Abstract
Background
As the major storage protein in rice seeds, glutelins are synthesized at the endoplasmic reticulum (ER) as proglutelins and transported to protein storage vacuoles (PSVs) called PBIIs (Protein body IIs), where they are cleaved into mature forms by the vacuolar processing enzymes. However, the molecular mechanisms underlying glutelin trafficking are largely unknown.
Results
In this study, we report a rice mutant, named glutelin precursor accumulation6 (gpa6), which abnormally accumulates massive proglutelins. Cytological analyses revealed that in gpa6 endosperm cells, proglutelins were mis-sorted, leading to the presence of dense vesicles (DVs) and the formation paramural bodies (PMBs) at the apoplast, consequently, smaller PBII were observed. Mutated gene in gpa6 was found to encode a Na+/H+ antiporter, OsNHX5. OsNHX5 is expressed in all tissues analyzed, and its expression level is much higher than its closest paralog OsNHX6. The OsNHX5 protein colocalizes to the Golgi, the trans-Golgi network (TGN) and the pre-vacuolar compartment (PVC) in tobacco leaf epidermal cells. In vivo pH measurements indicated that the lumens of Golgi, TGN and PVC became more acidic in gpa6.
Conclusions
Our results demonstrated an important role of OsNHX5 in regulating endomembrane luminal pH, which is essential for seed storage protein trafficking in rice.
Similar content being viewed by others
Background
Rice seeds accumulate large amount of storage proteins, including glutelin, prolamin, and α-globulin, which supply nutrients for seed germination and seedling growth. Up to 80% of the total seed storage proteins are made up of glutelins which are important protein sources for human consumption because of their easy digestibility. Glutelins are synthesized as 57 kD precursors on the rough endoplasmic reticulum (RER) and transported to PBIIs by DV-mediated post-Golgi transport pathway or ER-derived precursor-accumulating compartments [1,2,3,4,5,6].
Rice 57H mutants are characterized by over-accumulation of 57 kD glutelin precursors in seeds, which are excellent genetic resources to dissect the glutelin vacuolar transport pathway. Up till now, nine 57H mutants have been reported in rice, including gpa1/glup4, gpa2/glup6, gpa3, gpa4/glup2, W379/glup3, esp2, glup1/esp5, Glup5, and glup7 [5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20]. Among which four cloned genes have been shown to regulate the proglutelin trafficking events. GPA4 encodes GOLGI TRANSPORT 1B which regulates protein export from the ER [17, 20]. GPA3, a plant-specific Kelch-repeat domain containing protein, acts as a scaffold to recruit guanine-nucleotide exchange factor (GEF) GPA2/OsVPS9A, which in turn activates a small GTPase GPA1/OsRab5a [5, 6, 16, 18, 19]. GPA3, GPA2, and GPA1 proteins form a functional complex on the DVs to regulate the vacuolar trafficking of proglutelins [6]. In addition, ESP2 encodes protein disulfide isomerase-like1–1 (PDIL1–1) which regulates the disulfide bond formation in ER [3]. W379 encodes a vacuolar processing enzyme which processes proglutelins into acidic and basic subunits in the PBII [8, 15]. Despite these advances, molecular mechanisms underlying glutelin trafficking are still elusive.
Na+/H+ antiporters (NHX antiporters) are H+-coupled cotransporters that transfer Na+ or K+ across membrane in exchange for H+ [21]. In plants, NHX antiporters are essential for cellular pH and ion homeostasis. They play important roles in various cellular processes, such as Na+, K+ movement, pH homeostasis, vesicular trafficking and protein targeting, stress response, plant growth and development [22,23,24,25,26,27]. Based on the subcellular localization, Arabidopsis NHX antiporters are classified into three subgroups which localized to the vacuoles (AtNHX1–4) [28,29,30], plasma membrane (AtNHX7/8) [24, 30, 31], and endosomal compartments (AtNHX5/6) [32], respectively. Particularly, AtNHX5 and AtNHX6 are localized at the Golgi, TGN and required for cell expansion, response of salt stress as well as vesicular trafficking [32]. These two proteins are also localized at the PVC and important for maintaining endomembrane luminal pH and receptor-mediated protein trafficking to the vacuole [33]. In addition, AtNHX5 and AtNHX6 are require for seed storage protein processing [34]. In rice, overexpression of OsNHX1 enhanced tolerance to salt stress [35]. However, the function of endosomal NHX proteins in rice remain largely unknown.
In this study, we report the functional characterization of a rice gpa6 mutant that accumulated a large amount of proglutelins in the mutant endosperm cells, and demonstrate that GPA6 encodes a Golgi-, TGN- and PVC-localized Na+/H+ antiporter OsNHX5 which is essential for endomembrane luminal pH homeostasis and proglutelin vacuolar trafficking.
Results
gpa6 seeds accumulate proglutelins and develop abnormal endosperm
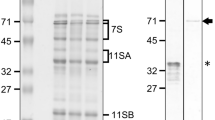
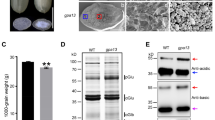
A 57H mutant named gpa6 was isolated during our continuous effort to dissect the glutelin trafficking pathway in rice. Unlike the transparent endosperm of wild type, gpa6 mutant endosperm appeared floury (Fig. 1a). Scanning electron microscopy (SEM) analysis revealed that gpa6 endosperm comprised round and loosely packaged compound starch granules instead of the tightly packaged, crystal-like structures observed in the wild type (Fig. 1b and c). Meanwhile, the 1000-grain weight was significantly decreased, and the amylose content was reduced approximately 20% and the lipid content was increased 81% in the gpa6 mutant. However, the total protein content in the endosperm was not changed (Additional file 8: Table S1). Compared with the wild type, gpa6 accumulated much higher level of unprocessed 57 kD proglutelins, accompanied by concomitant reduction of both 40 kD acidic and 20 kD basic subunits of the mature glutelins (Fig. 1d). The appearance of higher amount of proglutelins was further confirmed by immunoblotting using antibodies against 40-kD glutelin acidic subunits and 20-kD glutelin basic subunits (Fig. 1e). Time course studies revealed that the abnormal accumulation of proglutelins in gpa6 seeds occurred from ~ 12-DAF onwards (Additional file 1: Figure S1). In addition, the expression of representative genes coding for storage proteins, and the protein level of ER lumen BINDING PROTEIN1 (BiP1) and PDI1–1 showed almost no differences between gpa6 and wild-type seeds (Fig. 1e, Additional file 2: Figure S2), indicating that the accumulation of 57 KD proglutelins was not due to an increasement of storage protein coding gene expression and probably protein synthesis was normal as no ER stress was detected. Collectively these data suggested that in the gpa6 mutant might be defective in the trafficking of proglutelins.
Characterization of the gpa6 Mutant. a Transverse sections of representative wild-type (Indica cultivar N22) and gpa6 mutant dry seeds. Bars = 1 mm. b and c Scanning electron microscopy images of transverse sections of wild-type (b) and gpa6 mutant (c) seeds. Bars = 10 μm. d SDS-PAGE profiles of total seed storage proteins of the wild type and gpa6 mutant. pGlu, 57-kD proglutelins; αGlu, 40-kD glutelin acidic subunits; αGlb, 26-kD α-globulin; βGlu, 20-kD glutelin basic subunits; Pro, prolamins. e Immunoblot analysis of glutelins and the molecular chaperones BiP1 and PDI1–1. Arrowheads represent glutelin basic subunits. Arrows indicate the 57-kD proglutelins (red) and the glutelin acidic subunits (black). EF-1α was used as a loading control
The gpa6 mutant is defective in post-Golgi trafficking of storage proteins in developing endosperm
To gain an overview of glutelin deposition, semi-thin sections (1 μm) of 12-DAF wild-type and gpa6 mutant endosperm were prepared and subjected to the immunofluoresence staining with specific antibodies against prolamins and glutelin acidic subunits (Fig. 2). In gpa6 mutant, the PBIIs (containing glutelins) were reduced to 53% of the wild type (Fig. 2a-f, g), while the PBI sizes (containing prolamins) were comparable (Fig. 2a-f, h). Large glutelin-containing paramural bodies (PMBs) were readily observable (Fig. 2f). In addition, α-globulins were also transported incorrectly to the PMBs rather than PBIIs in gpa6 (Additional file 3: Figure S3). Consistent with the proglutelin trafficking defects, Pectins labeled with JIM7 were seen to accumulate inside the PMBs in gpa6 rather than display an even distribution along the wild type cell wall (Additional file 4: Figure S4) [36].
Immunofluorescence microscopy of protein bodies in the subaleurone cells of the wild type and gpa6 mutant. a to f Immunofluorescence microscopy images of storage proteins in wild-type (a-c) and gpa6 (d-f) 12 DAF seeds. a, d Secondary antibodies conjugated with Alexa fluor 488 (green) were used to trace the antigens recognized by the anti-glutelin antibodies. (b, e) Secondary antibodies conjugated with Alexa fluor 555 (red) were used to trace the antigens recognized by the anti-prolamin antibodies. (c, f) Merged images. White arrows in (f) indicate the PMB structures. Bars = 10 μm (a-f). (g) and (h) Statistical analysis of the diameters of PBIIs (g) and PBIs (h). Values are means ± SD. **P < 0.01 (n > 350, Student’s t test)
Next, subcellular observation by transmission electron microscopy (TEM) was performed using ultra-thin sections of 12-DAF developing endosperm. In wild-type endosperm cells, there are irregularly shaped, fully filled PBIIs and round spherical PBIs (Fig. 3a), however, in the gpa6 mutant, PBIIs were only partially filled with the storage proteins (Fig. 3b), which was accompanied by the presence of PMBs (formed by the clustered DVs) and secreted oval-shaped structures along the cell wall (Fig. 3g and i). To determine structural alterations in the endomembrane system that could account for the missorting of storage protein precursors to the apoplast, we analyzed 12-DAF developing endosperm by immunogold labeling. Indeed, in the gpa6 mutant, only part of glutelins was correctly transported to the PBIIs (Fig. 4a and b), meanwhile, mis-sorted DVs and PMBs were found to contain glutelins (Fig. 4c and d). These results demonstrated that proglutelin were mis-sorted, and delivered to the apoplast.
Ultrastructure of subaleurone cells of developing endosperm of the wild type and gpa6 mutant. a and b Two types of protein bodies were observed in wild-type (a) and gpa6 mutant (b) endosperm. Bars = 2 μm. CW, cell wall. c Wild type. Bars = 400 nm. d DVs bud off from the Golgi in the gpa6 mutant. Red Arrow indicates enlarged DVs. Bars = 400 nm. e Large clusters of DVs (blue arrow) in the gpa6 mutant. Bars = 400 nm. f and g Electron micrographs showing that DVs can fuse with the PM (f) and expel their contents into the apoplast forming oval-shaped structures (Arrowheads) (g) in the gpa6 mutant. Bars = 1 μm. h Two DVs are fused with each other (rectangular box) in the gpa6 mutant. Bars = 1 μm. (i) The PMB structures in the gpa6 mutant. Bars = 1 μm
Immunoelectron microscopy localization of glutelins in rice endosperm cells. a Glutelins were accumulated in PBIIs in the wild-type endosperm cells. CW, cell walls. Bars = 500 nm. b Size-reduced PBII containing glutelins. Bars = 500 nm. c Glutelins in DVs (red arrows) and oval-shaped structures (black arrows). Bars = 500 nm. d Glutelins in the PMBs. Bars = 500 nm. 10-nm gold particle conjugated secondary antibodies were used in (a–d)
Proglutelins are delivered to the PBII via DV-mediated trafficking pathway [5, 6], therefore, DV morphology are carefully examined. Statistical analysis showed that the average diameter of DVs near the Golgi apparatus, and presumably just budding from the Golgi apparatus in gpa6 is 193 nm (n = 75, Fig. 3d), which is larger than that of wild type (average 154 nm, n = 56, Fig. 3c), and these DVs tends to cluster in the cytosol (Fig. 3e). In addition, numerous DVs at an average diameter of 198 nm were found just fused with the plasma membrane, and kept spherical shapes (Fig. 3f), gradually, the secreted storage proteins became oval-shaped (Fig. 3g). Occasionally, fusion between two DVs was detected (Fig. 3h), this might account for the slight size increasement of DVs along the plasma membrane (198 nm) versus newly budded ones (193 nm). In short, DVs in gpa6 mutant are enlarged.
Altogether, these results demonstrated that in gpa6 mutant enlarged glutelin-containing DVs were mis-sorted to the apoplast, thus led to the reduction of PBII size.
Map-based cloning of GPA6
The gpa6 mutant was isolated from a 60Co-irradiated population of indica variety N22. Genetic analysis revealed that the mutant phenotype was inherited as a recessive mutation (Additional file 9: Table S2). For map-based cloning, we crossed gpa6 with the japonica variety Nipponbare to generate 208 F2 recessive individuals. The GPA6 locus was mapped to chromosome 9 and further fine-mapped to a 98-kb region (Fig. 5a). DNA sequencing revealed a 7 bp deletion in the sixth exon of Os09g0286400, generating a premature stop codon that led to a truncated product with 199 amino acids (Fig. 5b). Three independent transgenic lines bearing Ubiquitin promoter driven Os09g0286400 open reading frame (ORF) rescued the gpa6 mutant phenotypes, including the floury appearance of endosperm (Fig. 5c), the accumulation of proglutelins (Fig. 5d) and the abnormal glutelin deposit pattern (Fig. 5e and f). Therefore, Os09g0286400 is the gene responsible for gpa6 mutant phenotypes.
Map-based cloning of GPA6. a Fine mapping of the GPA6 locus. The molecular markers and the number of recombinants are shown. b Gene structure and the mutation site in Os09g0286400. Os09g0286400 comprises 22 exons (closed boxes) and 21 introns (lines). ATG and TGA represent the start and stop codons, respectively. A 7 bp deletion in the sixth exon of Os09g0286400 in gpa6. c-f The Os09g0286400 ORF under the control of ubiquitin promoter rescues the grain appearance (c), the storage protein composition pattern (d), the ultrastructures of endosperm cells in the wild type (e) and L1 (f). L1 to L3 denote the grains from three independent T1 transgenic lines. Red arrows in (d) indicate the 57-kD proglutelins. Bars = 1 mm in (c). Bars = 2 μm in (e) and (f)
GPA6 encodes OsNHX5 that localizes to the Golgi, TGN and PVC
GPA6 encodes a NHX antiporter homologous to the endosomal localized AtNHX5, and was named OsNHX5. OsNHX5 is predicted to have 9 putative transmembrane domains and the mutation in gpa6 leads to the deletion of the last four. Real-time PCR analysis revealed that OsNHX5 is expressed in all tissues examined (Fig. 6b). During endosperm development, the expression of OsNHX5 was low at the early stages, peaked at ~ 15-DAF, and decreased at ~ 18-DAF (Fig. 6b), which is correlated with the accumulation of glutelins. The rice genome has another putative endosomal antiporter OsNHX6 (Fig. 6a, Additional file 5: Figure S5). Although it is ubiquitiously expressed, its expression is much lower than OsNHX5 (ratio of expression level of OsNHX5/OsNHX6, root: 91; stem: 25; leaf: 232; leaf sheath: 15; panicle: 18; 12-DAF endosperm: 26) (Fig. 6b).
Phylogenetic analyses and spatial expression patterns of OsNHX5. a A neighbor-joining tree of OsNHX5 and its homologs. The tree was constructed using MEGA and bootstrapped with 1000 replicates. b Wild-type expression levels of OsNHX5 and OsNHX6 in various organs and different developmental stages of endosperm. R, root; S, stem; L, leaf; LS, leaf sheath; P, panicle; DAF, days after flowering. Actin1 was used as an internal control. For each RNA sample, three technical replicates were performed. Values are means ± SD
To determine the subcellular localization of OsNHX5, OsNHX5 coding sequence was fused to the N-terminus of GFP to obtain p35S:OsNHX5-GFP construct. After transformed into gpa6, it completely rescued the mutant phenotype, indicating that OsNHX5-GFP is functional in vivo (Additional file 6: Figure S6). Unfortunately, GFP fluorescence in the transgenic lines was too weak to be detected. Transiently expressed OsNHX5-GFP in N. benthamiana leaf epidermal cells was examined instead, and OsNHX5-GFP was shown to partially colocalized with the Golgi, TGN and PVC markers, respectively (Pearson’s correlation coefficient [PSC], Golgi: PSC = 0.608 ± 0.08; TGN: PSC = 0.753 ± 0.14; PVC: PSC = 0.587 ± 0.11, Fig. 7). Similarly, OsNHX6 was localized to the Golgi, TGN, and PVC as well (Additional file 7: Figure S7).
Subcellular Localization of OsNHX5 in N. benthamiana protoplasts. a to c Confocal microscopy images showing that OsNHX5-GFP is localized as punctate signals in the cytosol and its distribution partially overlaps with the markers for Golgi (ST-mRFP [a]), TGN (mRFP-SYP61 [b]) and PVC (mRFP-VSR2 [c]). Bars = 10 μm (a-c)
Given the fact that OsNHX5 mutation alone displayed phenotypes and OsNHX6 is much lower expressed, OsNHX5 might be a predominant endosomal localized NHX antiporter in rice.
The luminal pH of Golgi, TGN and PVC is more acidic in the gpa6 protoplasts
In order to determine whether the cellular pH was affected in gpa6, we use noninvasive live-cell imaging to measure pH by the pHluorin-based pH sensor [37, 38]. pH sensors ManI-PRpHluorin, PRpHluorin-BP80 (Y612A) and PRpHluorin-AtVSR2 were used to measure pH of Golgi, TGN and PVC, respectively in the rice protoplasts [38]. The calibration curve was acquired by calculating pH-dependent fluorescence ratios (Fig. 8a). Our results showed that the pH of the Golgi, TGN, and PVC was more acidic in gpa6 (Golgi: 6.45 ± 0.17; TGN: 6.01 ± 0.16; PVC: 5.87 ± 0.19) than in the wild type (Golgi: 6.95 ± 0.21; TGN: 6.36 ± 0.18; PVC: 6.31 ± 0.14) (Fig. 8d) where the △pH = 0.35–0.50. Representative pseudocoloured images of PRpHluorin-BP80 (Y612A) were shown (Fig. 8b and c). These results clearly indicated that OsNHX5 regulates the pH homeostasis of the Golgi, TGN, and PVC in rice protoplasts.
OsNHX5 regulates the pH of Golgi, TGN and PVC. a In vivo calibration curve of pH. pH calibration was achieved by equilibrating intracellular pH with 10 μM nigericin, 60 mM KCl, and 10 mM MES/HEPES Bis-Tris-propane, pH 5.0 to 8.0. (mean ± SD; n ≥ 25 protoplasts). b and c Representative pseudocolored images of PRpHluorin-BP80 (Y612A) in wild-type (b) or gpa6 (c) protoplasts. Bars = 5 μm. d pH of the Golgi, TGN and PVC. (mean ± SD; n ≥ 25 protoplasts; **P < 0.01; Student’s t test)
Discussion
gpa6 is defective in post-Golgi trafficking of storage proteins in rice endosperm cells
Characterization of 57H mutants facilitates us to understand the molecular mechanism of proglutelin trafficking and processing in rice endosperm cells. In this study, we isolated a 57H mutant gpa6 that accumulated large amount of proglutelins in rice endosperm. The comparable protein levels of BiP1 and PDI1–1 between wild type and the gpa6 mutant suggested a normal ER function in gpa6. Consistent with this notion, gpa6 developed normal ER-derived PBIs. In gpa6 mutant, large amount of DVs accumulated in the cytosol, suggesting that the trafficking rate is largely reduced. Furthermore, DVs were mis-sorted to the apoplast, leading to smaller PBIIs. These abnormalities are very similar to gpa1/osrab5a, gpa2/osvps9a, and gpa3/kelch mutants [5, 6, 16, 18, 19]. Taken together, gpa6 is defective in proglutelin post-Golgi vacuolar trafficking pathway in rice endosperm cells.
OsNHX5 is a predominant intracellular NHX antiporter that regulates luminal pH of several endomembrane compartments
Similar to animals, plant cells also have specific pH in different endomembrane compartments along the secretory pathway [37,38,39,40]. In this study, using noninvasive live-cell imaging and pH sensors, we found that the pH values of Golgi, TGN and PVC in wild-type rice protoplasts were not significantly different from their counterparts in Arabidopsis protoplasts (Golgi: 6.8; TGN: 6.3; PVC: 6.2) [38]. However, all three compartments had a more acidic pH (△pH = 0.35–0.50) in osnhx5, indicating that OsNHX5 plays an important role in the alkalization of these compartments. Given the fact that atnhx5 atnhx6 had a more acidic pH in those compartments as well (△pH = 0.25–0.40), endosomal antiporters seem to have conserved functions in rice and Arabidopsis cells.
In atnhx5 atnhx6 double mutant, lowered pH led to a compromised receptor–cargo association [33]. The phenotype of storage protein trafficking defects were similar between osnhx5 and atnhx5 atnhx6. Therefore, the acidification of Golgi, TGN, particularly PVC may result in reduced VSR (Vacuolar sorting receptor)-proglutelin association, although the receptors for proglutelins remains to be characterized. Previous studies showed that OsNHX5 has a K+ and Na+ transport activity [41], thus it is possible that ionic changes might also affect VSR-cargo interactions.
Previous studies implicate that maintaining pH homeostasis of TGN is required for protein trafficking in Arabidopsis [42]. DVs are unique carriers for proglutelin transport in rice presumably budded from the TGN [5, 6]. It is worth noting that the average size of DVs newly budded from TGN is enlarged to about 193 nm in gpa6, which is much bigger than 154 nm in the wild type (Fig. 3), 160 nm in gpa2 [5], and 153 nm in gpa3 mutants [6]. Thus, pH of TGN seems to have an impact on DV size control, although the detail mechanism remains to be explored.
Conclusions
In summary, our studies demonstrated that OsNHX5 is localized to the Golgi, TGN and PVC to maintain pH homeostasis, which is important for DV-mediated glutelin trafficking in rice endosperm.
Methods
Plant materials and growth conditions
The gpa6 mutant was identified from a 60Co-irradiated mutant pool of the indica cultivar N22 (Nagina22, an Indian traditional variety). An F2 population was produced from gpa6 and a japonica variety Nipponbare for mapping. The seeds of all accessions were collected, stored and supplied by the State Key Laboratory of Crop Genetics and Germplasm Enhancement of Nanjing Agricultural University, Jiangsu, China. All plants were grown in the paddy field during the normal growing seasons or in a greenhouse at Nanjing, China. Developing seeds of wild type (N22) and gpa6 at 6–21 days after fertilization (DAF) were used in the experiments.
Protein Extraction from Rice seeds and immunoblot analysis
Total protein extraction and immunoblot assay were performed as described previously [16].
Microscopy
Scanning electron microscopy, transmission electron microscopy, light and immunofluorescence microscopy and immunogold labeling analysis were performed as described previously [5, 6, 16, 17].
Map-based cloning
To map the GPA6 locus, an F2 population was generated from a cross between the gpa6 mutant and a japonica variety Nipponbare. Total proteins were extracted from half of an individual rice seed and resolved by SDS-PAGE gel to monitor the accumulation of the proglutelins. Meanwhile, the other half of the identified mutant seeds with embryos was grown for DNA extraction. In total, 208 recessive individuals were used for fine mapping of GPA6. The primers used in fine mapping are listed in Additional file 10: Table S3.
Real-time RT-PCR analysis
Total RNA was extracted from different tissues using an RNA Prep Pure Plant Kit (TIANGEN). First strand cDNA was synthesized using oligo (dT)18 as the primer (TaKaRa). Three biological replicates of real-time RT-PCR were performed with SYBR Premix Ex Taq II (TaKaRa) on an Applied Biosystems 7500 Real-Time PCR System. The primer sequences used for PCR are listed in Additional file 12: Table S5.
Subcellular localization
For transient expression analysis in N. benthamiana leaf epidermal cells, the coding region of OsNHX5 or OsNHX6 was amplified and inserted into the binary vector pCAMBIA1305GFP to produce the OsNHX5-GFP or OsNHX6-GFP fusion construct (Additional file 11: Table S4). Construct were introduced into the Agrobacterium strain EHA105 and then used to infiltrate N. benthamiana leaves, as described previously [43]. N. benthamiana protoplasts were isolated using the same method used with Arabidopsis [44]. Fluorescence was observed using a confocal laser scanning microscope (Leica TCS-SP8).
pH measurements
The rice protoplasts were isolated from 10-days-old N22 and gpa6 seedlings. pH sensors ManI–PRpHluorin, PRpHluorin-BP80 (Y612A) and PRpHluorin–AtVSR2 were transformed into rice protoplasts as previously described [45, 46]. The PRpHluorin signals at emission wavelength of 500 to 550 nm were recorded with dual-excitation wavelength at 405 and 488 nm, respectively, and used to calculate the pH using the calibration curve. In vivo calibration was achieved from the same protoplasts expressing the PRpHluorin for pH measurement. Protoplasts were incubated in WI protoplast buffer (0.5 M mannitol and 20 mM KCl) with 25 μM nigericin, 60 mM KCl, and 10 mM MES/HEPES Bis-Tris-propane adjusted to different pH values ranging from 5.0 to 8.0 for each calibration point [33, 37, 38, 47,48,49]. Fluorescence was observed using a confocal laser scanning microscope (Leica TCS-SP8).
Availability of data and materials
All data generated or analyzed during this study are included in this published article and its additional files.
Abbreviations
- DV:
-
Dense vesicle
- ER:
-
Endoplasmic reticulum
- GPA:
-
Glutelin precursor accumulation
- PBI:
-
Protein body I
- PBII:
-
Protein body II
- PMB:
-
Paramural body
- PSV:
-
Protein storage vacuole
- PVC:
-
Prevacuolar compartment
- TGN:
-
Trans-Golgi network
References
Yamagata H, Sugimoto T, Tanaka K, Kasai Z. Biosynthesis of storage proteins in developing rice seeds. Plant Physiol. 1982;70(4):1094–100.
Krishnan HB, Franceschi VR, Okita TW. Immunochemical studies on the role of the Golgi complex in protein-body formation in rice seeds. Planta. 1986;169(4):471–80.
Takemoto Y, Coughlan SJ, Okita TW, Satoh H, Ogawa M, Kumamaru T. The Rice mutant esp2 greatly accumulates the glutelin precursor and deletes the protein disulfide isomerase. Plant Physiol. 2002;128(4):1212–22.
Takahashi H, Saito Y, Kitagawa T, Morita S, Masumura T, Tanaka K. A novel vesicle derived directly from endoplasmic reticulum is involved in the transport of vacuolar storage proteins in rice endosperm. Plant Cell Physiol. 2005;46(1):245–9.
Liu F, Ren Y, Wang Y, Peng C, Zhou K, Lv J, Guo X, Zhang X, Zhong M, Zhao S, et al. OsVPS9A functions cooperatively with OsRAB5A to regulate post-Golgi dense vesicle-mediated storage protein trafficking to the protein storage vacuole in rice endosperm cells. Mol Plant. 2013;6(6):1918–32.
Ren Y, Wang Y, Liu F, Zhou K, Ding Y, Zhou F, Wang Y, Liu K, Gan L, Ma W, et al. GLUTELIN PRECURSOR ACCUMULATION3 encodes a regulator of post-Golgi vesicular traffic essential for vacuolar protein sorting in rice endosperm. Plant Cell. 2014;26(1):410–25.
Kumamaru T, Satoh H, Iwata N, Omura T, Ogawa M. Mutant for rice storage proteins. III. Genetic analysis of mutants for storage proteins of protein bodies in the starchy endosperm. Jpn J Genet. 1987;62(4):333–9.
Kumamaru T, Uemura Y, Inoue Y, Takemoto Y, Uddin Siddiqui S, Ogawa M, Hara-Nishimura I, Satoh H. Vacuolar processing enzyme plays an essential role in the crystalline structure of glutelin in rice seed. Plant Cell Physiol. 2010;51(1):38–46.
Satoh H, Kumamaru T, Yoshimura S, Ogawa M. New 57 kDa glutelin genes on chromosome 9 in rice. Rice Genet Newsl. 1994;11:158–61.
Satoh H, Kumamaru T, Ogawa M, Siraishi M, Im BG, Son MY, Takemoto Y. Spontaneous ‘57H’ mutants in rice. Rice Genet Newsl. 1995;12:194–6.
Satoh H, Li WX, Takemoto Y, Ito T, Kumamaru T, Qu LQ, Ogawa M. glup4 controlling a 57H character was located on chromosome 12 in rice. Rice Genet Newsl. 1999;16:98–100.
Tian HD, Kumamaru T, Takemoto Y, Ogawa M, Satoh H. Gene analysis of new 57H mutant gene, glup6, in rice. Rice Genet Newsl. 2001;18:48–9.
Ueda Y, Satoh H, Satoh M, Takemoto Y, Kumamaru T, Ogawa M. Inheritance mode of Glup5 gene and the genetic relationships with other 57 mutant genes. Rice Genet Newsl. 2004;21:49–50.
Ueda Y, Sugino A, Takemoto Y, Satoh M, Kumamaru T, Ogawa M. Satoh H. a novel 57H mutant gene, glup7, located on chromosome 4. Rice Genet Newsl. 2004;21:51–3.
Wang Y, Zhu S, Liu S, Jiang L, Chen L, Ren Y, Han X, Liu F, Ji S, Liu X, et al. The vacuolar processing enzyme OsVPE1 is required for efficient glutelin processing in rice. Plant J. 2009;58(4):606–17.
Wang Y, Ren Y, Liu X, Jiang L, Chen L, Han X, Jin M, Liu S, Liu F, Lv J, et al. OsRab5a regulates endomembrane organization and storage protein trafficking in rice endosperm cells. Plant J. 2010;64(5):812–24.
Wang Y, Liu F, Ren Y, Wang Y, Liu X, Long W, Wang D, Zhu J, Zhu X, Jing R, et al. GOLGI TRANSPORT 1B regulates protein export from the endoplasmic reticulum in rice endosperm cells. Plant Cell. 2016;28(11):2850–65.
Fukuda M, Satoh-Cruz M, Wen L, Crofts AJ, Sugino A, Washida H, Okita TW, Ogawa M, Kawagoe Y, Maeshima M, et al. The small GTPase Rab5a is essential for intracellular transport of proglutelin from the Golgi apparatus to the protein storage vacuole and endosomal membrane organization in developing rice endosperm. Plant Physiol. 2011;157(2):632–44.
Fukuda M, Wen L, Satoh-Cruz M, Kawagoe Y, Nagamura Y, Okita TW, Washida H, Sugino A, Ishino S, Ishino Y, et al. A guanine nucleotide exchange factor for Rab5 proteins is essential for intracellular transport of the proglutelin from the Golgi apparatus to the protein storage vacuole in rice endosperm. Plant Physiol. 2013;162(2):663–74.
Fukuda M, Kawagoe Y, Murakami T, Washida H, Sugino A, Nagamine A, Okita TW, Ogawa M, Kumamaru T. The dual roles of the Golgi transport 1 (GOT1B): RNA localization to the cortical endoplasmic reticulum and the export of proglutelin and α-globulin from the cortical ER to the Golgi. Plant Cell Physiol. 2016;57(11):2380–91.
Counillon L, Pouyssegur J. The expanding family of eucaryotic Na+/H+ exchangers. J Biol Chem. 2000;275:1–4.
Pardo JM, Cubero B, Leidi EO, Quintero FJ. Alkali cation exchangers: roles in cellular homeostasis and stress tolerance. J Exp Bot. 2006;57(5):1181–99.
Bassil E, Coku A, Blumwald E. Cellular ion homeostasis: emerging roles of intracellular NHX Na+/H+ antiporters in plant growth and development. J Exp Bot. 2012;63(16):5727–40.
Bassil E, Blumwald E. The ins and outs of intracellular ion homeostasis: NHX-type cation/H+ transporters. Curr Opin Plant Biol. 2014;22:1–6.
Reguera M, Bassil E, Blumwald E. Intracellular NHX-type cation/H+ antiporters in plants. Mol Plant. 2014;7(2):261–3.
Qiu Q. AtNHX5 and AtNHX6: roles in protein transport. Plant Signal Behav. 2016;11(6):e1184810.
Qiu Q. Plant endosomal NHX antiporters: activity and function. Plant Signal Behav. 2016;11(5):e1147643.
Apse MP, Aharon GS, Snedden WA, Blumwald E. Salt tolerance conferred by overexpression of a vacuolar Na+/H+ antiport in Arabidopsis. Science. 1999;285(5431):1256–8.
Bassil E, Tajima H, Liang Y, Ohto M, Ushijima K, Nakano R, Esumi T, Coku A, Belmonte M, Blumwald E. The Arabidopsis Na+/H+ antiporters NHX1 and NHX2 control vacuolar pH and K+ homeostasis to regulate growth, flower development, and reproduction. Plant Cell. 2011;23(9):3482–97.
Chanroj S, Wang G, Venema K, Zhang MW, Delwiche CF, Sze H. Conserved and diversified gene families of monovalent cation/H+ antiporters from algae to flowering plants. Front Plant Sci. 2012;3:25.
An R, Chen Q, Chai M, Lu P, Su Z, Qin Z, Chen J, Wang X. AtNHX8, a member of the monovalent cation:proton antiporter-1 family in Arabidopsis thaliana, encodes a putative Li+/H+ antiporter. Plant J. 2007;49(4):718–28.
Bassil E, Ohto M, Esumi T, Tajima H, Zhu Z, Cagnac O, Belmonte M, Peleg Z, Yamaguchi T, Blumwald E. The arabidopsis intracellular Na+/H+ antiporters NHX5 and NHX6 are endosome associated and necessary for plant growth and development. Plant Cell. 2011;23(1):224–39.
Reguera M, Bassil E, Tajima H, Wimmer M, Chanoca A, Otegui MS, Paris N, Blumwald E. pH regulation by NHX-type antiporters is required for receptor-mediated protein trafficking to the vacuole in Arabidopsis. Plant Cell. 2015;27(4):1200–17.
Ashnest JR, Huynh DL, Dragwidge JM, Ford BA, Gendall AR. Arabidopsis intracellular NHX-type sodium-proton antiporters are required for seed storage protein processing. Plant Cell Physiol. 2015;0(0):1–4.
Fukuda A, Nakamura A, Tagiri A, Tanaka H, Miyao A, Hirochika H, Tanaka Y. Function, intracellular localization and the importance in salt tolerance of a vacuolar Na+/H+ antiporter from rice. Plant Cell Physiol. 2004;45(2):146–59.
Chebli Y, Kaneda M, Zerzour R, Geitmann A. The cell wall of the Arabidopsis pollen tube--spatial distribution, recycling, and network formation of polysaccharides. Plant Physiol. 2012;160(4):1940–55.
Martiniere A, Bassil E, Jublanc E, Alcon C, Reguera M, Sentenac H, Blumwald E, Paris N. In vivo intracellular pH measurements in tobacco and Arabidopsis reveal an unexpected pH gradient in the endomembrane system. Plant Cell. 2013;25(10):4028–43.
Shen J, Zeng Y, Zhuang X, Sun L, Yao X, Pimpl P, Jiang L. Organelle pH in the Arabidopsis endomembrane system. Mol Plant. 2013;6(5):1419–37.
Llopis J, McCaffery JM, Miyawaki A, Farquhar MG, Tsien RY. Measurement of cytosolic, mitochondrial, and Golgi pH in single living cells with green fluorescent proteins. Proc Natl Acad Sci U S A. 1998;95(12):6803–8.
Paroutis P, Touret N, Grinstein S. The pH of the secretory pathway: measurement, determinants, and regulation. Physiology. 2004;19(4):207–15.
Fukuda A, Nakamura A, Hara N, Toki S, Tanaka Y. Molecular and functional analyses of rice NHX-type Na+/H+ antiporter genes. Planta. 2011;233(1):175–88.
Luo Y, Scholl S, Doering A, Zhang Y, Irani NG, Di Rubbo S, Neumetzler L, Krishnamoorthy P, Van Houtte I, Mylle E et al. V-ATPase activity in the TGN/EE is required for exocytosis and recycling in Arabidopsis. Nat Plants. 2015;1(7):15094.
Waadt R, Kudla J. In planta visualization of protein interactions using bimolecular fluorescence complementation (BiFC). CSH Protoc. 2008;3(4):prot4995.
Park M, Lee D, Lee G, Hwang I. AtRMR1 functions as a cargo receptor for protein trafficking to the protein storage vacuole. J Cell Biol. 2005;170(5):757–67.
Chiu W, Niwa Y, Zeng W, Hirano T, Kobayashi H, Sheen J. Engineered GFP as a vital reporter in plants. Curr Biol. 1996;6(3):325–30.
Chen S, et al. A highly efficient transient protoplast system for analyzing defence gene expression and protein-protein interactions in rice. Mol Plant Pathol. 2006;7(5):417–27.
Yoo S, Cho Y, Sheen J. Arabidopsis mesophyll protoplasts: a versatile cell system for transient gene expression analysis. Nat Protoc. 2007;2(7):1565–72.
Fan L, Zhao L, Hu W, Li W, Novák O, Strnad M, Simon S, Friml J, Shen J, Jiang L, et al. Na+, K+/H+ antiporters regulate the pH of endoplasmic reticulum and auxin-mediated development. Plant Cell Environ. 2018;41(4):850–64.
Zhu X, Pan T, Zhang X, Fan L, Quintero FJ, Zhao H, Su X, Li X, Villalta I, Mendoza I, et al. K+ efflux antiporters 4, 5, and 6 mediate pH and K+ homeostasis in endomembrane compartments. Plant Physiol. 2018;178(4):1657–78.
Acknowledgements
We thank Prof. Liwen Jiang (School of Life Sciences, The Chinese University of Hong Kong) for pH sensors ManI–PRpHluorin, PRpHluorin-BP80 (Y612A) and PRpHluorin–AtVSR2 vectors, Prof. Quansheng Qiu and Dr. Ting Pan (School of Life Sciences, Lanzhou University) for their kind help in pH measurement.
Funding
This research was supported by grant from the Ministry of Agriculture of China for Transgenic Research (2018ZX08009003 and 2018ZX0800920B), National Natural Science Foundation of China (31830064 and 31671652), Jiangsu Science and Technology Development Program (BE2017368), the Project for Major New Varieties of Agriculture in Jiangsu Province (PZCZ201701), Jiangsu Natural Science Foundation for Distinguished Young Scholars (BK30180024), and the Fundamental Research Funds for the Central Universities (KYTZ201601). This work was also supported by the Key Laboratory of Biology, Genetics, and Breeding of Japonica Rice in Mid-lower Yangtze River, Ministry of Agriculture, P.R. China, and the Jiangsu Collaborative Innovation Center for Modern Crop Production. The funding organizations played no role in the design of the study and collection, analysis, and interpretation of data and in writing the manuscript.
Author information
Authors and Affiliations
Contributions
JW, YHW, YR, and JZ designed the research. YLW, FL, XT, YZ, ED, MW, YH, XZ, JL, YFW, YY and TP performed the experiments. JZ wrote the manuscript, WJ, YB, and YHW revised the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Additional files
Additional file 1:
Figure S1. Time-course analysis of storage proteins during endosperm development of the wild-type N22 and the mutant gpa6. (a) SDS-PAGE analyses of seed storage proteins during wild-type and gpa6 endosperm development. DAF, days after flowering. (b) Immunoblot analysis of glutelins during wild-type and gpa6 endosperm development. EF-1α was used as a loading control. Red arrows in (a) and (b) indicate the 57-kD proglutelins. (DOCX 235 kb)
Additional file 2:
Figure S2. RT-qPCR assay of the expression of representative genes coding for storage proteins in 12-DAF endosperm. Glutelin genes: GluA1, GluB2, GluC1, GluD1; prolamin genes: pro10.1, pro16.2, pro13a.2, pro13b.2. Values are means ± SD. n = 3. (DOCX 81 kb)
Additional file 3:
Figure S3. Immunofluorescence microscopy of protein bodies in the subaleurone cells of the wild type and gpa6 mutant. (a) to (f) Immunofluorescence microscopy images of storage proteins in wild-type (a-c) and gpa6 (d-f) 12 DAF seeds. (a, d) Secondary antibodies conjugated with Alexa fluor 555 (red) were used to trace the antigens recognized by the anti-α-globulin antibodies. (b, e) Secondary antibodies conjugated with Alexa fluor 488 (green) were used to trace the antigens recognized by the anti-glutelin antibodies. (c, f) Merged images. White arrowheads in (f) indicate the mis-sorted α-globulins in the PMB. Bars = 10 μm (a-f). (DOCX 135 kb)
Additional file 4
Figure S4. Distribution of cell wall materials in 12 DAF endosperm cells. (a) to (f) Sections of 12 DAF endosperms from wild type (a-c) and gpa6 (d-f) plant were incubated with pectin (JIM7) or glutelin antibodies, followed by secondary antibodies conjugated to Alexa-555 or Alexa-488. Bars = 10 μm (a-f). (DOCX 152 kb)
Additional file 5:
Figure S5. Amino acid sequences alignment of OsNHX5 and OsNHX6. (DOCX 421 kb)
Additional file 6:
Figure S6. Complementation of gpa6 mutant phenotypes by p35S:OsNHX5-GFP. (a) Immunoblot analysis with monoclonal GFP antibodies. (b) p35S:OsNHX5-GFP transgene rescued the grain phenotype of the gpa6 mutant. Bars = 1 mm. (c) p35S:OsNHX5-GFP transgene in the gpa6 mutant reduce the amount of 57-KD proglutelins to a level comparable to the wild type. GL1 to GL3 denote the grains from three independent T1 transgenic lines. Red arrows indicate the 57-kD proglutelins. (DOCX 186 kb)
Additional file 7:
Figure S7. Subcellular Localization of OsNHX6 in N. benthamiana protoplasts. (a) to (c) Confocal microscopy images showing that OsNHX6-GFP is localized as punctate signals in the cytosol and its distribution partially overlaps with the markers for Golgi (ST-mRFP [a]), TGN (mRFP-SYP61 [b]) and PVC (mRFP-VSR2 [c]). Bars = 10 μm (a-c). (DOCX 112 kb)
Additional file 8:
Table S1. Properties of wild-type and gpa6 seeds. (DOCX 13 kb)
Additional file 9:
Table S2. Segregation of mutant phenotypes in reciprocal crosses between the wild type and gpa6 mutant. (DOCX 13 kb)
Additional file 10:
Table S3. Primers used for mapping. (DOCX 14 kb)
Additional file 11:
Table S4. Primers used for vector construction. (DOCX 14 kb)
Additional file 12:
Table S5. Primer used for real-time PCR analysis. (DOCX 14 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Zhu, J., Ren, Y., Wang, Y. et al. OsNHX5-mediated pH homeostasis is required for post-Golgi trafficking of seed storage proteins in rice endosperm cells. BMC Plant Biol 19, 295 (2019). https://doi.org/10.1186/s12870-019-1911-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12870-019-1911-y