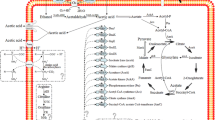
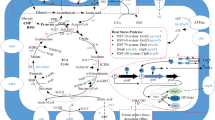
Abstract
Acetic acid is a weak organic acid exerting a toxic effect to most microorganisms at concentrations as low as 0.5 wt%. This toxic effect results mostly from acetic acid dissociation inside microbial cells, causing a decrease of intracellular pH and metabolic disturbance by the anion, among other deleterious effects. These microbial inhibition mechanisms enable acetic acid to be used as a preservative, although its usefulness is limited by the emergence of highly tolerant spoilage strains. Several biotechnological processes are also inhibited by the accumulation of acetic acid in the growth medium including production of bioethanol from lignocellulosics, wine making, and microbe-based production of acetic acid itself. To design better preservation strategies based on acetic acid and to improve the robustness of industrial biotechnological processes limited by this acid’s toxicity, it is essential to deepen the understanding of the underlying toxicity mechanisms. In this sense, adaptive responses that improve tolerance to acetic acid have been well studied in Escherichia coli and Saccharomyces cerevisiae. Strains highly tolerant to acetic acid, either isolated from natural environments or specifically engineered for this effect, represent a unique reservoir of information that could increase our understanding of acetic acid tolerance and contribute to the design of additional tolerance mechanisms. In this article, the mechanisms underlying the acetic acid tolerance exhibited by several bacterial strains are reviewed, with emphasis on the knowledge gathered in acetic acid bacteria and E. coli. A comparison of how these bacterial adaptive responses to acetic acid stress fit to those described in the yeast Saccharomyces cerevisiae is also performed. A systematic comparison of the similarities and dissimilarities of the ways by which different microbial systems surpass the deleterious effects of acetic acid toxicity has not been performed so far, although such exchange of knowledge can open the door to the design of novel approaches aiming the development of acetic acid-tolerant strains with increased industrial robustness in a synthetic biology perspective.

Similar content being viewed by others
References
Almeida B, Ohlmeier S, Almeida AJ, Madeo F, Leão C, Rodrigues F, Ludovico P (2009) Yeast protein expression profile during acetic acid-induced apoptosis indicates causal involvement of the TOR pathway. Proteomics 9:720–732
Ameyama M, Matsushita K, Ohno Y, Shinagawa E, Adachi O (1981) Existence of a novel prosthetic group, PQQ, in membrane-bound, electron transport chain-linked, primary dehydrogenases of oxidative bacteria. FEBS Lett 130:179–183
Andrés-Barrao C, Saas MM, Chappuis ML, Boffa M, Perret X, Ortega Pérez R, Barja F (2012) Proteome analysis of Acetobacter pasteurianus during acetic acid fermentation. J Proteomics 75:1701–1717
Arnold CN, McElhanon J, Lee AL, Leonhart R, Siegele DA (2001) Global analysis of Escherichia coli gene expression during the acetate-induced acid tolerance response. J Bacteriol 183:2178–2186
Axe DD, Bailey JE (1995) Transport of lactate and acetate through the energized cytoplasmic membrane of Escherichia coli. Biotechnol Bioeng 47:8–19
Bakker EP, Mangerich WE (1983) The effects of weak acids on potassium uptake by Escherichia coli K-12 inhibition by low cytoplasmic pH. Biochim Biophys Acta 730:379–386
Boenigk R, Dürre P, Gottschalk G (1989) Carrier-mediated acetate transport in Acetobacterium woodii. Arch Microbiol 152:589–593
Boesch C, Trček J, Sievers M, Teuber M (1998) Acetobacter intermedius, sp. nov. Syst Appl Microbiol 21:220–229
Booth IR (1985) Regulation of cytoplasmic pH in bacteria. Microbiol Rev 49:359–378
Booth IR, Statford N (2003) Acidulants and low pH. In: Gould GW, Russel NJ (eds). Food preservatives, Springer-Verlag US, pp 25–47
Breidt F Jr, Hayes JS, McFeeters RF (2004) Independent effects of acetic acid and pH on survival of Escherichia coli in simulated acidified pickle products. J Food Prot 67:12–18
Brudzinski L, Harrison MA (1998) Influence of incubation conditions on survival and acid tolerance response of Escherichia coli O157:H7 and non-O157:H7 isolates exposed to acetic acid. J Food Prot 61:542–546
Brul S, Coote P (1999) Preservative agents in foods: mode of action and microbial resistance mechanisms. Int J Food Microbiol 50:1–17
Carmelo V, Santos H, Sá-Correia I (1997) Effect of extracellular acidification on the activity of plasma membrane ATPase and on the cytosolic and vacuolar pH of Saccharomyces cerevisiae. Biochim Biophys Acta 1325:63–70
Chong H, Yeow J, Wang I, Song H, Jiang R (2013) Improving acetate tolerance of Escherichia coli by rewiring its global regulator cAMP receptor protein (CRP). PLoS One 8:e77422
Cleenwerck I, De Vos P (2008) Polyphasic taxonomy of acetic acid bacteria: an overview of the currently applied methodology. Int J Food Microbiol 125:2–14
Conner DE, Kotrola JS (1995) Growth and survival of Escherichia coli O157:H7 under acidic conditions. Appl Environ Microbiol 61:382–385
Constantine CZ, Starks CM, Mill CP, Ransome AE, Karpowicz SJ, Francois JA, Goodman RA, Kappock TJ (2006) Biochemical and structural studies of N 5-carboxyaminoimidazole ribonucleotide mutase from the acidophilic bacterium Acetobacter aceti. Biochemistry 45:8193–8208
Cummings JH, Pomare EW, Branch WJ, Naylor CPE, MacFarlane GT (1987) Short chain fatty acids in human large intestine, portal, hepatic and venous blood. Gut 28:1221–1227
Deeraksa A, Moonmangmee S, Toyama H, Yamada M, Adachi O, Matsushita K (2005) Characterization and spontaneous mutation of a novel gene, polE, involved in pellicle formation in Acetobacter tropicalis SKU1100. Microbiology 151:4111–4120
Diekert G (1992) The acetogenic bacteria. In: Balows A, Trüper HG, Dworkin M, Harder W, Schleifer KHE (eds) The prokaryotes. Springer Publishers, New York, pp 517–533
Diez-Gonzalez F, Russell JB (1997) The ability of Escherichia coli O157:H7 to decrease its intracellular pH and resist the toxicity of acetic acid. Microbiology 143:1175–1180
Diez-Gonzalez F, Russell JB (1999) Factors affecting the extreme acid resistance of Escherichia coli O157:H7. Food Microbiol 16:367–374
Ding J, Bierma J, Smith MR, Poliner E, Wolfe C, Hadduck AN, Zara S, Jirikovic M, van Zee K, Penner MH, Patton-Vogt J, Bakalinsky AT (2013) Acetic acid inhibits nutrient uptake in Saccharomyces cerevisiae: auxotrophy confounds the use of yeast deletion libraries for strain improvement. Appl Microbiol Biotechnol 97:7405–7416
Drake HL, Göβner AS, Daniel SL (2008) Old acetogens, new light. Ann N Y Acad Sci 1125:100–128
Durine JA, Frank J, van Zeeland JK (1979) Glucose dehydrogenase from Acinetobacter calcoaceticus: a quinoprotein. FEBS Lett 108:443–446
Fernandes AR, Mira NP, Vargas RC, Canelhas I, Sá-Correia I (2005) Saccharomyces cerevisiae adaptation to weak acids involves the transcription factor Haa1p and Haa1p-regulated genes. Biochem Biophys Res Commun 337:95–103
Fernández-Sandoval MT, Huerta-Beristain G, Trujillo-Martinez B, Bustos P, González V, Bolivar F, Gosset G, Martinez A (2012) Laboratory metabolic evolution improves acetate tolerance and growth on acetate of ethanologenic Escherichia coli under non-aerated conditions in glucose-mineral medium. Appl Microbiol Biotechnol 96:1291–1300
Francois JA, Starks CM, Sivanuntakorn S, Jiang H, Ransome AE, Nam JW, Constantine CZ, Kappock TJ (2006) Structure of a NADH-insensitive hexameric citrate synthase that resists acid inactivation. Biochemistry 45:13487–13499
Fukaya M, Takemura H, Okumura H, Kawamura H, Horinouchi S, Beppu T (1990) Cloning of genes responsible for acetic acid resistance in Acetobacter aceti. J Bacteriol 172:2096–2104
Fukaya M, Takemura H, Tayama K, Okumura H, Kawamura Y, Horinouchi S, Beppu T (1993) The aarC gene responsible for acetic acid assimilation confers acetic acid resistance on Acetobacter aceti. J Ferment Bioeng 76:270–275
Gancedo JM (1998) Yeast carbon catabolite repression. Microbiol Mol Biol Rev 62:334–361
Ghommidh C, Navarro JM, Durand G (1982) A study of acetic acid production by immobilized Acetobacter cells: oxygen transfer. Biotechnol Bioeng 24:605–617
Gibson BR, Lawrence SJ, Leclaire JPR, Powell CD, Smart KA (2007) Yeast responses to stresses associated with industrial brewery handling. FEMS Microbiol Rev 31:535–569
González Á, Hierro N, Poblet M, Rozès N, Mas A, Guillamón JM (2004) Application of molecular methods for the differentiation of acetic acid bacteria in a red wine fermentation. J Appl Microbiol 96:853–860
Guan N, Shin HD, Chen RR, Li J, Liu L, Du G, Chen J (2014) Understanding of how Propionibacterium acidipropionici respond to propionic acid stress at the level of proteomics. Sci Rep 4:6951
Gullo M, Caggia C, De Vero L, Giudici P (2006) Characterization of acetic acid bacteria in “traditional balsamic vinegar”. Int J Food Microbiol 106:209–212
Han K, Hong J, Lim HC (1993) Relieving effects of glycine and methionine from acetic acid inhibition in Escherichia coli fermentation. Biotechnol Bioeng 41:316–324
Hanada T, Kashima Y, Kosugi A, Koizumi Y, Yanagida F, Udaka S (2001) A gene encoding phosphatidylethanolamine N-methyltransferase from Acetobacter aceti and some properties of its disruptant. Biosci Biotechnol Biochem 65:2741–2748
Hasselbalch KA (1917) Die Berechnung der Wasserstoffzahl des Blutes aus der freien und gebundenen Kohlensäure desselben, und die Sauerstoffbindung des Blutes als Funktion der Wasserstoffzahl. Biochem Z 78:112–144
Heinrich Frings Information. http://www.frings.com/fileadmin/assets/Download_Essig/109_e_Produktion_Alkoholessig.pdf. Accessed 14 Jan 2015
Heipieper HJ, Weber FJ, Sikkema J, Keweloh H, de Bont JAM (1994) Mechanisms of resistance of whole cells to toxic organic solvents. Trends Biotechnol 12:409–415
Holyoak CD, Stratford M, McMullin Z, Cole MB, Crimmins K, Brown AJ, Coote PJ (1996) Activity of the plasma membrane H+-ATPase and optimal glycolytic flux are required for rapid adaptation and growth of Saccharomyces cerevisiae in the presence of the weak-acid preservative sorbic acid. Appl Environ Microbiol 62:3158–3164
Hosein AM, Breidt F Jr, Smith CE (2011) Modeling the effects of sodium chloride, acetic acid, and intracellular pH on survival of Escherichia coli O157:H7. Appl Environ Microbiol 77:889–895
Inaba T, Watanabe D, Yoshiyama Y, Tanaka K, Ogawa J, Takagi H, Shimoi H, Shima J (2013) An organic acid-tolerant HAA1-overexpression mutant of an industrial bioethanol strain of Saccharomyces cerevisiae and its application to the production of bioethanol from sugarcane molasses. AMB Express 3:74
Ingram LO (1976) Adaptation of membrane lipids to alcohols. J Bacteriol 125:670–680
Iyer R, Williams C, Miller C (2003) Arginine-agmatine antiporter in extreme acid resistance in Escherichia coli. J Bacteriol 185:6556–6561
Joachimsthal E, Haggett KD, Jang JH, Rogers PL (1998) A mutant of Zymomonas mobilis ZM4 capable of ethanol production from glucose in the presence of high acetate concentrations. Biotechnol Lett 20:137–142
Kanchanarach W, Theeragool G, Inoue T, Yakushi T, Adachi O, Matsushita K (2010) Acetic acid fermentation of Acetobacter pasteurianus: relationship between acetic acid resistance and pellicle polysaccharide formation. Biosci Biotechnol Biochem 74:1591–1597
Kaneshiro T, Law HJ (1964) Phosphatidylcholine synthesis in Agrobacterium tumefaciens. I. Purification and properties of a phosphatidylethanolamine N-methyltransferase. J Biol Chem 239:1705–1713
Kawahata M, Masaki K, Fujii T, Iefuji H (2006) Yeast genes involved in response to lactic acid and acetic acid: acidic conditions caused by the organic acids in Saccharomyces cerevisiae cultures induce expression of intracellular metal metabolism genes regulated by Aft1p. FEMS Yeast Res 6:924–936
Kirkpatrick C, Maurer LM, Oyelakin NE, Yoncheva YN, Maurer R, Slonczewski JL (2001) Acetate and formate stress: opposite responses in the proteome of Escherichia coli. J Bacteriol 183:6466–6477
Kleman GL, Strohl WR (1994) Acetate metabolism by Escherichia coli in high-cell-density fermentation. Appl Environ Microbiol 60:3952–3958
Kwon YM, Ricke SC (1998) Induction of acid resistance of Salmonella typhimurium by exposure to short-chain fatty acids. Appl Environ Microbiol 64:3458–3463
Lasko DR, Schwerdel C, Bailey JE, Sauer U (1997) Acetate-specific stress response in acetate-resistant bacteria: an analysis of protein patterns. Biotechnol Prog 13:519–523
Lasko DR, Zamboni N, Sauer U (2000) Bacterial response to acetate challenge: a comparison of tolerance among species. Appl Microbiol Biotechnol 54:243–247
Lee SY, Rhee MS, Dougherty RH, Kang DH (2010) Antagonistic effect of acetic acid and salt for inactivating Escherichia coli O157:H7 in cucumber puree. J Appl Microbiol 108:1361–1368
Lindberg L, Santos AX, Riezman H, Olsson L, Bettiga M (2013) Lipidomic profiling of Saccharomyces cerevisiae and Zygosaccharomyces bailii reveals critical changes in lipid composition in response to acetic acid stress. PLoS One 8:e73936
Lu P, Ma D, Chen Y, Guo Y, Chen GQ, Deng H, Shi Y (2013) L-glutamine provides acid resistance for Escherichia coli through enzymatic release of ammonia. Cell Res 23:635–644
Martinez A, Rodriguez ME, Wells ML, York SW, Preston JF, Ingram LO (2001) Detoxification of dilute acid hydrolysates of lignocelluloses with lime. Biotechnol Prog 17:287–293
Matsushita K, Toyama H, Adachi O (1994) Respiratory chain and bioenergetics of acetic acid bacteria. In: Rose AH, Tempest DW (eds) Advances in microbial physiology, vol. 35. Academic Press, London, pp 247−301
Matsushita K, Inoue T, Adachi O, Toyama H (2005) Acetobacter aceti possesses a proton motive force-dependent efflux system for acetic acid. J Bacteriol 187:4346–4352
McKellar RC, Knight KP (1999) Growth and survival of various strains of enterohemorrhagic Escherichia coli in hydrochloric and acetic acid. J Food Prot 62:1466–1469
Menzel U, Gottschalk G (1985) The internal pH of Acetobacterium wieringae and Acetobacter aceti during growth and production of acetic acid. Arch Microbiol 143:47–51
Mills TY, Sandoval NR, Gill RT (2009) Cellulosic hydrolysate toxicity and tolerance mechanisms in Escherichia coli. Biotechnol Biofuels 2:26
Mira NP, Becker JD, Sá-Correia I (2010a) Genomic expression program involving the Haa1p-regulon in Saccharomyces cerevisiae response to acetic acid. OMICS 14:587–601
Mira NP, Palma M, Guerreiro JF, Sá-Correia I (2010b) Genome-wide identification of Saccharomyces cerevisiae genes required for tolerance to acetic acid. Microb Cell Fact 9:79
Mira NP, Teixeira MC, Sá-Correia I (2010c) Adaptive response and tolerance to weak acids in Saccharomyces cerevisiae: a genome-wide view. OMICS 14:525–540
Miyazaki K, Hino T, Itabashi H (1991) Effects of extracellular pH on the intracellular pH, membrane potential, and growth yield of Megasphaera elsdenii in relation to the influence of monensin, ethanol, and acetate. J Gen Appl Microbiol 37:415–422
Mollapour M, Piper PW (2007) Hog1 mitogen-activated protein kinase phosphorylation targets the yeast Fps1 aquaglyceroporin for endocytosis, thereby rendering cells resistant to acetic acid. Mol Cell Biol 27:6446–6456
Mollapour M, Shepherd A, Piper PW (2008) Novel stress responses facilitate Saccharomyces cerevisiae growth in the presence of the monocarboxylate preservatives. Yeast 25:169–177
Mordukhova EA, Pan JG (2013) Evolved cobalamin-independent methionine synthase (MetE) improves the acetate and thermal tolerance of Escherichia coli. Appl Environ Microbiol 79:7905–7915
Mordukhova EA, Lee HS, Pan JG (2008) Improved thermostability and acetic acid tolerance of Escherichia coli via directed evolution of homoserine o-succinyltransferase. Appl Environ Microbiol 74:7660–7668
Moreau PL (2007) The lysine decarboxylase CadA protects Escherichia coli starved of phosphate against fermentation acids. J Bacteriol 189:2249–2261
Mullins EA, Kappock TJ (2013) Functional analysis of the acetic acid resistance (aar) gene cluster in Acetobacter aceti strain 1023. Acetic Acid Bacteria 2(s1):e3
Mullins E, Francois JA, Kappock TJ (2008) A specialized citric acid cycle requiring succinyl-coenzyme A (CoA):acetate CoA-transferase (AarC) confers acetic acid resistance on the acidophile Acetobacter aceti. J Bacteriol 190:4933–4940
Naber CK (2009) Staphylococcus aureus bacteremia: epidemiology, pathophysiology, and management strategies. Clin Infect Dis 48(Suppl 4):S231–S237
Nakano S, Fukaya M, Horinouchi S (2004) Enhanced expression of aconitase raises acetic acid resistance in Acetobacter aceti. FEMS Microbiol Lett 235:315–322
Nakano S, Fukaya M, Horinouchi S (2006) Putative ABC transporter responsible for acetic acid resistance in Acetobacter aceti. Appl Environ Microbiol 72:497–505
Nanda K, Taniguchi M, Ujike S, Ishihara N, Mori H, Ono H, Murooka Y (2001) Characterization of acetic acid bacteria in traditional acetic acid fermentation of rice vinegar (komesu) and unpolished rice vinegar (kurosu) produced in Japan. Appl Environ Microbiol 67:986–990
Oh DH, Pan Y, Berry E, Cooley M, Mandrell R, Breidt F Jr (2009) Escherichia coli O157:H7 strains isolated from environmental sources differ significantly in acetic acid resistance compared with human outbreak strains. J Food Prot 72:503–509
Ohmori S, Masai H, Arima K, Beppu T (1985) Isolation and identification of acetic acid bacteria for submerged acetic acid fermentation at high temperature. Agric Biol Chem 44:2901–2906
Ostling CE, Lindgren SE (1993) Inhibition of enterobacteria and Listeria growth by lactic, acetic and formic acids. J Appl Bacteriol 75:18–24
Osuga J, Mori A, Kato J (1984) Acetic acid production by immobilized Acetobacter aceti cells entrapped in a Κ-carrageenan gel. J Ferment Technol 62:139–149
Owen DH, Katz DF (1999) A vaginal fluid simulant. Contraception 59:91–95
Pampulha ME, Loureiro-Dias MC (1990) Activity of glycolytic enzymes of Saccharomyces cerevisiae in the presence of acetic acid. Appl Microbiol Biotechnol 34:375–380
Paraggio M, Fiore C (2004) Screening of Saccharomyces cerevisiae wine strains for the production of acetic acid. World J Microbiol Biotechnol 20:743–747
Park YS, Ohtake H, Fukaya M, Okumura H, Kawamura Y, Toda K (1989) Effects of dissolved oxygen and acetic acid concentrations on acetic acid production in continuous culture of Acetobacter aceti. J Ferment Bioeng 68:96–101
Piper P, Calderon CO, Hatzixanthis K, Mollapour M (2001) Weak acid adaptation: the stress response that confers yeasts with resistance to organic acid food preservatives. Microbiology 147:2635–2642
Polen T, Rittmann D, Wendisch VF, Sahm H (2003) DNA microarray analysis of the long-term adaptive response of Escherichia coli to acetate and propionate. Appl Environ Microbiol 69:1759–1774
Reed WM, Keller FA, Kite FE, Bogdan ME, Ganoung JS (1987) Development of increased acetic acid tolerance in anaerobic homoacetogens through induced mutagenesis and continuous selection. Enzyme Microb Technol 9:117–120
Richard H, Foster JW (2004) Escherichia coli glutamate- and arginine-dependent acid resistance systems increase internal pH and reverse transmembrane potential. J Bacteriol 186:6032–6041
Roe AJ, McLaggan D, Davidson I, O’Byrne C, Booth IR (1998) Perturbation of anion balance during inhibition of growth of Escherichia coli by weak acids. J Bacteriol 180:767–772
Roe AJ, O’Bryne C, McLaggan D, Booth IR (2002) Inhibition of Escherichia coli growth by acetic acid: a problem with methionine biosynthesis and homocysteine toxicity. Microbiology 148:2215–2222
Romano P, Fiore C, Paraggio M, Caruso M, Capece A (2003) Function of yeast species and strains in wine flavour. Int J Food Microbiol 86:169–180
Russell AD (1991a) Mechanisms of bacterial resistance to non-antibiotics: food additives and food and pharmaceutical preservatives. J Appl Bacteriol 71:191–201
Russell JB (1991b) Resistance of Streptococcus bovis to acetic acid at low pH: relationship between intracellular pH and anion accumulation. Appl Environ Microbiol 57:255–259
Saeki A, Matsushita S, Takeno S, Taniguchi M, Toyama H, Theeragool G, Lotong N, Adachi O (1999) Enzymes responsible for acetate oxidation by acetic acid bacteria. Biosci Biotechnol Biochem 63:2102–2109
Sakurai K, Arai H, Ishii M, Igarashi Y (2012) Changes in the gene expression profile of Acetobacter aceti during growth on ethanol. J Biosci Bioeng 113:343–348
Sakurai K, Yamazaki S, Ishii M, Igarashi Y, Arai H (2013) Role of the glyoxylate pathway in acetic acid production by Acetobacter aceti. J Biosci Bioeng 115:32–36
Sandoval NR, Mills TY, Zhang M, Gill RT (2011) Elucidating acetate tolerance in E. coli using a genome-wide approach. Metab Eng 13:214–224
Semchyshyn HM, Abrat OB, Miedzobrodzki J, Inoue Y, Lushchak VI (2011) Acetate but not propionate induces oxidative stress in bakers’ yeast Saccharomyces cerevisiae. Redox Rep 16:15–23
Serrano R (1991) Transport across yeast vacuolar and plasma membranes. In: Broach JR, Jones EW, Pringle JR (eds) The molecular and cellular biology of the yeast Saccharomyces: genome dynamics, protein synthesis, and energetics. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, pp 523–585
Shafiei R, Delvigne F, Babanezhad M, Thonart P (2013) Evaluation of viability and growth of Acetobacter senegalensis under different stress conditions. Int J Food Microbiol 163:204–213
Sievers M, Swings J (2005) Family Acetobacteraceae. In: Garrity GM (ed) Bergey’s manual of systematic bacteriology, vol 2. Springer-Verlag, New York, pp 41−95
Sievers M, Sellmer S, Teuber M (1992) Acetobacter europaeus sp. nov., a main component of industrial vinegar fermenters in central Europe. Syst Appl Microbiol 15:386–392
Simões T, Mira NP, Fernandes AR, Sá-Correia I (2006) The SPI1 gene, encoding a glycosylphosphatidylinositol-anchored cell wall protein, plays a prominent role in the development of yeast resistance to lipophilic weak acids food preservatives. Appl Environ Microbiol 72:7168–7175
Slapšak N, Cleenwerck I, De Vos P, Trček J (2013) Gluconacetobacter maltaceti sp. nov., a novel vinegar producing acetic acid bacterium. Syst Appl Microbiol 36:17–21
Sokollek SJ, Hertel C, Hammes WP (1998) Description of Acetobacter oboediens sp. nov. and Acetobacter pomorum sp. nov., two new species isolated from industrial vinegar fermentations. Int J Syst Bacteriol 48:935–940
Steiner P, Sauer U (2001) Proteins induced during adaptation of Acetobacter aceti to high acetate concentrations. Appl Environ Microbiol 67:5474–5481
Steiner P, Sauer U (2003a) Long term continuous evolution of acetate resistant Acetobacter aceti. Biotechnol Bioeng 84:40–44
Steiner P, Sauer U (2003b) Overexpression of the ATP-dependent helicase RecG improves resistance to weak organic acids in Escherichia coli. Appl Microbiol Biotechnol 63:293–299
Tahara Y, Yamada Y, Kondo K (1976) Phospholipid composition of Gluconobacter cerinus. Agric Biol Chem 40:2355–2360
Takahashi CM, Takahashi DF, Carvalhal ML, Alterthum F (1999) Effects of acetate on the growth and fermentation performance of Escherichia coli KO11. Appl Biochem Biotechol 81:193–203
Takemura H, Tsuchida T, Yoshinaga F, Matsushita K, Adachi O (1994) Prostetic group of aldehyde dehydrogenase in acetic acid bacteria is not pyrroloquinoline quinone. Biosci Biotechnol Biochem 58:2082–2083
Tanaka K, Ishii Y, Ogawa J, Shima J (2012) Enhancement of acetic acid tolerance in Saccharomyces cerevisiae by overexpression of the HAA1 gene, encoding a transcriptional activator. Appl Environ Microbiol 78:8161–8163
Taylor MP, Mulako I, Tuffin M, Cowan DA (2012) Understanding physiological responses to pre-treatment inhibitors in ethanologenic fermentations. Biotechnol J 7:1169–1181
Tenreiro S, Rosa PC, Viegas CA, Sá-Correia I (2000) Expression of the AZR1 gene (ORF YGR224w), encoding a plasma membrane transporter of the major facilitator superfamily, is required for adaptation to acetic acid and resistance to azoles in Saccharomyces cerevisiae. Yeast 16:1469–1481
Tenreiro S, Nunes PA, Viegas CA, Neves MS, Teixeira MC, Cabral MG, Sá-Correia I (2002) AQR1 gene (ORF YNL065w) encodes a plasma membrane transporter of the major facilitator superfamily that confers resistance to short-chain monocarboxylic acids and quinidine in Saccharomyces cerevisiae. Biochem Biophys Res Commun 292:741–748
Thurner C, Vela C, Thöny-Meyer L, Meile L, Teuber M (1997) Biochemical and genetic characterization of the acetaldehyde dehydrogenase complex from Acetobacter europaeus. Arch Microbiol 168:81–91
Trček J, Barja F (2015) Updates on quick identification of acetic acid bacteria with a focus on the 16S−23S rRNA gene internal transcribed spacer and the analysis of cell proteins by MALDI-TOF mass spectrometry. Int J Food Microbiol 196:137–144
Trček J, Ramuš J, Raspor P (1997) Phenotypic characterization and RAPD-PCR profiling of Acetobacter sp. isolated from spirit vinegar production. Food Technol Biotechnol 35:63–67
Trček J, Raspor P, Teuber M (2000) Molecular identification of Acetobacter isolates from submerged vinegar production, sequence analysis of plasmid pJK2-1 and application in development of a cloning vector. Appl Microbiol Biotechnol 53:289–295
Trček J, Toyama H, Czuba J, Misiewicz A, Matsushita K (2006a) Correlation between acetic acid resistance and characteristics of PQQ-dependent ADH in acetic acid bacteria. Appl Microbiol Biotechnol 70:366–373
Trček J, Toyama H, Czuba J, Misiewicz A, Matsushita K (2006b) Towards understanding the acetic acid resistance in Gluconacetobacter europaeus. In: Méndez-Vilas A (ed) Modern multidisciplinary applied microbiology: exploiting microbes and their interactions. Wiley-VCH Verlag, Weinheim, pp 674–678
Trček J, Jernejc K, Matsushita K (2007) The highly tolerant acetic acid bacterium Gluconacetobacter europaeus adapts to the presence of acetic acid by changes in lipid composition, morphological properties and PQQ-dependent ADH expression. Extremophiles 11:627–635
Ullah A, Chandrasekaran G, Brul S, Smits GJ (2013) Yeast adaptation to weak acids prevents futile energy expenditure. Front Microbiol 4:142
Wang J, Yu J (2000) Kinetic analysis on inhibited growth and poly(3-hydroxybutyrate) formation of Alcaligenes eutrophus on acetate under nutrient-rich conditions. Process Biochem 36:201–207
Wang H, Wang F, Wang W, Yao X, Wei D, Cheng H, Deng Z (2014) Improving the expression of recombinant proteins in E. coli BL21 (DE3) under acetate stress: an alkaline pH shift approach. PLoS One 9:e112777
Weber FJ, Ooijkaas LP, Schemen RMW, Hartmans S, De Bont JAM (1993) Adaptation of Pseudomonas putida S12 to high concentrations of styrene and other organic solvents. Appl Environ Microbiol 59:3502–3504
Wood JM (2011) Osmotic stress. In: Storz G, Hengee R (eds) Bacterial stress responses. ASM Press, Washington DC, pp 133–156
Wu J, Gullo M, Chen F, Giudici P (2010) Diversity of Acetobacter pasteurianus strains isolated from solid-state fermentation of cereal vinegars. Curr Microbiol 60:280–286
Yamada Y, Yukphan P, Vu HTL, Muramatsu Y, Ochaikul D, Tanasupawat S, Nakagawa Y (2012) Description of Komagataeibacter gen. nov., with proposals of new combinations (Acetobacteraceae). J Gen Appl Microbiol 58:397–404
Yamaguchi N, Sonoyama K, Kikuchi H, Nagura T, Aritsuka T, Kawabata J (2005) Gastric colonization of Candida albicans differs in mice fed commercial and purified diets. J Nutr 135:109–115
Yang S, Land ML, Klingeman DM, Pelletier DA, Lu TYS, Martin SL, Guo HB, Smith JC, Brown SD (2010) Paradigm for industrial strain improvement identifies sodium acetate tolerance loci in Zymomonas mobilis and Saccharomyces cerevisiae. Proc Natl Acad Sci U S A 107:10395–10400
Yang J, Russell TW, Hocking DM, Bender JK, Srikhanta YN, Tauschek M, Robins-Browne RM (2015) Control of acid resistance pathways of enterohemorrhagic Escherichia coli strain EDL933 by PsrB, a prophage-encoded AraC-like regulator. Infect Immun 83:346–353
Yuk HG, Marshall DL (2005) Influence of acetic, citric and lactic acids on Escherichia coli O157:H7 membrane lipid composition, verotoxin secretion, and acid resistance in simulated gastric fluid. J Food Prot 68:673–679
Zaldivar J, Ingram LO (1999) Effect of organic acids on the growth and fermentation of ethanologenic Escherichia coli LY01. Biotechnol Bioeng 66:203–210
Zhang X, Zhang Y, Li Z, Xia Y, Ye Q (2011) Continuous culture and proteomic analysis of Escherichia coli DH5α and its acetate-tolerant mutant DA19 under conditions of nitrogen source limitation. Bioprocess Biosyst Eng 34:179–187
Acknowledgments
LJ was supported by the NSF Engineering Research Center for Biorenewable Chemicals (CBiRC), NSF award number EEC-0813570 and Karen and Denny Vaughn. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. The research of JT has been funded by the Slovenian Research Agency through the programs IP-0552 and P2-0006. NPM acknowledges past and present members of the Biological Sciences Research Group from Instituto Superior Técnico, who have worked in the study of yeast adaptive responses to stress imposed by weak organic acids and have made significant contributions to most of the studies mentioned in this review. In particular, NPM would like to highlight the highly relevant contribution of Professor Isabel Sá-Correia, the head of BSRG, who pioneered much of the work herein reviewed in the field of organic acid toxicity in yeasts.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Trček, J., Mira, N.P. & Jarboe, L.R. Adaptation and tolerance of bacteria against acetic acid. Appl Microbiol Biotechnol 99, 6215–6229 (2015). https://doi.org/10.1007/s00253-015-6762-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-015-6762-3