic metabolites acetic acid and succinic acid. All the strains harboring H-NS mutants showed higher OD600 compared with strain MG when cultured in LBG medium acidified by acetic acid to pH 5.35 (Fig. 1c). Strain 3-36, which showed the highest growth when the medium was acidified by HCl, also showed the highest OD600 in acetic acid environment with a 39.7% improvement over strain MG. In LBG medium acidified by succinic acid to pH 4.5, only strains 5-30 and 9-36 had a slight growth advantage (Fig. 1d), with final OD600 values 9.6 and 22.1% higher than that of strain MG, respectively.
Transcriptional expression profile of H-NS mutant strain 3-36
To determine the transcriptional changes in strains harboring mutant H-NS and elucidate how the mutation of H-NS confers acid tolerance, strain 3-36, which showed the highest growth in moderate acid conditions, and strain MG were grown to exponential phase under moderate acid stress then their transcriptomes were determined and compared. The analysis showed that the mutation of H-NS changed the expression of a large number of genes (Additional file 3: Table S2). A total of 215 genes were differentially expressed with more than 2-fold change and p-value < 0.05 between strain 3-36 and strain MG, including 128 genes up-regulated and 87 genes down-regulated. Among them, only 24 genes have already been reported to be directly regulated by H-NS.
Noteworthy, many genes involved in the acid tolerance were highly up-regulated in strain 3-36 (Fig. 3a). The transcription of ybaS, ybaT, gadA, gadB and gadC, which compose the AR2 system, increased 9.1-, 9.4-, 41.4-, 61.8-, and 53.8-fold, respectively. This is consistent with the results obtained with the GAD activity assay and the GABA and ammonia release assays (see below). Similarly, the transcription of adiA, cadA and cadB, which belong to the AR3 and AR4 systems, increased 2.5-, 2.3-, 2.2-fold, respectively. Moreover, hdeA and hdeB, which express periplasmic chaperones and dps and hsp31, which express cytoplasmic chaperones, were upregulated 14.1-, 15.9-, 2.7-, 1.9-fold, respectively. The transcription of yciE and yciF in yciGFE operon was also highly up-regulated (8.1- and 15.1-fold, respectively) (Fig. 3b). The gene yciG is considered an important regulator for the tolerance to acid stress, oxidative stress and thermal stress in stationary phase [61]. yciE and yciF are located in the same operon expressing heat shock proteins. Additionally, the transcription of otsA and otsB, which convert UDP-glucose to trehalose, a disaccharide believed to protect the cells against various physical and chemical stresses [62], increased 3.3- and 3.9-fold (Additional file 3: Table S2).
Transcriptome analysis of strain 3-36 compared with strain MG. a Differentially regulated genes involved in acid resistance mechanisms. b Influence of 3-36 on the regulatory network of acid tolerance. The number below each gene is the log2(fold change) of strain 3-36 compared with strain MG. Red values: up-regulated in 3-36. AFI acid fitness island
The H-NS mutant also altered the expression of transcriptional regulators. Among them, the transcription of evgA, evgS, ydeO, gadE, gadW, gadX and cueR, which are crucial in acid tolerance [41], increased 2.3-, 5.2-, 2.0-, 48.5-, 10.6-, 9.7- and 2.2-fold, respectively (Fig. 3b). These results suggested that mutations of H-NS improve the acid tolerance of E. coli by activating multiple acid resistance systems through the regulatory network.
Activation of the AR2 system
Acid tolerance is a complex trait regulated by several acid resistance systems (ARs) [26]. Among the known ARs, the AR2 system, which acts by consuming intracellular protons, is considered to be the most important under fermentative conditions [63]. The AR2 system is originally reported as glutamate-dependent and consists of the glutamate decarboxylases GadA and GadB and the glutamate/GABA antiporter GadC [48]. To elucidate how the AR2 system was implicated in the mechanism behind the observed acid tolerance, the GAD activity of H-NS mutant strains cultured up to both exponential phase and stationary phase were assayed. The GAD activity of all the mutant strains was higher than that of the MG strain in both exponential phase and stationary phase. In particular, the GAD activity of strains 3-36, 5-30, 9-1, 9-36 and 10-21 was 4.9-, 2.1-, 3.6-, 2.2- and 3.4-fold higher than that of strain MG in exponential phase, and 4.1-, 2.1-, 2.7-, 3.0- and 3.8-fold higher than that of strain MG in stationary phase, respectively (Fig. 4a).
Activation of the AR2 system by H-NS mutants: GAD activity and GABA release. a GAD activity of cells harboring wild type H-NS and H-NS mutants in exponential phase (EP) and stationary phase (SP). b Concentration of GABA released by cells harboring wild type H-NS and H-NS mutants in EP and SP after 1 h incubation with excess of l-glutamate in citrate buffer (25 mM, pH 4.5). Error bars indicate the standard error of at least two biological replicates
GABA would be released to the medium during the process of AR2 functioning [64]. The amount of GABA released by the cells in exponential phase and stationary phase was measured to further explore the influence of AR2 in the acid tolerance displayed by H-NS mutants. As observed for the GAD activity, the release of GABA of all the mutant strains was higher than that of the MG strain in both exponential phase and stationary phase. In particular, the amount of GABA released by strains 3-36, 5-30, 9-1, 9-36 and 10-21 was 5.4-, 3.0-, 5.2-, 2.1- and 5.1-fold higher than that released by strain MG in exponential phase, and 1.6-, 5.4-, 2.5-, 0.7- and 6.1-fold than that released by strain MG in stationary phase, respectively (Fig. 4b).
Furthermore, the involvement of glutamine in the mechanism of acid tolerance operated by the AR2 system was recently reported [57]. According to this model, glutamine is imported into the cytoplasm by GadC [57] or YbaT [65], and converted into glutamate and ammonia by YbaS. The released ammonia is then protonated into ammonium ions while neutralizing the acids present in the cytoplasm. Therefore, the level of ammonium ions released by the cells in citrate buffer of pH 4.5 with l-glutamine in excess was monitored to further test the activation of the AR2 system in H-NS mutants. All strains harboring H-NS mutants had a higher rate of ammonium release except strain 3-36 in stationary phase (Fig. 5). In particular, the rate of ammonium release of strains 3-36, 5-30, 9-1, 9-36 and 10-21 in exponential phase was 2.8-, 1.4-, 3.1-, 1.4- and 4.4-fold higher than that of strain MG, respectively (Fig. 5a). The rate of ammonium release of strains 3-36, 5-30, 9-1, 9-36 and 10-21 in stationary phase was 0.8-, 2.0-, 1.9-, 1.1- and 2.0-fold of strain MG (Fig. 5b), respectively. Taken together, these results demonstrated the involvement of the AR2 system in the acid tolerance showed by the H-NS mutants.
Enzymatic release of ammonia of cells harboring H-NS mutants. Accumulation of ammonium ions obtained by incubating cells harboring wild type H-NS and H-NS mutants in citrate buffer (25 mM, pH 4.5) containing l-glutamine in excess: a cells in exponential phase (EP) and b cells in stationary phase (SP). Error bars indicate the standard error of at least two biological replicates
Sequence alignment and mutation analysis of the mutants
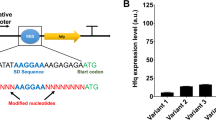
The full-length H-NS structure was previously determined by solid-state NMR [66]. H-NS is comprised of two functional domains, a N-terminal oligomerization domain (residues 1-79) and a C-terminal DNA binding domain (residues 95-137), separated by a flexible linker (residues 80-94) [67]. The amino acid mutations together with the mutations in the promoter region of the H-NS mutants were sequenced and aligned and are summarized in Fig. 6. Interestingly, all the mutants had mutations in the promoter region and coded region, a total of nine mutations (S2R, C21R, E27K, Q60R, T110S, I119F, A122V, M123V, L130P) were found. Among them, four mutations (S2R, C21R, E27K, Q60R) fell into the N-terminal oligomerization domain, while five mutations (T110S, I119F, A122V, M123V, L130P) were located in the C-terminal DNA binding domain. Given the number of mutations in the promoter region, qTR-PCR was further carried out to determine the transcription levels of these mutants, and it was found these mutations all increased the transcription by various degrees compared with strain MG (Additional file 4: Figure S2).
Summary of the mutations in the promoter region and the primary sequence of H-NS mutants. The red triangle indicates that the start codon was mutated from the native AUG to ACG without amino acid mutation [76]
Discussion
Acid stress arising from biomass hydrolysates and acidic metabolic products can damage cells and impair their growth and fermentation yield [9, 10, 68, 69]. Previous studies based on global transcription machinery engineering (gTME) employed tailored transcriptional regulators acting as activators to improve the tolerance of E. coli strains to environmental stress [14, 21, 30, 70, 71]. The possibility to use regulators acting as repressors to achieve the same goals has not yet been investigated. In this work, we investigated the influence of the global regulator and repressor H-NS on acid tolerance and we engineered H-NS to improve the acid tolerance of the commonly used workhorse E. coli. The five mutants obtained showed growth advantages in moderate acid environment and a higher survival rate in extreme acid environment compared to the wild type strain MG. Strain 3-36 harboring the best H-NS mutant in this study grew slightly better than a previously reported strain AcM1 harboring CRP mutant (53% versus 46%) under moderate acid stress [15].
The comparative transcriptome analysis showed that H-NS mutations can up-regulate several genes involved in well-studied acid resistance mechanisms including protective chaperones, and the proton-consuming AR2, AR3 and AR4 systems [26]. The results of GAD activity assay and ammonia and GABA release assays confirmed the up-regulation of the AR2 system in these H-NS mutants. The transcription of sodC and katE was also increased (2.5- and 2.8-fold, respectively) (Additional file 3: Table S2). SodC and katE, which express superoxide dismutase and catalase II, respectively, are involved in removing intracellular ROS (reactive oxygen species) which often increase significantly when cells are under stress conditions [21, 72, 73]. The up-regulation of these two genes in our H-NS mutants, together with the up-regulation of katE previously observed in another acid-tolerant strain [15], suggests that the removal of ROS is an important aspect of acid tolerance. Taken together, these findings confirms the crucial role of H-NS in the regulation of acid tolerance [41].
In addition to the tolerance showed towards HCl, the strains harboring H-NS mutants exhibited tolerance also towards acetic acid and succinic acid, although the performance depended on the type of acid used to induce the stress (Fig. 1). For instance, strain 3-36 showed the best growth performance in media acidified by HCl (pH 4.5) and acetic acid (pH 5.35) but it showed only a slight growth advantage in medium acidified by succinic acid (pH 4.5). This result was not surprising as a similar behavior was also observed in previous studies [15, 74]. Such dependence likely results from two factors: the different amount of acid that remains undissociated in the medium, which influences its passive transport across biological membranes, and the different toxicity of the conjugate bases [9].
Sequencing results provided some useful insights into the mechanism behind the acid tolerance of the H-NS mutants. H-NS acts almost exclusively as a repressor [75], and it is estimated to have approximately 20,000 copies per genome [75]. H-NS binds to about 250 loci, mostly in fimbriae- and phage-related genes and IS (insertion sequence element)-inserted genes, with a particular preference to horizontally acquired DNA [33]. H-NS directly represses more than 20 genes involved in acid tolerance and almost half of them are transcriptional regulators, including evgA, ydeO, gadE, gadW, gadX, adiY, cadC [41]. All the H-NS mutants of this study had mutations within the promoter region, which resulted in a modest increase of the transcription levels of H-NS, except for the mutant 9-36, whose transcription was enhanced by 16.8-fold (Fig. 6 and Additional file 4: Figure S2). This enhanced transcription, however, is unlikely to be reflected in an increased translation because the start codon for this mutant was changed from the native AUG to ACG, which can sharply decrease the translation initiation efficiency [76]. Indeed, as can be seen in Additional file 5: Figure S3, the expression level of each H-NS mutant, as determined by a GFP assay, was comparable to that of wild-type (50–89%), except for the mutant 9-36. For this mutant, as we suspected, the expression level was only 3–13% of the wild type. Among the nine mutations in the coded region of the H-NS mutants, five mutations (T110S, I119F, A122V, M123V and L130P) were located in the DNA binding region, and one of them (T110S) in the highly conserved core of the DNA binding motif [75]. It has been reported that several mutations at sites T110, I119, A122, M123 and L130 (DNA binding region) reduce the binding of H-NS to DNA or are involved in loss of function for H-NS [77, 78], while the mutation at site C21 (oligomerization region) increases the susceptibility of H-NS to Lon protease [42, 75]. Based on this information, we surmise that the H-NS mutants found in this study must be defective in DNA binding and since H-NS functions in a tetrameric form, the defective H-NS monomers might act as a spoiler in the formation of functional H-NS oligomers, thereby reducing the repression of H-NS. This mechanism would be consistent with the growth disadvantage that was observed in the exponential phase for the strain carrying the wild type H-NS on the plasmid (Fig. 1a). It cannot be excluded that these mutations may also induce more subtle changes to the binding of H-NS to other loci. While further investigation is required to fully understand the mechanism behind the acid tolerance of H-NS mutants, this work does suggest that fine-tuning the repressive action of H-NS can be a useful approach for improving acid tolerance in E. coli.
Conclusions
The influence of the global regulator H-NS on the acid tolerance of E. coli was studied. We showed that the H-NS mutants improved the acid tolerance by activating multiple acid resistance mechanisms, especially the acid resistance system AR2. This study proved that the engineering of global regulators that act as repressors can be a successful strategy to obtain strains with phenotypes of interest, thereby expanding the spectrum of application of gTME.
Abbreviations
- OD600 :
-
optical density at 600 nm
- gTME:
-
global transcription machinery engineering
- AR2:
-
acid resistance system 2
- AR3:
-
acid resistance system 3
- AR4:
-
acid resistance system 4
- ORF:
-
open reading frame
- GAD:
-
glutamate decarboxylase
- GABA:
-
γ-amino butyric acid
- EP:
-
exponential phase
- SP:
-
stationary phase
- AFI:
-
acid fitness island
References
Lin ZL, Zhang Y, Wang JQ. Engineering of transcriptional regulators enhances microbial stress tolerance. Biotechnol Adv. 2013;31:986–91.
Klinke HB, Thomsen AB, Ahring BK. Inhibition of ethanol-producing yeast and bacteria by degradation products produced during pre-treatment of biomass. Appl Microbiol Biotechnol. 2004;66:10–26.
Dien BS, Nichols NN, Bothast RJ. Recombinant Escherichia coli engineered for production of l-lactic acid from hexose and pentose sugars. J Ind Microbiol Biotechnol. 2001;27:259–64.
Vemuri GN, Eiteman MA, Altman E. Effects of growth mode and pyruvate carboxylase on succinic acid production by metabolically engineered strains of Escherichia coli. Appl Environ Microbiol. 2002;68:1715–27.
Warnecke TE, Lynch MD, Karimpour-Fard A, Lipscomb ML, Handke P, Mills T, et al. Rapid dissection of a complex phenotype through genomic-scale mapping of fitness altering genes. Metab Eng. 2010;12:241–50.
Guan NZ, Li JH, Shin HD, Du GC, Chen J, Liu L. Metabolic engineering of acid resistance elements to improve acid resistance and propionic acid production of Propionibacterium jensenii. Biotechnol Bioeng. 2015;113:1294–304.
Kirkpatrick C, Maurer LM, Oyelakin NE, Yoncheva YN, Maurer R, Slonczewski JL. Acetate and formate stress: opposite responses in the proteome of Escherichia coli. J Bacteriol. 2001;183:6466–77.
Roe AJ, McLaggan D, Davidson I, O’Byrne C, Booth IR. Perturbation of anion balance during inhibition of growth of Escherichia coli by weak acids. J Bacteriol. 1998;180:767–72.
Warnecke T, Gill RT. Organic acid toxicity, tolerance, and production in Escherichia coli biorefining applications. Microb Cell Fact. 2005;4:25.
Zaldivar J, Ingram LO. Effect of organic acids on the growth and fermentation of ethanologenic Escherichia coli LY01. Biotechnol Bioeng. 1999;66:203–10.
Choi SH, Baumler DJ, Kaspar CW. Contribution of dps to acid stress tolerance and oxidative stress tolerance in Escherichia coli O157:H7. Appl Environ Microbiol. 2000;66:3911–6.
Roe AJ, O’Byrne C, McLaggan D, Booth IR. Inhibition of Escherichia coli growth by acetic acid: a problem with methionine biosynthesis and homocysteine toxicity. Microbiology. 2002;148:2215–22.
van Maris AJ, Konings WN, Dijken JP, Pronk JT. Microbial export of lactic and 3-hydroxypropanoic acid: implications for industrial fermentation processes. Metab Eng. 2004;6:245–55.
Gao X, Jiang L, Zhu LY, Xu Q, Xu X, Huang H. Tailoring of global transcription sigma D factor by random mutagenesis to improve Escherichia coli tolerance towards low-pHs. J Biotechnol. 2016;224:55–63.
Basak S, Geng HF, Jiang RR. Rewiring global regulator cAMP receptor protein (CRP) to improve E. coli tolerance towards low pH. J Biotechnol. 2014;173:68–75.
Joachimsthal E, Haggett KD, Jang JH, Rogers PL. A mutant of Zymomonas mobilis ZM4 capable of ethanol production from glucose in the presence of high acetate concentrations. Biotechnol Lett. 1998;20:137–42.
Dragosits M, Mozhayskiy V, Quinones-Soto S, Park J, Tagkopoulos I. Evolutionary potential, cross-stress behavior and the genetic basis of acquired stress resistance in Escherichia coli. Mol Syst Biol. 2013;9:643.
Harden MM, He A, Creamer K, Clark MW, Hamdallah I, Martinez KA 2nd, et al. Acid-adapted strains of Escherichia coli K-12 obtained by experimental evolution. Appl Environ Microbiol. 2015;81:1932–41.
Creamer KE, Ditmars FS, Basting PJ, Kunka KS, Hamdallah IN, Bush SP, et al. Benzoate- and salicylate-tolerant strains of Escherichia coli K-12 lose antibiotic resistance during laboratory evolution. Appl Environ Microbiol. 2017;83:e02736–816.
Warner JR, Patnaik R, Gill RT. Genomics enabled approaches in strain engineering. Curr Opin Microbiol. 2009;12:223–30.
Wang JQ, Zhang Y, Chen YL, Lin M, Lin ZL. Global regulator engineering significantly improved Escherichia coli tolerances toward inhibitors of lignocellulosic hydrolysates. Biotechnol Bioeng. 2012;109:3133–42.
Negrete A, Shiloach J. Constitutive expression of the sRNA GadY decreases acetate production and improves E. coli growth. Microb Cell Fact. 2015;14:148.
Aiso T, Kamiya S, Yonezawa H, Gamou S. Overexpression of an antisense RNA, ArrS, increases the acid resistance of Escherichia coli. Microbiology. 2014;160:954–61.
Nicolaou SA, Gaida SM, Papoutsakis ET. A comparative view of metabolite and substrate stress and tolerance in microbial bioprocessing: from biofuels and chemicals, to biocatalysis and bioremediation. Metab Eng. 2010;12:307–31.
Alper H, Stephanopoulos G. Global transcription machinery engineering: a new approach for improving cellular phenotype. Metab Eng. 2007;9:258–67.
Kanjee U, Houry WA. Mechanisms of acid resistance in Escherichia coli. Annu Rev Microbiol. 2013;67:65–81.
Alper H, Moxley J, Nevoigt E, Fink GR, Stephanopoulos G. Engineering yeast transcription machinery for improved ethanol tolerance and production. Science. 2006;314:1565–8.
Santos CNS, Xiao WH, Stephanopoulos G. Rational, combinatorial, and genomic approaches for engineering l-tyrosine production in Escherichia coli. Proc Natl Acad Sci USA. 2012;109:13538–43.
Kim HJ, Turner TL, Jin YS. Combinatorial genetic perturbation to refine metabolic circuits for producing biofuels and biochemicals. Biotechnol Adv. 2013;31:976–85.
Chen TJ, Wang JQ, Yang R, Li JC, Lin M, Lin ZL. Laboratory-evolved mutants of an exogenous global regulator, IrrE from Deinococcus radiodurans, enhance stress tolerances of Escherichia coli. PLoS ONE. 2011;6:e16228.
Martı́nez-Antonio A, Collado-Vides J. Identifying global regulators in transcriptional regulatory networks in bacteria. Curr Opin Microbiol. 2003;6:482–9.
Hommais F, Krin E, Laurent-Winter C, Soutourina O, Malpertuy A, Le Caer JP, et al. Large-scale monitoring of pleiotropic regulation of gene expression by the prokaryotic nucleoid-associated protein, H-NS. Mol Microbiol. 2001;40:20–36.
Oshima T, Ishikawa S, Kurokawa K, Aiba H, Ogasawara N. Escherichia coli histone-like protein H-NS preferentially binds to horizontally acquired DNA in association with RNA polymerase. DNA Res. 2006;13:141–53.
Dorman CJ. H-NS, the genome sentinel. Nat Rev Microbiol. 2006;5:157–61.
Dorman CJ. H-NS-like nucleoid-associated proteins, mobile genetic elements and horizontal gene transfer in bacteria. Plasmid. 2014;75:1–11.
Dorman CJ, Hinton JCD, Free A. Domain organization and oligomerization among H-NS-like nucleoid-associated proteins in bacteria. Trends Microbiol. 1999;7:124–8.
Fang FC, Rimsky S. New insights into transcriptional regulation by H-NS. Curr Opin Microbiol. 2008;11:113–20.
Landick R, Wade JT, Grainger DC. H-NS and RNA polymerase: a love–hate relationship? Curr Opin Microbiol. 2015;24:53–9.
Schroder O, Wagner R. The bacterial regulatory protein H-NS—a versatile modulator of nucleic acid structures. Biol Chem. 2002;383:945–60.
Hong SH, Wang X, Wood TK. Controlling biofilm formation, prophage excision and cell death by rewiring global regulator H-NS of Escherichia coli. Microb Biotechnol. 2010;3:344–56.
Krin E, Danchin A, Soutourina O. Decrypting the H-NS-dependent regulatory cascade of acid stress resistance in Escherichia coli. BMC Microbiol. 2010;10:273.
Johansson J, Uhlin BE. Differential protease-mediated turnover of H-NS and StpA revealed by a mutation altering protein stability and stationary-phase survival of Escherichia coli. Proc Natl Acad Sci USA. 1999;96:10776–81.
Small P, Blankenhorn D, Welty D, Zinser E, Slonczewski JL. Acid and base resistance in Escherichia coli and Shigella flexneri: role of rpoS and growth pH. J Bacteriol. 1994;176:1729–37.
Cheville AM, Arnold KW, Buchrieser C, Cheng CM, Kaspar CW. rpoS regulation of acid, heat, and salt tolerance in Escherichia coli O157: H7. Appl Environ Microbiol. 1996;62:1822–4.
Zhou YN, Gottesman S. Modes of regulation of RpoS by H-NS. J Bacteriol. 2006;188:7022–5.
Battesti A, Tsegaye Y, Packer D, Majdalani N, Gottesman S. H-NS regulation of IraD and IraM antiadaptors for control of RpoS degradation. J Bacteriol. 2012;194:2470–8.
Battesti A, Majdalani N, Gottesman S. The RpoS-mediated general stress response in Escherichia coli. Annu Rev Microbiol. 2011;65:189–213.
Foster JW. Escherichia coli acid resistance: tales of an amateur acidophile. Nat Rev Microbiol. 2004;2:898–907.
Yoshida T, Ueguchi C, Mizuno T. Physical map location of a set of Escherichia coli genes (hde) whose expression is affected by the nucleoid protein H-NS. J Bacteriol. 1993;175:7747–8.
Shin M, Song M, Rhee JH, Hong Y, Kim YJ, Seok YJ, et al. DNA looping-mediated repression by histone-like protein H-NS: specific requirement of Eσ70 as a cofactor for looping. Genes Dev. 2005;19:2388–98.
Jiang Y, Chen B, Duan CL, Sun BB, Yang JJ, Yang S. Multigene editing in the Escherichia coli genome via the CRISPR-Cas9 system. Appl Environ Microbiol. 2015;81:2506–14.
Xu LH, Li S, Ren C, Cai Z, Lin ZL. Heat-inducible autolytic vector for high-throughput screening. Biotechniques. 2006;41:319–23.
Joo H, Lin ZL, Arnold FH. Laboratory evolution of peroxide-mediated cytochrome P450 hydroxylation. Nature. 1999;399:670–3.
Yu K, Hu S, Huang J, Mei LH. A high-throughput colorimetric assay to measure the activity of glutamate decarboxylase. Enzyme Microb Technol. 2011;49:272–6.
Tsukatani T, Higuchi T, Matsumoto K. Enzyme-based microtiter plate assay for γ-aminobutyric acid: application to the screening of γ-aminobutyric acid-producing lactic acid bacteria. Anal Chim Acta. 2005;540:293–7.
Thu Ho NA, Hou CY, Kim WH, Kang TJ. Expanding the active pH range of Escherichia coli glutamate decarboxylase by breaking the cooperativeness. J Biosci Bioeng. 2013;115:154–8.
Lu PL, Ma D, Chen YL, Guo YY, Chen GQ, Deng HT, et al. l-Glutamine provides acid resistance for Escherichia coli through enzymatic release of ammonia. Cell Res. 2013;23:635–44.
Trapnell C, Hendrickson DG, Sauvageau M, Goff L, Rinn JL, Pachter L. Differential analysis of gene regulation at transcript resolution with RNA-seq. Nat Biotechnol. 2013;31:46–53.
Spurio R, Dürrenberger M, Falconi M, Teana A, Pon CL, Gualerzi CO. Lethal overproduction of the Escherichia coli nucleoid protein H-NS: ultramicroscopic and molecular autopsy. Mol Gen Genet. 1992;231:201–11.
Lee C, Kim J, Shin SG, Hwang S. Absolute and relative QPCR quantification of plasmid copy number in Escherichia coli. J Biotechnol. 2006;123:273–80.
Kalyanaraman G. Construction and characterization of yciGFE mutants in Escherichia coli. College Station: Texas A&M University; 2003.
Bianco C, Imperlini E, Calogero R, Senatore B, Amoresano A, Carpentieri A, et al. Indole-3-acetic acid improves Escherichia coli’s defences to stress. Arch Microbiol. 2006;185:373–82.
Lund P, Tramonti A, De Biase D. Coping with low pH: molecular strategies in neutralophilic bacteria. FEMS Microbiol Rev. 2014;38:1091–125.
Feehily C, Karatzas KAG. Role of glutamate metabolism in bacterial responses towards acid and other stresses. J Appl Microbiol. 2013;114:11–24.
Djoko KY, Phan M-D, Peters KM, Walker MJ, Schembri MA, McEwan AG. Interplay between tolerance mechanisms to copper and acid stress in Escherichia coli. Proc Natl Acad Sci USA. 2017;114:6818–23.
Renault M, Garcia J, Cordeiro TN, Baldus M, Pons M. Protein oligomers studied by solid-state NMR-the case of the full-length nucleoid-associated protein histone-like nucleoid structuring protein. FEBS J. 2013;280:2916–28.
Gao YF, Foo YH, Winardhi RS, Tang QN, Yan J, Kenney LJ. Charged residues in the H-NS linker drive DNA binding and gene silencing in single cells. Proc Natl Acad Sci USA. 2017;114:12560–5.
Jarboe LR, Royce LA, Liu P. Understanding biocatalyst inhibition by carboxylic acids. Front Microbiol. 2013;4:272.
Trcek J, Mira NP, Jarboe LR. Adaptation and tolerance of bacteria against acetic acid. Appl Microbiol Biotechnol. 2015;99:6215–29.
Geng HF, Jiang RR. cAMP receptor protein (CRP)-mediated resistance/tolerance in bacteria: mechanism and utilization in biotechnology. Appl Microbiol Biotechnol. 2015;99:4533–43.
Tan FR, Wu B, Dai LC, Qin H, Shui ZX, Wang JL, et al. Using global transcription machinery engineering (gTME) to improve ethanol tolerance of Zymomonas mobilis. Microb Cell Fact. 2016;15:4.
Chiang SM, Schellhorn HE. Regulators of oxidative stress response genes in Escherichia coli and their functional conservation in bacteria. Arch Biochem Biophys. 2012;525:161–9.
Chen TJ, Wang JQ, Zeng LL, Li RZ, Li JC, Chen YL, et al. Significant rewiring of the transcriptome and proteome of an Escherichia coli strain harboring a tailored exogenous global regulator IrrE. PLoS ONE. 2012;7:e37126.
Rau MH, Calero P, Lennen RM, Long KS, Nielsen AT. Genome-wide Escherichia coli stress response and improved tolerance towards industrially relevant chemicals. Microb Cell Fact. 2016;15:176.
Dorman CJ. H-NS: a universal regulator for a dynamic genome. Nat Rev Microbiol. 2004;2:391–400.
Hecht A, Glasgow J, Jaschke PR, Bawazer LA, Munson MS, Cochran JR, et al. Measurements of translation initiation from all 64 codons in E. coli. Nucleic Acids Res. 2017;45:3615–26.
Ueguchi C, Suzuki T, Yoshida T, Tanaka K, Mizuno T. Systematic mutational analysis revealing the functional domain organization of Escherichia coli nucleoid protein H-NS. J Mol Biol. 1996;263:149–62.
Williams RM, Rimsky S, Buc H. Probing the structure, function, and interactions of the Escherichia coli H-NS and StpA proteins by using dominant negative derivatives. J Bacteriol. 1996;178:4335–43.
Authors’ contributions
XXG designed part of the experiments, performed most of the experiments, analyzed the data and prepared the manuscript draft. PC, XFY, JHL and YZ contributed to the design of experiments and part of the experiments. ZLL conceived the study, supervised the experiments, and revised the manuscript. All authors read and approved the final manuscript.
Acknowledgements
This work was supported by a Grant from the National Basic Research program of China (2013CB733902).
We are grateful to Professor Sheng Yang at Institute of Plant Physiology and Ecology in Shanghai Institutes for Biological Sciences for the gift of plasmid pCas and pTargetF-cadA.
Competing interests
The authors declare that they have no competing interests.
Availability of data and materials
All data generated or analysed during this study are included in this published article and its Additional information files.
Consent for publication
Not applicable.
Ethics approval and consent to participate
Not applicable.
Funding
This Project was funded by the National Basic Research program of China (2013CB733902).
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Author information
Authors and Affiliations
Corresponding authors
Additional files
Additional file 1: Table S1.
Maximum growth rate of strains harboring H-NS mutants under acid stress.
Additional file 2: Figure S1.
Cell morphology of strains MG, and strains harboring H-NS wild type or mutants.
Additional file 3: Table S2.
Genes differently expressed at transcriptional level between strain 3-36 and wild type strain MG.
Additional file 4: Figure S2.
Fold change of the expression of H-NS at transcriptional level obtained by qRT-PCR.
Additional file 5: Figure S3.
Quantification of protein expression level of H-NS wild type and mutants in exponential phase (EP) and stationary phase (SP). Green fluorescent protein (GFP) was fused to the C-terminal of H-NS wild type and mutants. Cells expressing the fusion protein were cultured to exponential phase or stationary phase in LBG medium acidified by HCl to pH 4.5. Fluorescence was determined to quantify the protein expression level.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Gao, X., Yang, X., Li, J. et al. Engineered global regulator H-NS improves the acid tolerance of E. coli. Microb Cell Fact 17, 118 (2018). https://doi.org/10.1186/s12934-018-0966-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12934-018-0966-z