Abstract
Background
Increase of pulmonary vascular resistance (PVR) is an efficient method of modulating pulmonary and systemic blood flows (Qp/Qs) for patients with left-to-right (L-R) shunt, and is also closely associated with insufficient oxygen exchange for pulmonary hypoperfusion. So that it might be a preferred regime of maintaining arterial partial pressure of carbon dioxide tension (PaCO2) within an optimal boundary via ventilation management in congenital heart disease (CHD) patients for the inconvenient measure of the PVR and Qp/Qs. However, the appropriate range of PaCO2 and patient-specific mechanical ventilation settings remain controversial for CHD children with L-R shunt.
Methods
Thirty-one pediatric patients with L-R shunt, 1–6 yr of age, were included in this observation study. Patients were ventilated with tidal volume (VT) of 10, 8 and 6 ml/kg in sequence, and 15 min stabilization period for individual VT. The velocity time integral (VTI) of L-R shunt, pulmonary artery (PA) and descending aorta (DA) were measured with transesophageal echocardiography (TEE) after an initial 15 min stabilization period for each VT, with arterial blood gas analysis. Near-infrared spectroscopy sensor were positioned on the surface of the bilateral temporal artery to monitor the change in regional cerebral oxygen saturation (rScO2).
Results
PaCO2 was 31.51 ± 0.65 mmHg at VT 10 ml/kg vs. 37.15 ± 0.75 mmHg at VT 8 ml/kg (P < 0.03), with 44.24 ± 0.99 mmHg at VT 6 ml/kg significantly higher than 37.15 ± 0.75 mmHg at VT 8 ml/kg. However, PaO2 at a VT of 6 ml/kg was lower than that at a VT of 10 ml/kg (P = 0.05). Meanwhile, 72% (22/31) patients had PaCO2 in the range of 40-50 mmHg at VT 6 ml/kg. VTI of L-R shunt and PA at VT 6 ml/kg were lower than that at VT of 8 and 10 ml/kg (P < 0.05). rScO2 at a VT of 6 ml/kg was higher than that at a VT of 8 and 10 ml/kg (P < 0.05), with a significantly correlation between rScO2 and PaCO2 (r = 0.53). VTI of PA in patients with defect diameter > 10 mm was higher that that in patients with defect diameter ≤ 10 mm.
Conclusions
Maintaining PaCO2 in the boundary of 40-50 mmHg with VT 6 ml/kg might be a feasible ventilation regime to achieve better oxygenation for patients with L-R shunt. Continue raising PaCO2 should be careful.
Trail registration
Clinical Trial Registry of China (http://www.chictr.org.cn) identifier: ChiCTR-OOC-17011338, prospectively registered on May 9, 2017.
Similar content being viewed by others
Background
Pulmonary oxygen exchange and cardiac output (CO) are closely associated with adequate tissue oxygenation, which could be evaluated by the ratio of pulmonary and systemic blood flow (Qp/Qs) [1]. In congenital heart disease (CHD) children with left-to-right (L-R) shunt, the ratio of Qp/Qs often more than one due to the steal of pulmonary blood flow from systemic blood flow [2], and result in pulmonary hyperperfusion and poor systemic perfusion, which was associated with seriously complications, including pulmonary hemorrhage and necrotizing enterocolitis [3].
Increasing pulmonary vascular resistance (PVR) is a double-blade sword, since it would not only augment right ventricular afterload and lessen L-R shunt [4], but also lead to the insufficient pulmonary oxygenation and deteriorate tissue oxygenation [5]. Therefore, the key regime of achieving better oxygenation in patients with L-R shunt is to balance the PVR for a favorable ratio of Qp/Qs [6, 7]. However, precisely measuring PVR and Qp/Qs is complicated and time-consuming in the operating room. In Reddy’s study, increased PVR, CO and reduced ratio of Qp/Qs through enhancing PaCO2 was testified [8], similar clinical phenomenon was also observed after 4 % CO2 added to the fresh gas flow in a 6-year-old patient with a 4 mm Blalock-Taussing shunt [9]. These indicated that PaCO2 which could be noninvasively measured via blood gases is more likely to be an indicator of unstable PVR and Qp/Qs [10]. However, the definite PaCO2 level that would cause intended change in L-R shunt at patient with congenital heart lesions remain unknown. Besides, adjusting PaCO2 by ventilation management is preferred by anesthesiologists for its available and handy, compare to adding CO2 to the fresh gas. Whereas, how to maintain a favorable level of PaCO2 by regulating mechanical ventilation parameters is still unclear, and largely derived from anesthesiologist’s personal experience to alleviate this unequal Qp/Qs distribution in patients with L-R shunt.
The aim of our study was to compare the VTI of L-R shunt, PA and DA blood flow, cerebral oxygen saturation (rScO2) by interfering with common mechanical ventilation parameters for pediatrics: VT 10, 8 and 6 ml/kg respectively. We attempted to find a perioperative ventilation strategy with a proper range of PaCO2 which could provide an appropriate Qp/Qs with optimal oxygen supply for children with L-R shunt.
Methods
Patient
This study was approved by Ethics Committee of West China Hospital of Sichuan University and then registered on Clinical Trial Registry of China (ChiCTR-OOC-17011338). Eligible subjects were clinically stable children of ASA II-III and aged 1-6 years with a diagnosis of L-R shunt confirmed by echocardiography and scheduled for elective cardiac surgery. Written informed consent from parents or legal guardians was obtained. Children with pulmonary diseases, heart failure or severe arrhythmia were excluded. The case would be cancelled if had one of the followings: difficult intubation, bronchial spasm and unfinished experiment before cardiopulmonary bypass.
A standardized anesthetic protocol was administered. Electrocardiogram (ECG), peripheral oxygen saturation (SpO2) and mean blood pressure (MAP) were performed on arrival at the operating room. General anesthesia was induced with midazolam (0.2~0.3 mg/kg), sufentanil (1~1.5 μg/kg) and rocuronium (0.6~1 mg/kg). After intubated a tracheal tube, bilateral lung ventilations were evaluated by auscultation. Then patients were meachanically ventilated with volume control mode (Aisys CS2, Datex-Ohmeda, WI, USA). Initial settings were VT 10 ml/kg, ratio of inspiratory to expiratory at 1:2 and FiO2 at 0.6, with ventilator rate adjusted according to age (1 to 3 years old: 20–25 rates/min, 3 to 6 years old: 16–20 rates/min). After induction of anesthesia, a radial arterial catheter was inserted for invasive arterial blood pressure monitoring and gas sampling. During next procedure, end-tidal carbon dioxide partial pressure (EtCO2) and invasive arterial pressure (IAP) were monitored continuously. In addition, two sensors of cerebral oximeter (EGOS-600, Enginmed, Suzhou, China) were placed bilaterally on forehead to detect rScO2 [11].
Anesthesia was maintained with 1~2% sevofurane, additional rocuronium and sufentanil were given intravenously when necessary. Moreover, all patients received 10~15 ml/kg of crystalloid in the first hour. Core body temperature remained stable at 36° to 37 °C throughout the study.
Experiment protocol
After intubation, all children were ventilated in three level of VT in the order of 10 ml/kg, 8 ml/kg and 6 ml/kg. Each VT level was maintained for 15 min. TEE examinations and blood gas measurement were performed after 15 min of stabilization ventilation at each VT level. Data of rScO2 was automatic recording every 3 s during the operation and downloaded to a storage disk for further analysis after surgery.
TEE measurements
TEE, regarding as a reliable and minimally invasive monitoring technology, had been used in cardiac surgery [12]. Descending aorta blood flow (DA) which occupies almost 70% of CO could reflect the change in systemic blood flow. The velocity time integral (VTI) of L-R shunt, pulmonary artery (PA) and DA were measured respectively to represent blood flow assuming that the diameters of arteries changed subtly than arterioles by PaCO2 [13, 14].
Detailed TEE measurements as follows: a TEE probe was inserted after intubation, and connected to an ultrasound system (Philips iE33, Bothell, WA, USA). M-line, color flow and spectral Doppler were used to measure the VTI of blood flow, assuring the angel between beam M-line and flow smaller than 20°. Once 15 min ventilation of VT 10 ml/kg completed, the L-R shunt and PA flows were obtained at optimal TEE views, such as mid-esophageal (ME) four chamber and ME right ventricle inflow-outflow. The blood flow of DA was acquired via long-axis view of ascending aorta with the depth of TEE probe kept at mid-esophageal (ME) [15]. After 15 min of stabilization at following VT level, the identical TEE views with VT 10 ml/kg were scanned to acquire the VTI in each patient. All VTI of L-R shunt, PA and DA flows were traced and averaged over three consecutive cardiac cycles (Fig. 1a, b). All echocardiography recordings were obtained and analyzed by the same manipulator.
Statistical analysis
A preliminary test of 5 patients revealed that at least 24 children were needed to detect a significant difference among three VT assuming α = 0.05 and ß = 0.8. Data were tested for distribution and presented as mean ± SD. Differences among three VT were analyzed using Related-Samples Wilcoxon Signed Rank test. The differences of between-group were evaluated by Student’s t-test. Besides, Spearman correlation test was performed to access correlations. P value of < 0.05 was considered statistically significant. All statistical analyses were performed with IBM SPSS Statistics 24 (SPSS, Inc., IL, USA).
Results
All enrolled patients had completed and 93 TEE measurements was successfully obtained (Table 1). There was a statistically significant increase in PaCO2 and rScO2 as VT progressive decreased from 10 ml/kg to 8 ml/kg and 6 ml/kg (Table 2). Low PaCO2 (below than 30 mmHg) occurred in 11/31 patients at VT 10 ml/kg, and 5/31 patients occurred high PaCO2 (above than 50 mmHg) at VT 6 ml/kg. In addition, the proportion of PaCO2 between 40 and 50 mmHg increased from 0% in VT 10 ml/kg to 34% in VT 8 ml/kg, and 72% among the VT 6 ml/kg.
Figure 2 delineated a significantly stepwise decline in VTI of L-R shunt (from 60.16 ± 6.0 cm to 50.35 ± 5.12 cm and 43.44 ± 5.11 cm). Meanwhile, the corresponding VTI of PA markedly descended from 26.36 ± 1.68 cm to 26.33 ± 1.82 cm and 23.23 ± 1.55 cm with VT 6 ml/kg compared to 10 and 8 ml/kg. Nevertheless, no obvious changes was found in the VTI of DA flow. Concurrently, the hypoventilation also resulted in a relatively slight but consistent reduction in the ratio of VTI PA/VTI DA (Table 2).
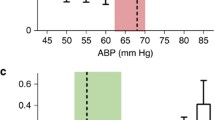
The mean value of VTIPA/VTIDA was lower in the group of PaCO2 higher than 40 mmHg (1.85 vs 1.67), but without statistical significance. Meanwhile, the rScO2 risen 2.78% significantly following PaCO2 higher than 40 mmHg (from 62.14 to 64.92%), with a significant linear correlation between PaCO2 and rScO2 (r = 0.53) depicted in Fig. 3.
Children with defect> 10 mm were younger, accompanied by higher VTI of PA flow and longer hospitalization days (Table 3). In addition, the decrease of L-R shunt caused by rising PaCO2 was more striking in children whose defect≤10 mm rather than children with defect> 10 mm, although without significant difference.
Discussion
In the present study, hyperventilation with VT 10 ml/kg aggravated the VTI of L-R shunt and reduced rScO2 for pediatric patients with CHD, inversely, hypoventilation with VT 6 ml/kg evoked moderate hypercapnia indicated with mitigating the excessive L-R shunt and raising rScO2.
Hyperventilation has traditionally been preferred to improve pulmonary oxygen exchange and reduce the incidence of perioperative desaturation. However, it may be harmful for CHD children with L-R shunt as the fragile balance of Qp/Qs might be exacerbated by decrease of PaCO2 [16, 17]. Seriously, the increase of Qp/Qs may result in imbalance of oxygen supply and postoperative mortality [18, 19]. CO2 could increase the extracellular concentration of Ca2+, constrict pulmonary arterioles and lead to an increase of PVR [20], which is likely to result in a reduction in L-R shunt and redistribute the blood from PA to the systemic blood flow. In this study, we founded the VTI of L-R shunt decreased by 27.8% as PaCO2 increased from 31 mmHg to 44 mmHg, meanwhile the VTI of PA decreased by 11.9%, without significant increase in the DA blood flow and MAP. The possible explanation was that the DA blood flow represented about 70% of CO, which may underestimate the slightly increase of systemic blood flow.
Previous studies demonstrated that adjusting Qp/Qs via ventilation strategies was an effective method in children with intra-cardiac shunt [21, 22]. We decreased the VT from 10 ml/kg to 6 ml/kg in this population, and the increase of PaCO2 led to a decrease in VTIPA/VTIDA, Fajardo et al. also came to the same conclusion [7]. Despite the ventilation strategies of increased PVR differed in some way, but the ultimately outcomes of lower L-R shunt was coincident. The decrease of VTIPA/VTIDA in our results mainly attributed to the decreased L-R shunt and PA blood flow, which was also testified in another study, in which an increase in PaCO2 from 55 mmHg even 90 mmHg incurred statistically significant reduction in Qp/Qs [23]. Nevertheless, our modest but insignificant decrease of VTIPA/VTIDA was the consequent of confined fluctuate range of PaCO2 in our study: merely 30 mmHg to 50 mmHg considering the clinical safety.
Bradely et al.demonstrated that hypoventilation improves cerebral blood flow velocity in infants with bidirectional superior cavopulmonary connection [24], and rSCO2 was associated with the change of cerebral blood perfusion [25]. In this study, we found increase of rSCO2 in accordance of increase of PaCO2. One reason might be that dilated cerebrovascular induced by increased PaCO2 [26, 27]. Another possible reason was increased CO for decrease of L-R shunt and PA flow while DA remain unchanged. It’s seems that the cerebrovascular dilation component was predominated contributor to the increased rSCO2.
Previous studies have suggested that hypocarbia alkalosis should be vigilant in children with elevated pulmonary artery tension due to pulmonary vasodilation [28]. But it need be cautious that although hypercarbia could induce a series of advantages, including mitigated the pulmonary over-circulation and dilated cerebral vascular bed, as indicated by decreased L-R shunt, PA blood flows and VTIPA/VTIDA and increased rSCO2 in our study. Nonetheless, hypercarbia would also cause disadvantage of decreased PaO2 by alveolar hypoventilation. Pervious study founded systemic oxygen saturation initially improved as the Qp/Qs declined in animals modes, whereas decreased reversely after the Qp/Qs below than 0.7 [29]. Moreover, the hypercarbic probably induced an increase in heart rate, even arrhythmia and acute right heart failure which might be undesirable for the adequate oxygen supply during perioperative. As our results manifested, PaO2 decreased from 216.89 to 203.74 mmHg as PaCO2 increased, accompanied by a small rise in HR. Therefore maintaining the ratio of Qp/Qs near 1 both balance systemic blood flow and pulmonary venous oxygen content is a focus for anesthesiologists during surgery of children with L-R shunt. As seen in our results, rising PaCO2 from 31.51 to 44.24 mmHg decreased the VTIPA/VTIDA absolute 0.12 and increased rSCO2 almost 2%, meanwhile, PaCO2 in 22 children were between 40 and 50 mmHg at VT 6 ml/kg. Hence, maintaining PaCO2 between 40 and 50 mmHg by VT 6 ml/kg is favorable for children with L-R shunt, in part because of reductions in L-R shunt and pulmonary blood perfusion and in part because of an increase in rSCO2. And it may be unwise to concentrate on minimizing L-R shunt, PA and VTIPA/VTIDA through limitless increasing PaCO2, ignoring risks of pulmonary hypoperfusion [30]. Besides, consecutively monitoring EtCO2 could be used as a convenient method to prevent excessively high PaCO2 [31].
For children with defect> 10 mm, their higher VTI of PA and lower intraoperative rSCO2 may result from the excessive pulmonary hyperperfusion and relatively insufficient of cerebral blood flow [32]. The prolonged length of stays in defect> 10 mm was consistent with previous postoperative follow-up study, in which an increased risk of postoperative cognitive impairment and a significantly prolonged LOS in patients with low intraoperative rSCO2 were revealed [33]. The possible reason of the weaker vascular reactivity to higher PaCO2 in children with defect> 10 mm may accounted for their excessive pulmonary blood flow [34], which has been suggested produce alterations in the pulmonary vasculature, including vasoocclusive intimal thicking and medical hypertrophy [35]. Extremely patients finally evolved with obliterative pulmonary vascular disease. In previous case, children who may already been developed in pulmonary vascular obstructive disease only improved lower CO via banding the pulmonary artery, but without reactivity at a high level of PaCO2 [36]. Thus, we need realized that adjusting the ratio of Qp/Qs by increasing PaCO2 may not always be applicable to all patients, especially for children older than 18 month with defect> 10 mm. Other measures of changing PVR or SVR should take into consideration individually. Furthermore, the various pulmonary vascular response to PaCO2 may be used to estimate the magnitude of pulmonary hypertension and severity of disease.
One of the limitations of this explorative study is the limited range of PaCO2 concerning patients’ safety, which contributed to the weeny decrease in VTIPA/VTIDA in our results didn’t achieved statistical significance. Moreover, control the enrollment age within 18 month may prevent pulmonary arteriosclerosis from affecting outcomes. Therefore, further studies are still need to evaluate the effects of hypoventilation on children of different ages and defect types with L-R shunt, and develop individualized ventilation settings to optimize the Qp/Qs under various pulmonary vascular reactivity.
Conclusions
In conclusion, our findings recommended a feasible perioperative ventilation strategy for children with L-R shunt. That is maintaining PaCO2 at 40-50 mmHg by VT 6 ml/kg would be helpful to mitigate the excessive L-R shunt after anesthesia, and achieve a favorable VTIPA/VTIDA accompanied by an improvement in cerebral blood perfusion.
Availability of data and materials
All data generated or analyzed during this study are available from the corresponding author on reasonable request.
Abbreviations
- CHD:
-
Congenital heart disease
- DA:
-
Descending aorta
- EtCO2 :
-
End-tidal carbon dioxide partial pressure
- IAP:
-
Invasive arterial pressure
- L-R:
-
Left-to-Right
- NIRS:
-
Near-infrared spectroscopy
- PA:
-
Pulmonary artery
- PaCO2 :
-
Arterial partial pressure of carbon dioxide tension
- PVR:
-
Pulmonary vascular resistance
- rScO2 :
-
Regional cerebral oxygen saturation
- TEE:
-
Transesophageal echocardiography
- VT :
-
Tidal volume
- VTI:
-
Velocity time integral
References
Brienza N, Biancofiore G, Cavaliere F, Corcione A, De Gasperi A, De Rosa RC, et al. Clinical guidelines for perioperative hemodynamic management of non cardiac surgical adult patients. Minerva Anestesiol. 2019. https://doi.org/10.23736/S0375-9393.19.13584-5.
Yamasaki Y, Kawanami S, Kamitani T, Sagiyama K, Sakamoto I, Hiasa KI, et al. Noninvasive quantification of left-to-right shunt by phase contrast magnetic resonance imaging in secundum atrial septal defect: the effects of breath holding and comparison with invasive oximetry. Int J Cardiovasc Imaging. 2018;34(6):931–7.
Kitterman JA, Edmunds LH Jr, Gregory GA, Heymann MA, Tooley WH, Rudolph AM. Patent ducts arteriosus in premature infants. Incidence, relation to pulmonary disease and management. N Engl J Med. 1972;287(10):473–7.
Wheller J, George BL, Mulder DG, Jarmakani JM. Diagnosis and management of postoperative pulmonary hypertensive crisis. Circulation. 1979;60(7):1640–4.
Liu W, Huang Q, Lin D, Zhao L, Ma J. Effect of lung protective ventilation on coronary heart disease patients undergoing lung cancer resection. J Thorac Dis. 2018;10(5):2760–70.
Pinsky MR. The hemodynamic consequences of mechanical ventilation: an evolving story. Intensive Care Med. 1997;23(5):493–503.
Fajardo MF, Claure N, Swaminathan S, Sattar S, Vasquez A, D'Ugard C, et al. Effect of positive end-expiratory pressure on ductal shunting and systemic blood flow in preterm infants with patent ductus arteriosus. Neonatology. 2014;105(1):9–13.
Reddy VM, Liddicoat JR, Fineman JR, McElhinney DB, Klein JR, Hanley FL. Fetal model of single ventricle physiology: hemodynamic effects of oxygen, nitric oxide, carbon dioxide, and hypoxia in the early postnatal period. J Thorac Cardiovasc Surg. 1996;112(2):437–49.
Jobes DR, Nicolson SC, Steven JM, Miller M, Jacobs ML, Norwood WI Jr. Carbon dioxide prevents pulmonary overcirculation in hypoplastic left heart syndrome. Ann Thorac Surg. 1992;54(1):150–1.
Misra S, Koshy T, Mahaldar DA. Sudden decrease in end-tidal carbon-dioxide in a neonate undergoing surgery for type B interrupted aortic arch. Ann Card Anaesth. 2011;14(3):206–10.
Dix LM, van Bel F, Lemmers PM. Monitoring cerebral oxygenation in neonates: an update. Front Pediatr. 2017;5:46.
Schober P, Loer SA, Schwarte LA. Perioperative hemodynamic monitoring with transesophageal Doppler technology. Anesth Analg. 2009;109(2):340–53.
Nagi MM, Ward ME. Modulation of myogenic responsiveness by CO2 in rat diaphragmatic arterioles: role of the endothelium. Am J Phys. 1997;272(3 Pt 2):H1419–25.
Green JF, Schmidt ND. Mechanism of hyperpnea induced by changes in pulmonary blood flow. J Appl Physiol Respir Environ Exerc Physiol. 1984;56(5):1418–22.
Sreedhar R. Acyanotic congenital heart disease and transesophageal echocardiography. Ann Card Anaesth. 2017;20(Supplement):S36–s42.
Sousse LE, Herndon DN, Andersen CR, Ali A, Benjamin NC, Granchi T, et al. High tidal volume decreases adult respiratory distress syndrome, atelectasis, and ventilator days compared with low tidal volume in pediatric burned patients with inhalation injury. J Am Coll Surg. 2015;220(4):570–8.
Bradley SM, Simsic JM, Mulvihill DM. Hyperventilation impairs oxygenation after bidirectional superior cavopulmonary connection. Circulation. 1998;98(19 Suppl):II372–6 discussion II6–7.
McElhinney DB, Marianeschi SM, Reddy VM. Additional pulmonary blood flow with the bidirectional Glenn anastomosis: does it make a difference? Ann Thorac Surg. 1998;66(2):668–72.
Mainwaring RD, Lamberti JJ, Uzark K, Spicer RL, Cocalis MW, Moore JW. Effect of accessory pulmonary blood flow on survival after the bidirectional Glenn procedure. Circulation. 1999;100(19 Suppl):Ii151–6.
Horimoto M. Measurements of blood flow velocity in pulmonary microvessels with laser-Doppler microscope and investigation of several factors affecting the blood flow velocity (author's transl). Hokkaido Igaky Zasshi. 1981;56(5):507–18.
Lellouche F, Delorme M, Bussieres J, Ouattara A. Perioperative ventilatory strategies in cardiac surgery. Best Pract Res Clin Anaesthesiol. 2015;29(3):381–95.
White FN, Hicks JW, Ishimatsu A. Relationship between respiratory state and intracardiac shunts in turtles. Am J Phys. 1989;256(1 Pt 2):R240–7.
Tabbutt S, Ramamoorthy C, Montenegro LM, Durning SM, Kurth CD, Steven JM, et al. Impact of inspired gas mixtures on preoperative infants with hypoplastic left heart syndrome during controlled ventilation. Circulation. 2001;104(12 Suppl 1):I159–64.
Bradley SM, Simsic JM, Mulvihill DM. Hypoventilation improves oxygenation after bidirectional superior cavopulmonary connection. J Thorac Cardiovasc Surg. 2003;126(4):1033–9.
Yagi Y, Yamamoto M, Saito H, Mori T, Morimoto Y, Oyasu T, et al. Changes of cerebral oxygenation in sequential Glenn and Fontan procedures in the same children. Pediatr Cardiol. 2017;38(6):1215–9.
Hoskote A, Li J, Hickey C, Erickson S, Van Arsdell G, Stephens D, et al. The effects of carbon dioxide on oxygenation and systemic, cerebral, and pulmonary vascular hemodynamics after the bidirectional superior cavopulmonary anastomosis. J Am Coll Cardiol. 2004;44(7):1501–9.
Park CS, Kwak JG, Lee C, Lee CH, Lee SK, Kim YL. Near-infrared spectroscopy as a possible device for continuous monitoring of arterial carbon dioxide tension during cardiac surgery. Perfusion. 2011;26(6):524–8.
Schuller JL, Bovill JG, Nijveld A. End-tidal carbon dioxide concentration as an indicator of pulmonary blood flow during closed heart surgery in children. A report of two cases. Br J Anaesth. 1985;57(12):1257–9.
Barnea O, Austin EH, Richman B, Santamore WP. Balancing the circulation: theoretic optimization of pulmonary/systemic flow ratio in hypoplastic left heart syndrome. J Am Coll Cardiol. 1994;24(5):1376–81.
Li J, Zhang G, Holtby H, Bissonnette B, Wang G, Redington AN, et al. Carbon dioxide--a complex gas in a complex circulation: its effects on systemic hemodynamics and oxygen transport, cerebral, and splanchnic circulation in neonates after the Norwood procedure. J Thorac Cardiovasc Surg. 2008;136(5):1207–14.
Smolinsky AK, Shinfeld A, Paret G, Bar-El Y, Glauber V, Shabtai EL, et al. End-tidal CO2 levels are a reliable indicator of band tightness in pulmonary artery banding. Ann Thorac Surg. 1995;60(6 Suppl):S523–4.
Matthews IL, Bjornstad PG, Kaldestad RH, Heiberg L, Thaulow E, Gronn M. The impact of shunt size on lung function in infants with univentricular heart physiology. Pediatr Crit Care Med. 2009;10(1):60–5.
Meng L, Xiao J, Gudelunas K, Yu Z, Zhong Z, Hu X. Association of intraoperative cerebral and muscular tissue oxygen saturation with postoperative complications and length of hospital stay after major spine surgery: an observational study. Br J Anaesth. 2017;118(4):551–62.
Dorrington KL, Talbot NP. Human pulmonary vascular responses to hypoxia and hypercapnia. Pflugers Arch. 2004;449(1):1–15.
Heath D, Edwards JE. The pathology of hypertensive pulmonary vascular disease; a description of six grades of structural changes in the pulmonary arteries with special reference to congenital cardiac septal defects. Circulation. 1958;18(4 Part 1):533–47.
Wong RS, Baum VC, Sangwan S. Truncus arteriosus: recognition and therapy of intraoperative cardiac ischemia. Anesthesiology. 1991;74(2):378–80.
Acknowledgements
The authors thank all the teachers, students and the research participants who took part in the data collection. We would like to acknowledge Dr. Yiping Bai and Dr. Hong Yu for their assistance in making this a better article. We also thank Mr. Jiapei Yang and Mrs. Haili Zhang for the assistance in the research.
Funding
This research was financially supported by research grants from the 1.3.5 project for disciplines of excellence, West China Hospital, Sichuan University (Zy2016101) and Taipei Cheng Hsin General Hospital (CHGH105–1). None of funding bodies had any influence on the design of the study and collection, analysis, and interpretation of data and in writing the manuscript.
Author information
Authors and Affiliations
Contributions
PY-L: ethics approve, study design, data collection and analysis, drafted the manuscript; JZ: methodology, reviewed manuscript for important intellectual content; WW: study design, data collection, data curation and reviewed it for important intellectual content; JL: reviewed it for important intellectual content. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was approved by Ethics Committee of West China Hospital of Sichuan University and then registered on Clinical Trial Registry of China (OOC17011338. Peiyi Li/Wei Wei. Registered 9 May, 2017). Written informed consent from parents or legal guardians was obtained prior to enrollment.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Li, P., Zeng, J., Wei, W. et al. The effects of ventilation on left-to-right shunt and regional cerebral oxygen saturation: a self-controlled trial. BMC Anesthesiol 19, 178 (2019). https://doi.org/10.1186/s12871-019-0852-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12871-019-0852-1