Abstract
Background
The adipokine adipsin contributes to insulin resistance (IR), inflammation, and obesity, which are all regarded as high-risk factors for mild cognitive impairment (MCI) in patients with type 2 diabetes mellitus. This research aimed to uncover the role of adipsin in Chinese type 2 diabetes mellitus (T2DM) population with early cognitive dysfunction and determine whether adipsin contributes to diabetic MCI caused by IR.
Methods
In our study, 126 patients with T2DM were enrolled. The Montreal Cognitive Assessment (MoCA) was used to assess cognitive impairment. Demographic data and neuropsychological test results were evaluated. Plasma adipsin level was measured by enzyme-linked immunosorbent assay.
Results
The MCI group (n = 57) presented higher plasma adipsin levels compared with the healthy controls (p = 0.018). After adjustment for educational attainment, and age, begative correlations were found between plasma adipsin levels and MoCA, Mini Mental State Exam, and Verbal Fluency Test scores(r = − 0.640, p < 0.001; r = − 0.612, p < 0.001; r = − 0.288, p = 0.035; respectively). Correlation analysis demonstrated that adipsin levels were significantly positively correlated with fasting C-peptide; homeostasis model of assessment for insulin resistance (HOMA-IR) (r = 0.368, p < 0.001; r = 0.494, p < 0.001; respectively). Multivariable regression analysis further indicated that high plasma adipsin level was a significant independent determinant of MCI in the Chinese population withT2DM (p = 0.017).
Conclusions
Elevated plasma adipsin level was associated with MCI in Chinese T2DM patients. Further large-scale studies should be designed to determine whether adipsin is linked to IR-associated susceptibility to early cognitive decline in T2DM patients.
Similar content being viewed by others
Background
Given its prevalence, type 2 diabetes mellitus (T2DM) is expected to affect 552 million people worldwide by 2030 according to the International Diabetes Federation (IDF) [1]. With its growing chronic complications, diabetes-induced cognitive dysfunction has received considerable attention from researches [2]. Previous researches demonstrated that patients with T2DM have an increased incidence of dementia and mild cognitive impairment (MCI), a transition phase between dementia and regular aging [3, 4]. T2DM results in a 60% increase in Alzheimer’s disease (AD) risk [5]. The exact mechanisms of diabetes-induced cognitive dysfunction are multifactorial. Insulin resistance (IR), dyslipidemia, neuroinflammation, hyperphosphorylation of TAU and abnormal accumulation of amyloid-beta (Aβ) peptide were reported [6, 7]. Nevertheless, the potential etiology and pathological mechanisms remain unclear.
IR is one of the principal distinctive features of T2DM, which exists throughout the entire diabetes course [8]. IR itself also leads to the production of Aβ and hyperphosphorylation of tau protein [9]. Prior studies suggested that systemic IR actuates brain IR [9], and leads to the reduction of cerebral glucose metabolic rate and worsened memory [10]. Indeed, accumulated evidence has suggested that AD is usually accompanied by profound IR; moreover, IR abnormalities also participate in the occurrence of T2DM-related early cognitive dysfunction and contribute to the progression of MCI to AD [11]. However, the precise mechanisms about diabetic MCI caused by IR remain uncertain.
Partial adipocytokines were thought to be involved in diabetic-related MCI. Pathological mechanisms such as cerebral IR, hyperinsulinemia, and inflammation have been discussed. Certain adipocytokines including leptin and adiponectin were reported to medicate early cognitive impairment caused by IR [12, 13]. Leptin-deficient mice with T2DM [14] show impaired cerebral insulin signaling, thereby leading to the activation of glycogen synthase kinase 3β(GSK3β), the production of Aβ, the hyperphosphorylation of tau protein, and subsequent cognitive impairment. The adipocytokine adiponectin can ameliorate insulin sensitivity by activating protein kinase (AMPK) phosphorylation, reaulting in neuroinflammation, neurodegeneration, Aβ production, and tau protein hyperphosporylation. Thus, IR plays an important role in T2DM, adipokine levels, and cognitive impairment. The adipokine adipsin (complement factor D), is a serine protease that was first found in 3 T3 adipocytes [15]. Patients suffering from DM have high serum and cerebrospinal fluid (CSF) levels of adipsin [16]. In mice, Lo et al. found that adipsin, together with its downstream receptor of C3a, C3aR1, acts on islets and finally stimulates insulin secretion [17]. This finding provided a link between IR and adipsin. The association between adipsin and IR has been confirmed in some investigations. Many human clinical studies presented a positive correlation between adipsin and IR, although contradictory clinical reports were found by Wang et al. [18]. Moreover, adipsin has also been reported to modulate lipid metabolism [19], ischemia-reperfusion [20], and insulin secretion [17], which are all implicated as risk factors of cognitive dysfunction. Thus, adipsin, probably plays a previously unrecognized role in T2DM-related cognitive dysfunction. Therefore, we hypothesized that adipsin might regulate IR - related susceptibility to early cognitive dysfunction in T2DM patients.
The present cross-sectional study aimed to evaluate the latent correlation between plasma adipsin levels and diabetes-related cognitive impairment. Further analysis may reveal the potential mechanisms of IR- related susceptibility to early cognitive impairment in T2DM patients.
Methods
Clinical subjects and study design
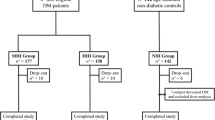
The present cross-sectional research was designed and implemented in T2DM patients from 2013 to 2017. The Endocrinology Department of the Affiliated Zhongda Hospital of Southeast University provided recruiters. Altogether, 126 right-handed, hospitalized T2DM individuals were recruited (71 men and 55 women, aged 40–75 years). All subjects had at least three years of diabetes duration and met the diagnostic criteria for T2DM based on the World Health Organization in 1999 [21]. Among these individuals, 57 patients (28 females, 29 males, mean ± SE age = 59.98 ± 0.919 years) were diagnosed as MCI and 69 patients (27 females, 42 males, mean ± SE age = 58.28 ± 1.035 years) were diabetic patients with healthy cognition. The recruited individuals with MCI met the 2006 diagnostic criteria: 1) Cognitive complaints, come from patients themselves or family members; 2) Clinical Dementia Rating (CDR) score ≥ 0.5); 3) Cognitive dysfunction certified by professional clinicians without dementia and major repercussions in daily life [22]. Exclusion criteria include: 1) diabetic ketoacidosis, severe hypoglycemia coma or other acute diabetic complication, 2) acute cardiovascular and cerebrovascular events, known history stroke within one year (Hachinski score ≥ 4), epilepsy, head injury, moderate depression or other psychiatric illness; 3) Severe systemic disease (i.e., thyroid disease, serious infection and anemia); 4) Severe visual or hearing loss.
Clinical data collection
Demographic data were gathered including age, sex, education levels, height, hip circumference, waist circumference, weight, and blood pressure. Physical data were measured by a professional research staff based on a standard and uniform method. The body mass index (BMI) = body weight in kilograms / the square of the height in meters (kg/m2). Systolic blood pressure ≥ 140 mmHg or diastolic blood pressure ≥ 90 mmHg would be defined as hypertension, according to the 2010 Chinese Hypertension Management Guidelines [23]. Medical histories such as diabetes duration (calculated from the time when diabetes was diagnosed by a professional doctor), insulin use, lifestyle factors (including smoking and drinking) were obtained through self-report or medical records. Fatty liver was detected by the Color Doppler ultrasound. The blood samples were assayed for fasting and 2-h postprandial glucose (FBG and 2hPG), glycosylated hemoglobin (HbA1c), total cholesterol (TC), fasting C-peptide (FCP), triglyceride (TG), high-density and low-density lipoprotein cholesterol (HDL-C, LDL-C), apolipoprotein A1 (ApoA1) and apolipoprotein B (ApoB). The homeostasis model of assessment for insulin resistance (HOMA-IR) was calculated by the formula HOMA-IR = fasting glucose (mmol/L) x fasting peptide (nmol/L) / 22.5 [24]. All the samples were measured by the Center of the Zhongda Hospital in accordance with the internal and external quality management procedures implemented by the Chinese Laboratory of Quality Control.
Neuropsychological test data
An experienced neuropsychiatry specialist conducted the neuropsychological test by using a single-blind method. The present research employed a neuropsychological battery, including Montreal Cognitive Assessment (MoCA), Verbal Fluency Test (VFT), Mini Mental State Exam (MMSE), Clock Drawing Test (CDT), Digit Span Test (DST), Auditory Verbal Learning Test (AVLT), Stroop color word test (SCWT), Trail Making Test-A and B (TMT-A and TMT-B). Overall cognitive function, executive abilities, calculation ability, attention and information processing speed were covered.
Measurement of plasma adipsin level
After overnight fasting, 2 ml blood samples were drawn from the anterior elbow vein between 6 and 7 A. M into tubes anticoagulated by heparin and then centrifuged at 100×g at least 15 min. After that, the samples were separated and refrigerated at − 80 °C before measured. The plasma levels of adipsin were detected by the enzyme-linked immunosorbent assay kits [USCN, Wuhan, China] based on the manufacturer’s instructions. The Intra-Assay CV was < 10% and the Inter-Assay CV < 12%. The minimum detectable value of this kit was 0.257 ng/ ml. Each sample was measured 2 times and then taking the average value. All samples were measured on the same day to minimize test variation.
Statistical analysis
All the data were tested in the form of the means ± standard error (SE), n (%), or the median (interquartile range) according to the characteristics. SPSS version 22.0 was conducted. The Kolmogorov –Smirnov (KS) test was performed to validate the normality of data. Analysis of variance (ANOVA) and Student’s tests were performed for normally distributed variables, otherwise, non-parametric Mann-Whitney U or Kruskal-Wallis tests would be performed. Besides, the Chi-squared analysis (χ2) was taken to analyze categorical data. The partial correlation analysis was used after adjustment for age and some other confounding factors to determine the correlation of plasma adipsin levels and cognitive performance. The Regression model was conducted to establish a predictive model of MCI. MCI group was recommended with a MoCA score less than 26, patients with education levels < 12 years would have a one-point adjustment. All analyses were bilateral. P < 0.05 was considered statistically significant.
Results
Demographic, clinical and neuropsychological characteristics
The demographic, clinical and neuropsychological tests are shown in Table 1. A total of 126 Chinese subjects with T2DM were recruited and further divided into two groups. Among these patients, 57 were diagnosed as MCI and 69 showed healthy cognition.
The two groups well matched in terms of age, sex, educational attainment, smoking, drinking, hypertension, insulin use and duration of diabetes (all p > 0.05). No significant differences were discovered in both groups in BMI, weight, WC, HC, WHR, TG, TC, HDL, LDL, ApoA1 and ApoB (all p > 0.05). Compared with the normal group, the MCI group exhibited increased plasma FCP, FBG, HOMA-IR and HbA1c levels (all p < 0.05). Moreover, increased adipsin levels were found in the MCI group (13.532 ± 0.948 vs. 10.4274 ± 0.877, p < 0.05). T2DM patients with MCI presented poorer cognitive performance than healthy controls (all p < 0.05).
Relationship between plasma adipsin level and cognitive performance
The correlation between the plasma levels of adipsin and cognitive performance were determined by partial correlation analysis for all subjects. After adjusting for age, sex and education levels, the adipsin level was statistically significant negatively correlated with MoCA scores (r = − 0.640, p < 0.001), MMSE scores (r = − 0.612, p < 0.001), SCWT-A Time(r = 0.290, p = 0.034) and VFT scores (r = − 0.288, p = 0.035) in T2DM patients with cognition dysfunction. However, only the SCWT-A Number(r = − 0.299, p = 0.015) was interrelated in the normal cognitive group (Table 2).
Binary logistic regression analysis for all individuals
We elucidated the major determinants related to MCI prevalence in the enrolled populations. A regression analysis was constructed. All variables from Table 1 were entered into the model. The model was finallydeveloped by a stepwise approach. Adipsin (β = 0.063, p = 0.017) and HbA1c (β = 0.196, p = 0.031) were eventually imported to the model (Table 3), and analysis revealed that high plasma levels of adipsin and HbA1c were the independent risk factors that increased the diagnosis of MCI.
Multivariable regression analysis among patients with cognitive impairment
Simple linear regression models and multivariable linear regression models were constructed to evaluate the independent factors that might affect the neuropsychological test results. The MoCA score was taken as a dependent variable, while sex, age, education level, diabetes duration, HbA1c, FBG, and adipsin were included as independent variables in the multiple stepwise regression models. The analysis results suggested that MoCA score was negatively associated with adipsin, (standardized B = -0.623, p < 0.001; β = − 0.286, p = 0.02; respectively; Table 4). Similar results were obtained with MMSE and VFT as the dependent variables (Table 4).
Correlations of adipsin with clinical variables
Table 5 shows the correlations between the plasma adipsin level and different clinical data. Spearman rank correlation or Pearson correlation analyses were performed. Remarkable positive correlations were found in FCP (r = 0.368, p = 0.005; r = 0.525, p < 0.001) and HOMA-IR (r = 0.494, p < 0.001, r = 0.437;p < 0.001) in all participants. In addition, positive correlations were found between plasma adipsin level and SBP (r = 0.285, p = 0.032), hypertension (r = 0.463, p < 0.001), smoking (r = 0.317, p = 0.016), BMI(r = 0.336, p = 0.011), and weight (r = 0.295, p = 0.029) in the MCI group only. No statistically significant differences were found between adipsin and WC and adipsin and fatty liver.
Multiple linear regression analysis
Multiple linear regression analyses were performed to evaluate the independent associations between adipsin and clinical parameters (Table 6). All the independent parameters were entered in the model at step one. APoB, FCP, HOMA-IR, TC, and FBG independently predicted adipsin levels.
Discussion
The foremost results of this study were as follows: (1) Compared with the normal controls, individuals with cognitive dysfunction exhibited higher plasma adipsin levels. (2) After controlling for potential confounders such as levels of education, age, and sex, the plasma adipsin level was remarkably negatively correlated with MoCA, MMSE and VFT scores, which represent executive function [25] in the MCI group. (3) Increased plasma adipsin level was a major independent determinant for diabetic MCI. (4) Plasma adipsin level was positively associated with FCP and HOMA-IR in all subjects. In addition, FCP and HOMA-IR independently predicted adipsin levels.
Consistent with previous studies [26], our study demonstrated that T2DM patients with MCI exhibited worsened glucose homeostasis, as indicated by increased HbA1c and FBG in the MCI group. Moreover, HbA1c is an independent risk factor for poor cognitive performance. Yaffe et al. [27] reported that for patients with HbA1c ≥ 7%, the risk for developing MCI was increased by nearly fourfold in a large sample size study with a female population. Several mechanisms have been investigated, including accumulation of advanced glycation end-products, activation of protein kinase C and increased flux of hexosamine in brain endothelial cells that lead to vessel occlusion, alteration of angiogenesis and permeability, production of NF-κB that causes neuroinflammation, obstruction of Akt/CREB signaling pathway, and impaired insulin homeostasis of the brain [26, 28].
Moreover, increased plasma adipsin level was correlated with higher BMI and weight in the MCI group. Adipose tissue dysfunction and obesity exists in diabetes and contributes to the development of lipid metabolism disorder and MCI in patients with T2DM [29,30,31]. Animal experiments indicated that circulating adipsin levels decreased in obese models [32], while human studies present diametrically opposite results [33]. The exact explanation for these discrepancies remains uncertain. One possible reason is that the expansion of fat mass in obesity may compensate to maintain higher circulating levels of adipsin [17]. Our results were consistent with most human studies. Maslowska et al. [34] reported that an obese group presented a high plasma adipsin levels, and a strong positive association was discovered between BMI and plasma adipsin. Analogous results were found in a cross-sectional study carried out on Arabs [35],in which a positive correlation was also discovered between adipsin and waist circumference. Schrover et al. [36] reported a strong positive relation between adipsin and BMI; moreover, visceral adipose tissue was related to higher plasma concentrations of adipsin. Conversely, adipsin is a serine protein in triglyceride synthesis through the ASP/adipsin pathway [19, 37]. Maslowska et al. [34] also verified that free fatty acidand BMI predicted adipsin levels. However, insignificant correlations were found between adipsin and TG and adipsin and WC in our study.
We also noted significantly higher FCP and HOMA-IR in the MCI group, thereby suggesting an association between elevated IR and MCI. Our findings are in line with previous research supporting the concept that IR contributes to the pathological mechanism of cognitive dysfunction [38]. Consistent with our study, Ekblad et al. [39] reported that IR predicted poor verbal fluency and acted as an independent risk factor of mild cognitive dysfunction in a population-based cohortwith an 11-year large follow-up survey. Insulin signaling in the brain induces the suppression ofGSK-3, which results in tau hyperphosphorylation and neurofibrillary formation, thereby causing damage or apoptosis of neurons [9].
Our correlation study of plasma adipsin level and IRrevealed that higher plasma adipsin level was correlated with higher FCP and HOMA-IR. Further multivariate analysis confirmed that the degree of HOMA-IR was an independent predictor of adipsin levels. Research on animals suggested that adipsin cleaves factor B, thereby catalyzing the formation of C3bBb, which cleaves C3 to liberate C3a. Then, together with its downstream receptor of C3a, C3aR1 acts on islets through augmenting intracellular adenosine-triphosphate (ATP) levels, thus motivating ATP-coupled respirations, increasing the concentration of cytosolic free Ca2+, and finally stimulating insulin secretion [17]. Interestingly, a dispute exists about the relationship between adipsin and IR. Our findings are consistent with some human studies. One recent cross-sectional study displayed significant positive correlations between adipsin levels and the HOMA-IR index in patients with polycystic ovary syndrome. In another study, IR was found to be an independent predictive factor of adipsin levels [40]. Similar findings were obtained by Derosa et al. [41], who suggested that increased HOMA-IR index was profoundly correlated with higher adipsin levels in obese subjects. However, an insignificant correlation was reported in a study on t Arabs with cardiovascular disease. Lo et al. [17] revealed that adipsin−/− mice are insulinopenic, and diabetic db/db mice injected with adipsin presented increased insulin secretion and improved glucose homeostasis. Wang et al. reported that serum adipsin levels were negatively correlated with IR, especially in subjects with BMI ≥ 25 kg/m2. The disparity between results was not fully understood. One possible explanation is that the patients with T2DM employed in our research have not developed severe metabolic disorders, and a compensatory mechanism may have been triggered to retain normal insulin secretion. In addition, different study populations (patients with MCI in our report vs. subjects with cardiovascular disease by Calan et al. [41]), different races (Asians vs. Arabs), and different species (human vs. mice) may at least partly explain such discrepancies. Additionally, MCI subjects presented high adipsin levels. Further multivariate analyses demonstrated that, besides HbA1c, adipsin is an independent determinant for MCI individuals with T2DM. This outcome implies that a higher plasma adipsin level might play a previously unrecognized role in diabetic MCI caused by IR. Neuropsychological tests conducted in our study also indicated that the MMSE and MoCA scores, which represented global cognition, were inversely correlated to plasma adipsin levels. The influences on cognitive dysfunction were consistent with cognitive decline caused by insulin resistance in the past study conducted by Zhong Y et al. [42]. In our present study, although lower HOMA-IR was found in the MCI group, the further multivariable linear regression analyses discovered insignificant correlations about HOMA-IR and MoCA, MMSE, VFT scores. This may be due to the insufficient population. Furthermore, adipsin acts as an independent predictor of VFT, which represents the executive function. To the best of our knowledge, this is the unique research estimating the expressing of adipsin in cognitive impairment with T2DM.
One or more possible explanations may be reasonable for the observed results: 1) glucose dysregulation in T2DM patients with cognitive dysfunction promotes adipsin production as a compensatory mechanism to maintain normal insulin secretion [17], that leads to compensatory IR, which, in turn may contribute to impairment of cellular insulin signaling [43], reduction of brain insulin uptake, and increased levels of Aβ [10]. In addition, abnormal insulin signaling represents a risk to the dysfunction of the entorhinal cortex and hippocampus, finally leading to impaired memory and executive function [44]. 2) adipsin itself is related to CSF inflammation and increases grades of disturbed blood-CSF barrier function [16].
Several limitations exist in our research. First, the cross-sectional study design itself failed to explain any causal relationship between adipsin and cognitive impairment. Large-scale and multi-center prospective studies should be conducted to verify our inferences. Second, some clinical parameters such as education levels were collected through self-report and medical records, which could lead to recall bias. Third, the sample size in this study is not large enough and consists of the Chinese Han population, which might reduce the strength of the results. Furthermore, while we adjusted some confounding factors that may influence the logistic regression, the results may have been affected by other possible confounders (e.g., living environment and habits), which could not be controlled. Finally, we used the less invasive HOMA-IR method to evaluate IR; thus, more accurate indicators should be used to gain a better evaluation of IR.
Conclusion
In summary, the current study demonstrated that T2DM individuals with cognitive dysfunction presented increased plasma adipsin levels. Furthermore, high plasma adipsin level is an independent risk predictor of overall cognitive function and executive function. The data implied that adipsin could be a potential predictor of early cognitive dysfunction among Chinese patients with T2DM. Additionally, we obtained evidence that plasma adipsin level is significantly positively associated with FCP and HOMA-IR, which suggested that adipsin might facilitate the development of diabetic MCI caused by IR. Further prospective studies with large sample size should be conducted to confirmed our hypotheses and clarify whether adipsin is involved in diabetic MCI caused by IR.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- 2hPG :
-
2-h postprandial blood glucose
- AD:
-
Alzheimer’s disease
- ApoA1:
-
apolipoprotein A1
- ApoB:
-
apolipoprotein B
- AVLT:
-
Auditory Verbal Learning Test
- BMI:
-
body mass index
- CDT:
-
Clock Drawing Test
- CI:
-
confidence interval for odds ratio
- DBP:
-
Diastolic blood pressure
- DST:
-
Digit Span Test
- FBG:
-
fasting blood-glucose
- FCP:
-
fasting C-peptide
- HbA1c:
-
glycosylated hemoglobin
- HC:
-
hip circumference
- HDL:
-
high-density lipoprotein
- HOMA-IR:
-
homeostasis model of assessment for insulin resistance
- IR:
-
insulin resistance
- LDL:
-
low-density lipoprotein
- LMT:
-
Logical Memory Test
- MCI:
-
mild cognitive impairment
- MMSE:
-
Mini-mental State Examination
- MoCA:
-
Montreal Cognitive Assessment
- SBP:
-
systolic blood pressure
- SCWT:
-
Stroop Color Word Test
- SE:
-
standard error
- T2DM:
-
type 2 diabetes mellitus
- TC:
-
total cholesterol
- TG:
-
triglyceride
- TMT-A:
-
Trail Making Test-A
- TMT-B:
-
Trail Making Test-B
- VFT:
-
Verbal Fluency Test
- WC:
-
waist circumference
- β:
-
regression coefficient
References
Whiting DR, Guariguata L, Weil C, Shaw J. IDF diabetes atlas: global estimates of the prevalence of diabetes for 2011 and 2030. Diabetes Res Clin Pract. 2011;94(3):311–21.
van Harten B, de Leeuw FE, Weinstein HC, Scheltens P, Biessels GJ. Brain imaging in patients with diabetes: a systematic review. Diabetes Care. 2006;29(11):2539–48.
Mariani E, Monastero R, Mecocci P. Mild cognitive impairment: a systematic review. J Alzheimers Dis. 2007;12(1):23–35.
Li W, Huang E. An update on type 2 diabetes mellitus as a risk factor for dementia. J Alzheimers Dis. 2016;53(2):393–402.
Kimm H, Lee PH, Shin YJ, Park KS, Jo J, Lee Y, Kang HC, Jee SH. Mid-life and late-life vascular risk factors and dementia in Korean men and women. Arch Gerontol Geriatr. 2011;52(3):e117–22.
De Felice FG, Ferreira ST. Inflammation, defective insulin signaling, and mitochondrial dysfunction as common molecular denominators connecting type 2 diabetes to Alzheimer disease. Diabetes. 2014;63(7):2262–72.
Ceriello A, Motz E. Is oxidative stress the pathogenic mechanism underlying insulin resistance, diabetes, and cardiovascular disease? The common soil hypothesis revisited. Arterioscler Thromb Vasc Biol. 2004;24(5):816–23.
Kahn SE. The relative contributions of insulin resistance and beta-cell dysfunction to the pathophysiology of type 2 diabetes. Diabetologia. 2003;46(1):3–19.
Umegaki H, Makino T, Uemura K, Shimada H, Hayashi T, Cheng XW, Kuzuya M. The associations among insulin resistance, hyperglycemia, physical performance, diabetes mellitus, and cognitive function in relatively healthy older adults with subtle cognitive dysfunction. Front Aging Neurosci. 2017;9:72.
Baker LD, Cross DJ, Minoshima S, Belongia D, Watson GS, Craft S. Insulin resistance and Alzheimer-like reductions in regional cerebral glucose metabolism for cognitively normal adults with prediabetes or early type 2 diabetes. Arch Neurol. 2011;68(1):51–7.
Talbot K, Wang HY, Kazi H, Han LY, Bakshi KP, Stucky A, Fuino RL, Kawaguchi KR, Samoyedny AJ, Wilson RS, et al. Demonstrated brain insulin resistance in Alzheimer's disease patients is associated with IGF-1 resistance, IRS-1 dysregulation, and cognitive decline. J Clin Invest. 2012;122(4):1316–38.
Berglund ED, Vianna CR, Donato J Jr, Kim MH, Chuang JC, Lee CE, Lauzon DA, Lin P, Brule LJ, Scott MM, et al. Direct leptin action on POMC neurons regulates glucose homeostasis and hepatic insulin sensitivity in mice. J Clin Invest. 2012;122(3):1000–9.
Davis C, Mudd J, Hawkins M: Neuroprotective effects of leptin in the context of obesity and metabolic disorders. Neurobiology of disease 2014, 72 Pt A:61–71.
Gao C, Liu Y, Li L, Holscher C. New animal models of Alzheimer's disease that display insulin desensitization in the brain. Rev Neurosci. 2013;24(6):607–15.
Rosen BS, Cook KS, Yaglom J, Groves DL, Volanakis JE, Damm D, White T, Spiegelman BM. Adipsin and complement factor D activity: an immune-related defect in obesity. Science (New York, NY). 1989;244(4911):1483–7.
Schmid A, Hochberg A, Berghoff M, Schlegel J, Karrasch T, Kaps M, Schaffler A. Quantification and regulation of adipsin in human cerebrospinal fluid (CSF). Clin Endocrinol. 2016;84(2):194–202.
Lo JC, Ljubicic S, Leibiger B, Kern M, Leibiger IB, Moede T, Kelly ME, Chatterjee Bhowmick D, Murano I, Cohen P, et al. Adipsin is an adipokine that improves beta cell function in diabetes. Cell. 2014;158(1):41–53.
Wang JS, Lee WJ, Lee IT, Lin SY, Lee WL, Liang KW, Sheu WH. Association between serum Adipsin levels and insulin resistance in subjects with various degrees of glucose intolerance. J Endocr Soc. 2019;3(2):403–10.
Song NJ, Kim S, Jang BH, Chang SH, Yun UJ, Park KM, Waki H, Li DY, Tontonoz P, Park KW. Small molecule-induced complement factor D (Adipsin) promotes lipid accumulation and adipocyte differentiation. PLoS One. 2016;11(9):e0162228.
Stahl GL, Xu Y, Hao L, Miller M, Buras JA, Fung M, Zhao H. Role for the alternative complement pathway in ischemia/reperfusion injury. Am J Pathol. 2003;162(2):449–55.
Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med. 1998;15(7):539–53.
Portet F, Ousset PJ, Visser PJ, Frisoni GB, Nobili F, Scheltens P, Vellas B, Touchon J. Mild cognitive impairment (MCI) in medical practice: a critical review of the concept and new diagnostic procedure. Report of the MCI working Group of the European Consortium on Alzheimer's disease. J Neurol Neurosurg Psychiatry. 2006;77(6):714–8.
Liu LS. Chinese guidelines for the management of hypertension. Zhonghua xin xue guan bing za zhi 2011. 2010;39(7):579–615.
Kim JD, Kang SJ, Lee MK, Park SE, Rhee EJ, Park CY, Oh KW, Park SW, Lee WY. C-Peptide-Based Index Is More Related to Incident Type 2 Diabetes in Non-Diabetic Subjects than Insulin-Based Index. Endocrinology and metabolism (Seoul, Korea). 2016;31(2):320–7.
Herbert V, Brookes RL, Markus HS, Morris RG. Verbal fluency in cerebral small vessel disease and Alzheimer's disease. J Int Neuropsychol Soc. 2014;20(4):413–21.
Xiang Q, Zhang J, Li CY, Wang Y, Zeng MJ, Cai ZX, Tian RB, Jia W, Li XH. Insulin resistance-induced hyperglycemia decreased the activation of Akt/CREB in hippocampus neurons: molecular evidence for mechanism of diabetes-induced cognitive dysfunction. Neuropeptides. 2015;54:9–15.
Yaffe K, Blackwell T, Whitmer RA, Krueger K, Barrett Connor E. Glycosylated hemoglobin level and development of mild cognitive impairment or dementia in older women. J Nutr Health Aging. 2006;10(4):293–5.
SR-F J, Sa-Roriz TM, Rosset I, Camozzato AL, Santos AC, Chaves ML, Moriguti JC, Roriz-Cruz M. (pre) diabetes, brain aging, and cognition. Biochim Biophys Acta. 2009;1792(5):432–43.
Sanz CM, Ruidavets JB, Bongard V, Marquie JC, Hanaire H, Ferrieres J, Andrieu S. Relationship between markers of insulin resistance, markers of adiposity, HbA1c, and cognitive functions in a middle-aged population-based sample: the MONA LISA study. Diabetes Care. 2013;36(6):1512–21.
Tangvarasittichai S, Pongthaisong S, Meemark S, Tangvarasittichai O. Abdominal obesity associated with elevated serum Butyrylcholinesterase activity, insulin resistance and reduced high density lipoprotein-cholesterol levels. Indian J Clinical Biochem. 2015;30(3):275–80.
Sallam HS, Tumurbaatar B, Zhang WR, Tuvdendorj D, Chandalia M, Tempia F, Laezza F, Taglialatela G, Abate N. Peripheral adipose tissue insulin resistance alters lipid composition and function of hippocampal synapses. J Neurochem. 2015;133(1):125–33.
Flier JS, Cook KS, Usher P, Spiegelman BM. Severely impaired adipsin expression in genetic and acquired obesity. Science (New York, NY). 1987;237(4813):405–8.
Napolitano A, Lowell BB, Damm D, Leibel RL, Ravussin E, Jimerson DC, Lesem MD, Van Dyke DC, Daly PA, Chatis P, et al. Concentrations of adipsin in blood and rates of adipsin secretion by adipose tissue in humans with normal, elevated and diminished adipose tissue mass. Int J Obes Relat Metab Disord. 1994;18(4):213–8.
Maslowska M, Vu H, Phelis S, Sniderman AD, Rhode BM, Blank D, Cianflone K. Plasma acylation stimulating protein, adipsin and lipids in non-obese and obese populations. Eur J Clin Investig. 1999;29(8):679–86.
Abu-Farha M, Behbehani K, Elkum N. Comprehensive analysis of circulating adipokines and hsCRP association with cardiovascular disease risk factors and metabolic syndrome in Arabs. Cardiovasc Diabetol. 2014;13:76.
Schrover IM, van der Graaf Y, Spiering W, Visseren FL. The relation between body fat distribution, plasma concentrations of adipokines and the metabolic syndrome in patients with clinically manifest vascular disease. Eur J Prev Cardiol. 2018;25(14):1548–57.
Cianflone K. The acylation stimulating protein pathway: clinical implications. Clin Biochem. 1997;30(4):301–12.
Kim TE, Lee DH, Kim YJ, Mok JO, Kim CH, Park JH, Lee TK, Yoo K, Jeong Y, Lee Y, et al. The relationship between cognitive performance and insulin resistance in non-diabetic patients with mild cognitive impairment. Int J Geriatr Psychiatry. 2015;30(6):551–7.
Ekblad LL, Rinne JO, Puukka P, Laine H, Ahtiluoto S, Sulkava R, Viitanen M, Jula A. Insulin resistance predicts cognitive decline: an 11-year follow-up of a nationally representative adult population sample. Diabetes Care. 2017;40(6):751–8.
Gursoy Calan O, Calan M, Yesil Senses P, Unal Kocabas G, Ozden E, Sari KR, Kocar M, Imamoglu C, Senses YM, Bozkaya G, et al. Increased adipsin is associated with carotid intima media thickness and metabolic disturbances in polycystic ovary syndrome. Clin Endocrinol. 2016;85(6):910–7.
Derosa G, Fogari E, D'Angelo A, Bianchi L, Bonaventura A, Romano D, Maffioli P. Adipocytokine levels in obese and non-obese subjects: an observational study. Inflammation. 2013;36(4):914–20.
Zhong Y, Miao Y, Jia WP, Yan H, Wang BY, Jin J. Hyperinsulinemia, insulin resistance and cognitive decline in older cohort. Biomed Environ Sci. 2012;25(1):8–14.
Sripetchwandee J, Chattipakorn N, Chattipakorn SC. Links between obesity-induced brain insulin resistance, brain mitochondrial dysfunction, and dementia. Front Endocrinol. 2018;9:496.
Zhou Y, Fang R, Liu LH, Chen SD, Tang HD. Clinical characteristics for the relationship between Type-2 diabetes mellitus and cognitive impairment: a Cross-sectional study. Aging Dis. 2015;6(4):236–44.
Acknowledgments
We would also sincerely appreciate all participants and care organization for their contribution to this study.
Funding
This work was partially supported by the National Natural Science Foundation of China (No.81570732, Shaohua Wang; and No.81870568, Shaohua Wang), the National Nature Science Youth Foundation of China (No. 81200635, Yang Yuan), and the Jiangsu Provincial Medical Youth Talent (QNRC2016819, Yang Yuan).
The sponsors provided financial support and did not participate in the research design, data collection, analysis, interpretation, or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
SHW, DG, YY, JH carried out the study design; DG, RH, ST, were in charge of the experiments; DG, SHW, RH, ST, HYL, JQW, KA collected the clinical data, analyzed the experimental data and give the interpretation of data; DG was the main contributor in composing the manuscript. All authors read and approved the final manuscript.
Authors’ information
Not applicable.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The cross-sectional study was ratified by the IEC for clinical Research of Zhongda Hospital, Affiliated to Southeast University (reference number: 2013ZDSYLL040.0). All participants and their legal guardians will provided written informed consents before study related activities commenced according to a protocol approved by the Research Ethics Committee of the Affiliated Zhongda Hospital of Southeast University .
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Guo, D., Yuan, Y., Huang, R. et al. Association between plasma adipsin level and mild cognitive impairment in Chinese patients with type 2 diabetes: a cross-sectional study. BMC Endocr Disord 19, 108 (2019). https://doi.org/10.1186/s12902-019-0431-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12902-019-0431-y