Abstract
Background
Bacterial chondronecrosis with osteomyelitis (BCO) develops in the growth plate (GP) of the proximal femur and tibia and is initiated by damage to the less mineralized chondrocytes followed by colonization of opportunistic bacteria. This condition affects approximately 1% of all birds housed, being considered one of the major causes of lameness in fast growing broilers. Although several studies have been previously performed aiming to understand its pathogenesis, the molecular mechanisms involved with BCO remains to be elucidated. Therefore, this study aimed to generate a profile of global differential gene expression involved with BCO in the tibia of commercial broilers, through RNA sequencing analysis to identity genes and molecular pathways involved with BCO in chickens.
Results
Our data showed 192 differentially expressed (DE) genes: 63 upregulated and 129 downregulated in the GP of the tibia proximal epiphysis of BCO-affected broilers. Using all DE genes, six Biological Processes (BP) were associated with bone development (connective tissue development, cartilage development, skeletal system development, organ morphogenesis, system development and skeletal system morphogenesis). The analyses of the upregulated genes did not indicate any significant BP (FDR < 0.05). However, with the downregulated genes, the same BP were identified when using all DE genes in the analysis, with a total of 26 coding genes explaining BCO in the tibia: ACAN, ALDH1A2, CDH7, CHAD, CHADL, COL11A1, COMP, CSGALNACT1, CYR61, FRZB, GAL3ST1, HAPLN1, IHH, KIF26B, LECT1, LPPR1, PDE6B, RBP4A, SERINC5, SFRP1, SOX8, SOX9, TENM2, THBS1, UCHL1 and WFIKKN2. In addition, seven transcription factors were also associated to BCO: NFATC2, MAFB, HIF1A-ARNT, EWSR1-FLI1, NFIC, TCF3 and NF-KAPPAB.
Conclusions
Our data show that osteochondral downregulated genes are potential molecular causes of BCO in broilers, and the bacterial process seems to be, in fact, a secondary condition. Sixteen genes responsible for bone and cartilage formation were downregulated in BCO-affected broilers being strong candidate genes to trigger this disorder.
Similar content being viewed by others
Background
The broiler chickens have undergone intense genetic and nutritional improvement, exponentially increasing their weight gain and decreasing the slaughter age. However, the improvement focused on the high performance has overlooked some physiological characteristics, such as the skeletal structure [1]. The bacterial chondronecrosis with osteomyelitis (BCO) affects up to 1% of all birds housed, being considered a worldwide major cause of lameness in commercial broilers, generating economic losses and impacting negatively the animal welfare [2,3,4]. The prevalence of lameness due the BCO can extent up to 50%, and 5% of mortality [5], however the incidence of BCO is unknown in most countries [2,3,4, 6].
BCO affect the chicken GP, where the chondrocyte columns are irregularly aligned due to the high longitudinal growth rates, being associated with the growth plate turnover in this specie [7]. The BCO pathogenesis is supposed to be initiated by the damage of the poorly mineralized chondrocytes followed by colonization of opportunistic bacteria, such as Staphylococcus aureus, Escherichia coli, Coagulase-negative Staphylococcus and Enterococcus spp., in the osteochondrotic clefts [2, 6, 7] of both femur and tibia [8, 9].
Despite the fact that many opportunistic microorganisms have been identified in studies focused on the involvement of bacteria in the process of BCO [3, 6,7,8,9,10,11,12], there are no studies exploring the genetics and molecular mechanisms involved with BCO in tibia, although, previous studies have shown the importance of some genes involved with BCO in the femur [13,14,15]. Therefore, the aim of this study was to identify the global differential gene expression profile in the tibia GP involved with BCO in chickens, through the transcriptome analysis using RNA-seq of normal and BCO-affected broilers.
Results
RNA-Seq data
An average of 17,5 million reads/sample (2 × 100 base paired-end reads) was generated and about an average of 13 million reads/sample were kept after the data quality control. The transcriptome had in average more than 99% of the reads mapped against the chicken reference genome Galgal5, ranging from 98.83 to 99.26% to each individual sample, with 73% of the reads present in genes.
Differential gene expression
A total of 12,576 transcripts were obtained. From those, 1018 transcripts were selected with FDR < 0.05, and grouped in three categories: protein-coding (973), lncRNA (44) and snoRNA (1). The transcripts with logFC < − 2.0 and > 2.0 were selected, obtaining 192 DE genes (12 lncRNA and 180 protein-coding), being 63 upregulated (logFC> 2.0) (8 lncRNA and 55 protein-coding) and 129 downregulated (logFC<− 2.0) (4 lncRNA and 125 protein-coding (112 annotated genes) in the affected compared to healthy broilers (Additional File 1).
Gene ontology
In the first step, 192 DE genes were used in the GO analysis and a total of 304 Biological Processes (BP) were identified. From those, only nine BP were significant with FDR ≤ 0.05 (Table 1). Among these nine BP, only six were specifically related to bone development: connective tissue development, cartilage development, skeletal system development, organ morphogenesis, system development, and skeletal system morphogenesis.
In a second step, the GO analyses were performed separating the up and downregulated genes. Using the 63 upregulated genes, the GO did not show any significant BP. However, when the 129 downregulated genes were used, the GO showed the same six BP that were identified when all 192 DE genes were used, with a total of 26 coding genes among the BPs (Table 2). The most enriched BP were system development (26 genes), skeletal system development (15 genes), organ morphogenesis (14 genes), connective tissue development (12 genes), cartilage development (11 genes) and skeletal system morphogenesis (9 genes) (Table 1).
Gene and Transcription factor (TF) network
A gene network using Cytoscape v.3.6 [16]. was performed to identify connections among genes enriched in bone bioprocesses (Fig. 1). Three genes (COMP, COL11A1 and SOX9) were shared in all six BP, four genes (CDH7, CSGALNACT1, ACAN and IHH) were shared by five BP, three genes (CHADL, CYRG1 and LECT1) were shared by four BPs, four genes (SFRP1, CHAB, FRZB and SOX8) were shared by three BPs and three genes (TENM2, WFIKKN2 and ALDH1A2) were shared by two BPs (Additional file 2). The other genes were in only one BP (Fig. 1). In addition, regulatory sequence analyses for all detected genes were performed for the 26 genes used as input at TFM-explorer [17]. A total of 21 transcription factors (TF) were identified from the downregulated genes. Based on the BP from DAVID and the literature review, we selected the most putative BCO associated TF. The main TF, from the most to the least connected were: NFATC2, MAFB, HIF1A::ARNT, EWSR1-FLI1, NFIC, TCF3 and NF-KAPPAB, which were used to construct a TF network highlighting the most connected genes (Fig. 2).
Gene network showing connections between downregulated genes (circles) and Biological Process (diamonds). The size (low values are small size) and the colors (low values are in bright colors) of the nodes indicate the number of directed edges and the neighborhood connectivity, respectively. The Edge colors indicate the betweenness of the edges (low values are in bright colors)
Transcription Factor network showing connections between downregulated genes (circles) and Transcription Factors (diamonds). The size (low values are small size) and the colors (low values are in bright colors) of the nodes indicate the number of directed edges and the neighborhood connectivity, respectively. The Edge colors indicate the betweenness of the edges (low values are in bright colors)
Discussion
Tibia is a high-mineralized bone in the body, being considered a good indicator of mineralization in ossification studies [18, 19]. The bacterial chondronecrosis with osteomyelitis (BCO) has been diagnosed worldwide and its pathogenesis seems to be related to the poor mineralization of chondrocytes and cartilage of the femur and tibia bones in fast-growing chicken, with proliferation of opportunistic bacteria [7]. Although several studies are available evaluating femur, it is known that the lesion status differ between bones [3], and that high incidences of tibia BCO has been previously reported in broilers [20]. Nowadays, there are just few studies addressing the molecular mechanisms involved with femur BCO [13,14,15] and no information regarding tibia BCO is available which highlights the importance of our study, since it brings novel and relevant knowledge to the field. Furthermore, this condition is observed in males and females, and an influence of sire line on susceptibility for this trait has already been found [20]. However, it is consensus that high performance broilers are widely affected by BCO [3, 20,21,22], which reinforces the importance of new studies considering tibia BCO.
Although only one commercial line was used in this study, it is expected common molecular pathways involved in the BCO development in different lines, since this is a health condition. However, the line or sex effect could influence the gene expression profile related to this condition, leading to a variation in the BCO manifestation, and should be further confirmed. According to Wideman et al. (2012) [3], no difference was found on the incidence of lameness between males and females and among lines in the same type of floor. Regarding tibia BCO, no differences between males and females were found until 40 days, but an effect of sire was observed, especially when broilers were older than 6 weeks of age [20]. In our study, we used males from a high performance commercial broiler line since they are fast growing, heavy weight and therefore more probable to get affected with BCO. Therefore, if we have used other high performance commercial line, we expect that similar results would be obtained.
In the tibia GP transcriptome, 1018 genes were DE between normal and BCO-affects broilers. A total of 44 lncRNA were identified and these transcripts may be related to the regulation of adjacent genes [23]. According to the position in the genome of the NCBI database, the lncRNAs ENSGALG00000040226, ENSGALG00000033872 and ENSGALG00000032326 are close to the genes SOX9, COL11A1 and SOX8, respectively, and may be involved in the regulation of these important genes for bone development. The SOX9 and SOX8, two transcriptions factors acting on endochondral ossification, have recently been associated to femur epiphysiolysis [15]. In the current study, it was possible to identify several DE genes involved with BCO development, even using a small number of samples per group, as can be seen in several RNA-Seq studies, when groups are well characterized [24,25,26,27,28].
Initially, using 112 downregulated genes, a total of 26 genes enriched six BP directly related to bone and cartilage development (Table 2): system development (GO: 0048731), skeletal system development (GO: 0001501), organ morphogenesis (GO: 0009887), connective tissue development (GO: 0061448), cartilage development (GO: 0051216) and skeletal system morphogenesis (GO: 0048705). These are important BP for bone development and their malfunction can cause poor ossification, leading to cases of lameness, commonly observed in BCO-affected chickens [12]. The genes related to these biological pathways are ACAN, ALDH1A2, CDH7, CHAD, CHADL, COL11A1, COMP, CSGALNACT1, CYR61, FRZB, GAL3ST1, HAPLN1, IHH, KIF26B, LECT1, LPPR1, PDE6B, RBP4A, SERINC5, SFRP1, SOX8, SOX9, TENM2, THBS1, UCHL1 and WFIKKN2 (Fig. 1). These are functional candidate genes associated to BCO, as discussed below.
The aggrecan gene (ACAN) is one of the most important cartilage aggregating chondroitin [29]. This gene is essential to the regulation of growth factors and cartilage development [30, 31], and it is associated with diseases such as skeletal dysplasia [32], osteoarthritis [33], osteochondritis [34, 35] and chondrodystrophy in chickens [36]. In addition, SOX8 and SOX9 play an important role as transcription factors in the development of cartilage, controlling the differentiation of chondrocytes and osteoblasts in the development of progenitor cells [30, 31, 37,38,39,40,41,42].
The CHAD gene (Chondroadherin) is highly expressed in cartilage and was first isolated in cattle [43], direct binding with calcium phosphate [44]. Hessle et al. (2013) [38] showed that CHAD has an influence on the enlargement of the epiphyseal plate and its inactivation compromises the hypertrophic differentiation of the chondrocytes, as well as the cartilage composition in rats. An important CHAD paralog is CHADL, which can negatively regulate chondrocyte differentiation, inhibiting cartilage fibrillogenesis and can be used as a marker for cartilage diseases [45].
Collagen type XI (COL11) constitutes a triple helix formed by COL11a1, COL11a2 and COL12a1, being an essential component of the extracellular matrix-regulating the diameter of the fibrils [46,47,48,49]. In the absence of COL11a1, an alternate triple helix is formed with COL11a2 and COL5a1, but it is unable to compensate for the functional deficiency of Collagen 11a1 [50]. COL11 is essential for the cartilage collagen fibrils formation and for differentiation and organization of growth plate chondrocytes [51]. The COL11a1 plays a key role in endochondral ossification, and the low expression of this gene results in alterations in the mineralization of newly formed bone [40].
The Cartilage Oligomeric Matrix Protein (COMP) is a non-collagenous protein present in the extracellular matrix of bone and cartilage, and mutation in this gene can cause death of the chondrocytes [52]. Chondrocyte death can be caused by the retention of COMP and other extracellular matrix proteins within an enlarged rugged endoplasmic reticulum, and this can lead to an abnormal matrix that is easily eroded over time [53]. In addition, COMP may be a potential molecular marker for bone diseases [54,55,56].
The CSGALNACT1 gene encodes the N-acetilgalactosaminiltransferase enzyme, which has activity in beginning and the elongation of the chondroitin sulfate synthesis [57]. In rats, reduced levels of CSGALNACT1 may cause post-natal lethality due to respiratory failure, mild dwarfism and the cartilage has an abnormality of endochondral ossification [58, 59].
Cysteine-Rich Angiogenic Inducer 61 (CYR61 or CCN1) is part of the group of matricellular proteins [60], being associated with extracellular matrix secretion [61]. Furthermore, CYR61 has important role in regulating inflammation and possible cell repair [62]. This gene potentiates DNA synthesis for cell proliferation induced by other mitogens [63] in response to bacterial or viral infection [64, 65].
Frizzled Related Protein (FRZB/SFRP-3) is part of the set of Secreted Frizzled-Related Protein (SFRP1), which comprises five members that modulate negatively and positively the Wingless-type (Wnt) signaling cascade [66]. Wnt signaling cascade is important for regulating the development, maintenance and homeostasis of bone and cartilage [67]. Therefore, dysregulation of Wnt signaling may lead to the development of osteoarthritis in rodents [67,68,69,70,71].
Hyaluronan and Proteoglycan Link Protein 1 (HAPLN1/CRTL1) is an abundant polysaccharide in the extracellular matrix of cartilage under normal conditions and is involved in cell differentiation and morphogenesis [72,73,74]. Recent studies have shown that in bone diseases in humans, such as osteoarthritis and allograft transplantation, there is always a low HAPLN1 expression [75, 76].
The Indian hedgehog (IHH) gene is expressed in cartilage, and it is recognized as regulator of bone development and morphogenesis [77]. IHH induces the differentiation of the osteogenic cell of the periosteum, being essential in the differentiation of the osteoblasts and in the maintenance of the growth plate, articular cartilage and adjacent endochondral bone formation [78,79,80]. Jin et al. [81] demonstrated that a deletion in the IHH gene was responsible for the Creeper phenotype in broilers, which is characterized by short and stunted legs. In addition, a mutation in this gene causes brachydactyly in humans [82].
Chondromodulin (CNMD) regulates the rapid growth of cartilage and vascular invasion prior to the process of cartilage replacement by the endochondral bone [83]. New evidence on the mechanism of differentiation of mesenchymal stem cells (CTMs) into chondrocytes induced by ChM-I shows that the main pathways involved in the process are focal adhesion, glycolysis, regulation of the actin cytoskeleton and the ribosome [84]. In osteoarthritis condition, the expression of ChM-I is decreased. Therefore, the regulation of this gene in cartilage may be a potential treatment, because it protects the chondrocytes from hypertrophy and delays the progression of osteoarthritis [85].
Retinol-binding protein (RBP4A) has the function of transporting vitamin A in the blood, from the liver to the peripheral tissues [86, 87]. Vitamin A plays an important role in the development of various organs, including bone growth [88], contributing to bone health [89, 90], and abnormal levels of Vitamin A may have a negative impact on bone growth [91,92,93,94]. Moreover, RBP4 is expressed during limb growth and this gene is also involved in chondrogenesis, collagen X transcription and bone mineral density [95,96,97].
Thrombospondin (THBS1) is a glycoprotein that regulates the structure of the extracellular matrix [98] being involved in the protection of chondrocytes, since it exerts pro-chondrogenic and anti-inflammatory function [99]. Furthermore, THBS1 seems to be important in homeostasis and maintenance of bone matrix integrity, and in the regulation of osteoclast formation [100].
From the 26 DE genes enriching the bone and cartilage development BP, 10 (ALDH1A2, CDH7, GAL3ST1, KIF26B, PLPPR1, PDE6B, SERINC5, TENM2, UCHL1 and WFIKKN2) appear to have no direct relationship to bone or cartilage formation. However, due to the complexity of these processes, the function of these genes in the regulation of bone cells could still be unknown, especially in chicken.
Transcription factors
Nine TF associated to downregulated DE genes were found (Fig. 2), being three of those highly connected (NFATC2, MAFB and HIF1A:TNA). Several studies have shown that these TF were related to osteoblast and osteoclast differentiation, and osteolysis [101,102,103,104,105,106,107,108] The NFATC2 plays important role in the immune response, and in the differentiation and regulation of osteoclast growth [109]. Zanoti and Canalis [102] concluded that the activation of NFATC2 may inhibit the function of osteoblasts and decrease the volume of spongy bone. Furthermore, NFATC2 was associated with 24 out of 26 DE genes detected as downregulated in the affected chickens (Fig. 2). Moreover, the MAFB TF, although expressed selectively in monocytes [110], can be found in several tissues, and has been related to osteolysis in humans and rats [111] by the negative regulation of important cytokines for osteoclast differentiation [105]. HIF1A regulates oxygen homeostasis, glucose transport and generation of anaerobic energy in joints and chondrocytes, and may play an important role in the osteoarthritis metabolism [107, 112]. Four other TF were also found with a high number of connections and appear to be associated with osteosarcoma (EWSR1-FLI1 and NFIC), cranium formation (TCF3), soft tissue calcification and chondrocyte differentiation (NF-KAPPAB) [113,114,115,116,117].
Clinical implications related to BCO include, but are not limited to claudication. There is no efficient treatment and the diagnosis is not possible in early stages, being necessary to perform a necropsy. The DE genes and transcription factors found in this study play a fundamental role in bone and cartilaginous development in broilers. Their low expression in the affected chickens seems to be related to incomplete bone formation, initiating the BCO process in the tibia growth plate of fast-growing chickens. The current findings confirm our hypothesis and indicate that improvement in ossification and cartilage formation have to be addressed in poultry breeding programs.
Conclusions
We found 16 differentially expressed genes in the tibia GP transcriptome that are directly responsible for bone and cartilage formation, which were downregulated in BCO-affected broilers. According to our data, the lack of ossification might be the main cause of BCO in broilers, and the bacterial process seems to be a secondary condition. Moreover, our results highlight the pathogenesis of BCO, and show that to pursue prevention and control of such condition, breeding strategies have to focus on the improvement of ossification and cartilage formation.
Methods
Animals and sample collection
This study was performed at the Embrapa Swine and Poultry National Research Center. Approximately 50 male chicks from Cobb500 commercial broiler line were raised from 1 to 42 days of age, with a density of 12 broilers/m2 with infrared lamps. The drinkers, feeders, curtains, light, and chickens were managed following the recommendations for the commercial line. Diets containing 3150 kcal/kg AME and 21% CP (1–21 days), 3200 kcal/kg AME and 20% CP (22–35 days), and 3200 kcal/kg AME and 18.5% CP (36–42 days) were provided. The animals had free access to feed and water. Before sample collection, the broilers were weighted at 42 days of age and euthanized by cervical dislocation followed by bleeding, according to the approval of the Embrapa Swine and Poultry Ethical Committee of Animal Use (CEUA), under protocol number 012/2012.
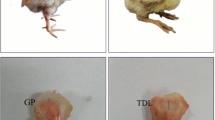
A classification of the tibia was performed according to the presence or absence of different levels of BCO, by visual observation of compatible necrosis lesions, according to Wideman et al. (2012) [3]. Tibia samples with adhesion between the growth plate (GP) and cartilage (CA) were considered in the normal group and those presenting separation between GP and CA were classified as the affected group. Only those with the initial level of BCO and present in both tibias were used in this study. For sample collection of the normal group, all CA was removed to access the GP. The entire GP of samples were collected, stored in liquid nitrogen and transferred to the − 80 °C freezer for further RNA analysis. Six samples (three normal and three affected) were collected and prepared for RNA-Seq analysis.
RNA extraction and library preparation
About 100 mg of the tibia GP tissue was used for RNA extraction using Trizol Reagent (Invitrogen, Carlsbad, CA) following the manufacturer’s instructions. The RNA cleanup was performed using Rneasy mini kit (Qiagen, Germany) following manufacturer’s instructions. After RNA extraction, the RNA was quantified in Nanodrop spectrophotometer (Thermo Scientific; Waltham, MA, USA) and the Agilent 2100 BioAnalyzer (Agilent Technologies; Santa Clara, CA, USA) was used for integrity measurement, where samples with RNA integrity number (RIN) higher than 8 were used for library preparation. A total of six tibia samples, three normal and three BCO-affected were prepared for RNA-sequencing using the TruSeq™ RNA Sample Prep Kit v2 (Illumina, Inc.; San Diego CA, USA), according to the manufacturer’s recommendations, with 2 μg of total RNA.
Sequencing, quality control, assembly and differential expression analysis
The libraries were sent to the Functional Genomics Center, ESALQ, University of São Paulo, Piracicaba, SP, Brazil for sequencing in Illumina HiSeq2500 equipment (Illumina, Inc.; San Diego CA, EUA), all in the same lane, following the 2x100bp paired-end protocol.
The quality control was performed using SeqyClean tool (https://github.com/ibest/seqyclean) with the raw FASTQ data for removing short reads (<70pb), low quality reads (Qphred < 24), PCR artifacts and adapter sequences. The sequence reads were mapped against the chicken reference genome (Gallus gallus, assembly 5.0) available in (www.ensembl.org), using BWA-MEM software [118]. The read counting was performed with Htseq software [119] using Ensembl annotation release 89. The edgeR package [120] in R environment [121] was used to identify differentially expressed (DE) genes from BCO-affected and unaffected groups. Significance threshold for DE genes was set at a False Discovery Rate (FDR) ≤ 0.05 after multiple correction tests to reduce type I error. Smear plots of DE genes were generated using the expression data for each gene within each sample in the edgeR package from the R environment [121].
Gene ontology (GO) and network analyses
In a first step, a total of 192 DE genes with log Fold Change (logFC) < − 2.0 and > 2.0 was used as input on DAVID Bioinformatics Resources 6.8 tool (https://david.ncifcrf.gov/summary), and all the identified expressed genes were used as background. Genes with positive or negative logFC values were considered, respectively, upregulated or downregulated when the BCO-affected group was compared to the unaffected group. In a second step, a GO analysis was performed using only upregulated DE genes (63 genes) and only downregulated DE genes (129 genes).
The gene and transcription factor (TF) network analysis was built using Cytoscape 3.6.1 [16]. The output of GO analysis was used as input on Cytoscape with each gene associated with the correspondent biological process. Twenty-six genes were submitted to the TFM-Explorer program (http://bioinfo.lifl.fr/tfm-explorer/form.php) to identify the TF related to them. From sequence of a set of gene promoters, TFM-Explorer searches for locally overrepresented transcription factor binding sites (TFBS) using weight matrices from JASPAR database [122] to detect all potential TFBS, and extracts significant clusters by calculating a score function.
Availability of data and materials
All data generated or analyzed during this study are included in this published article (and its additional files). The transcriptome sequences are available in the SRA database with BioProject number PRJNA352716 and biosample numbers: SAMN05992341, SAMN05992340, SAMN05992339, SAMN05992338, SAMN05992337 and SAMN05992336.
Abbreviations
- BCO:
-
Bacterial Chondronecrosis with Osteomyelitis
- DE:
-
Differentially Expressed
- BP:
-
Biological Processes
- lncRNA:
-
Long Non-Coding RNAs
- snoRNA:
-
Small Nucleolar RNAs
- GO:
-
Gene Ontology
- FDR:
-
False Discovery Rate
- TF:
-
Transcription Factors
References
Julian RJ. Rapid growth problems: ascites and skeletal deformities in broilers. Poult Sci. 1998;77:1773–80.
McNamee PT, Smyth JA. Bacterial chondronecrosis with osteomyelitis ('femoral head necrosis’) of broiler chickens: a review. Avian Pathol. 2000;29:253–70.
Wideman RF, Hamal KR, Stark JM, Blankenship J, Lester H, Mitchell KN, et al. A wire-flooring model for inducing lameness in broilers: evaluation of probiotics as a prophylactic treatment. Poult Sci. 2012;91:870–83 https://doi.org/10.3382/ps.2011-01907.
Cook ME. Skeletal deformities and their causes: introduction. Poult Sci. 2000;79:982–4 http://www.ncbi.nlm.nih.gov/pubmed/10901198.
Nairn ME, Watson ARA. Leg weakness of poultry—a clinical and PATHOLOGICAL characterisation. Aust Vet J. 1972;48:645–56 https://doi.org/10.1111/j.1751-0813.1972.tb09237.x.
Wijesurendra DS, Chamings AN, Bushell RN, Rourke DO, Stevenson M, Marenda MS, et al. Pathological and microbiological investigations into cases of bacterial chondronecrosis and osteomyelitis in broiler poultry. Avian Pathol. 2017;46:683–94.
Wideman RF, Prisby RD. Bone circulatory disturbances in the development of spontaneous bacterial chondronecrosis with osteomyelitis: a translational model for the pathogenesis of femoral head necrosis. Front Endocrinol (Lausanne). 2013;3:1–15 https://doi.org/10.3389/fendo.2012.00183.
Jiang T, Mandal RK, Wideman RF, Khatiwara A, Pevzner I, Kwon YM. Molecular survey of bacterial communities associated with bacterial chondronecrosis with osteomyelitis (BCO) in broilers. PLoS One. 2015;10:1–20.
Mandal RK, Jiang T, Al-Rubaye AA, Rhoads DD, Wideman RF, Zhao J, et al. An investigation into blood microbiota and its potential association with Bacterial Chondronecrosis with Osteomyelitis (BCO) in Broilers. Sci Rep. 2016;6:1–11 https://doi.org/10.1038/srep25882.
Al-Rubaye AAK, Ekesi NS, Zaki S, Emami NK, Wideman RF, Rhoads DD. Chondronecrosis with osteomyelitis in broilers: further defining a bacterial challenge model using the wire flooring model. Poult Sci. 2017;96:332–40.
Gaußmann B, Hess C, Grafl B, Kovacs M, Troxler S, Stessl B, et al. Escherichia coli isolates from femoral bone marrow of broilers exhibit diverse pheno- and genotypic characteristics that do not correlate with macroscopic lesions of bacterial chondronecrosis with osteomyelitis. Avian Pathol. 2018;9457:1–10.
Dinev I. Clinical and morphological investigations on the prevalence of lameness associated with femoral head necrosis in broilers. Br Poult Sci. 2009;50:284–90.
Petry B, Savoldi IR, Ibelli AMG, Paludo E, de Oliveira PJ, Jaenisch FRF, et al. New genes involved in the bacterial Chondronecrosis with osteomyelitis in commercial broilers. Livest Sci. 2018;208(December 2017):33–9 https://doi.org/10.1016/j.livsci.2017.12.003.
Paludo E, Ibelli AMG, Peixoto JO, Tavernari FC, Lima-Rosa CAV, Pandolfi JRC, et al. The involvement of RUNX2 and SPARC genes in the bacterial chondronecrosis with osteomyelitis in broilers. Animal. 2017;11:1063–70.
de Oliveira PJ, Savoldi IR, Ibelli AMG, Cantão ME, Jaenisch FRF, Giachetto PF, et al. Proximal femoral head transcriptome reveals novel candidate genes related to epiphysiolysis in broiler chickens. BMC Genomics. 2019;20:1031 https://doi.org/10.1186/s12864-019-6411-9.
Shannon P, Markiel A, Ozier O, Baliga NS, Wang JT, Ramage D, et al. Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res. 2003;13:2498–504 https://doi.org/10.1101/gr.1239303.
Tonon L, Touzet H, Varre J-S. TFM-Explorer: mining cis-regulatory regions in genomes. Nucleic Acids Res. 2010;38(Web Server):W286–92 https://doi.org/10.1093/nar/gkq473.
Angel R. Metabolic disorders: limitations to growth of and mineral deposition into the broiler skeleton after hatch and potential implications for leg problems. J Appl Poult Res. 2007;16:138–49 https://doi.org/10.1093/japr/16.1.138.
Sanchez-Rodriguez E, Benavides-Reyes C, Torres C, Dominguez-Gasca N, Garcia-Ruiz AI, Gonzalez-Lopez S, et al. Changes with age (from 0 to 37 D) in tibiae bone mineralization, chemical composition and structural organization in broiler chickens. Poult Sci. 2019; https://doi.org/10.3382/ps/pez363.
Wideman RF, Al-Rubaye A, Reynolds D, Yoho D, Lester H, Spencer C, et al. Bacterial chondronecrosis with osteomyelitis in broilers: influence of sires and straight-run versus sex-separate rearing. Poult Sci. 2014;93:1675–87 https://doi.org/10.3382/ps.2014-03912.
Gilley AD, Lester H, Pevzner IY, Anthony NB, Wideman RF. Evaluating portable wire-flooring models for inducing bacterial chondronecrosis with osteomyelitis in broilers1. Poult Sci. 2014;93:1354–67 https://doi.org/10.3382/ps.2013-03781.
Wideman RF. Bacterial chondronecrosis with osteomyelitis and lameness in broilers: a review. Poult Sci. 2016;95:325–44.
Milligan MJ, Lipovich L. Pseudogene-derived lncRNAs: emerging regulators of gene expression. Front Genet. 2015;5 https://doi.org/10.3389/fgene.2014.00476.
Yu S, Wang G, Liao J, Tang M. Transcriptome profile analysis identifies candidate genes for the melanin pigmentation of breast muscle in Muchuan black-boned chicken. Poult Sci. 2018;97:3446–55 https://doi.org/10.3382/ps/pey238.
Ding X, Zhang S, Li X, Feng C, Huang Q, Wang S, et al. Profiling expression of coding genes, long noncoding RNA, and circular RNA in lung adenocarcinoma by ribosomal RNA-depleted RNA sequencing. FEBS Open Bio. 2018;8:544–55 https://doi.org/10.1002/2211-5463.12397.
Jaing C, Rowland RRR, Allen JE, Certoma A, Thissen JB, Bingham J, et al. Gene expression analysis of whole blood RNA from pigs infected with low and high pathogenic African swine fever viruses. Sci Rep. 2017;7:10115 https://doi.org/10.1038/s41598-017-10186-4.
Kwon SG, Hwang JH, Park DH, Kim TW, Kang DG, Kang KH, et al. Identification of differentially expressed genes associated with litter size in Berkshire pig placenta. PLoS One. 2016;11:e0153311 https://doi.org/10.1371/journal.pone.0153311.
Li HL, Lin HR, Xia JH. Differential gene expression profiles and alternative isoform regulations in gill of Nile Tilapia in response to acute hypoxia. Mar Biotechnol. 2017;19:551–62 https://doi.org/10.1007/s10126-017-9774-4.
Ovadia BM, Parker CH, Lash JW. Changing patterns of proteoglycan synthesis during chondrogenic differentiation. J Embryol Exp Morpholog. 1980;56:59–70.
Hu G, Codina M, Fisher S. Multiple enhancers associated with ACAN suggest highly redundant transcriptional regulation in cartilage. Matrix Biol. 2012;31:328–37 https://doi.org/10.1016/j.matbio.2012.06.001.
Lauing KL, Cortes M, Domowicz MS, Henry JG, Baria AT, Schwartz NB. Aggrecan is required for growth plate cytoarchitecture and differentiation. Dev Biol. 2014;396:224–36 https://doi.org/10.1016/j.ydbio.2014.10.005.
Tompson SW, Merriman B, Funari VA, Fresquet M, Lachman RS, Rimoin DL, et al. A recessive skeletal dysplasia, SEMD Aggrecan type, results from a missense mutation affecting the C-type Lectin domain of Aggrecan. Am J Hum Genet. 2009;84:72–9 https://doi.org/10.1016/j.ajhg.2008.12.001.
Stattin E-L, Tegner Y, Domellöf M, Dahl N. Familial osteochondritis dissecans associated with early osteoarthritis and disproportionate short stature. Osteoarthr Cartil. 2008;16:890–6 https://doi.org/10.1016/j.joca.2007.11.009.
Stattin E-L, Wiklund F, Lindblom K, Önnerfjord P, Jonsson B-A, Tegner Y, et al. A missense mutation in the Aggrecan C-type Lectin domain disrupts extracellular matrix interactions and causes dominant familial Osteochondritis Dissecans. Am J Hum Genet. 2010;86:126–37 https://doi.org/10.1016/j.ajhg.2009.12.018.
Xu M, Stattin E-L, Shaw G, Heinegård D, Sullivan G, Wilmut I, et al. Chondrocytes derived from Mesenchymal stromal cells and induced pluripotent cells of patients with familial Osteochondritis Dissecans exhibit an endoplasmic reticulum stress response and defective matrix assembly. Stem Cells Transl Med. 2016;5:1171–81 https://doi.org/10.5966/sctm.2015-0384.
Primorac D, Stover ML, Clark SH, Rowe DW. Molecular basis of nanomelia, a heritable chondrodystrophy of chicken. Matrix Biol. 1994;14:297–305 https://doi.org/10.1016/0945-053X(94)90195-3.
Bi W, Deng JM, Zhang Z, Behringer RR, de Crombrugghe B. Sox9 is required for cartilage formation. Nat Genet. 1999;22:85–9 https://doi.org/10.1038/8792.
Dy P, Wang W, Bhattaram P, Wang Q, Wang L, Ballock RT, et al. Sox9 directs hypertrophic maturation and blocks osteoblast differentiation of growth plate chondrocytes. Dev Cell. 2012;22:597–609 https://doi.org/10.1016/j.devcel.2011.12.024.
Serrano RL, Chen L-Y, Lotz MK, Liu-Bryan R, Terkeltaub R. Proteasomal function is impaired in human osteoarthritic chondrocytes and this can contribute to decreased SOX9 and aggrecan. Arthritis Rheum. 2018; https://doi.org/10.1002/art.40456.
Schmidt K, Schinke T, Haberland M, Priemel M, Schilling AF, Mueldner C, et al. The high mobility group transcription factor Sox8 is a negative regulator of osteoblast differentiation. J Cell Biol. 2005;168:899–910 https://doi.org/10.1083/jcb.200408013.
Finzsch M, Stolt CC, Lommes P, Wegner M. Sox9 and Sox10 influence survival and migration of oligodendrocyte precursors in the spinal cord by regulating PDGF receptor expression. Development. 2008;135:637–46 https://doi.org/10.1242/dev.010454.
Umeda K, Oda H, Yan Q, Matthias N, Zhao J, Davis BR, et al. Long-term expandable SOX9+ chondrogenic ectomesenchymal cells from human pluripotent stem cells. Stem Cell Reports. 2015;4:712–26 https://doi.org/10.1016/j.stemcr.2015.02.012.
Larsson T, Sommarin Y, Paulsson M, Antonsson P, Hedbom E, Wendel M, et al. Cartilage matrix proteins. A basic 36-kDa protein with a restricted distribution to cartilage and bone. J Biol Chem. 1991;266:20428–33.
H-Y ZHOU. Proteomic analysis of hydroxyapatite interaction proteins in bone. Ann N Y Acad Sci. 2007;1116:323–6 https://doi.org/10.1196/annals.1402.023.
Tillgren V, Ho JCS, Önnerfjord P, Kalamajski S. The novel small leucine-rich protein chondroadherin-like (CHADL) is expressed in cartilage and modulates chondrocyte differentiation. J Biol Chem. 2015;290:918–25.
Van Der Rest M, Garrone R. Collagen family of proteins. FASEB J. 1991;5:2814–23.
Holmes DF, Kadler KE. The 10+4 microfibril structure of thin cartilage fibrils. Proc Natl Acad Sci. 2006;103:17249–54 https://doi.org/10.1073/pnas.0608417103.
Blaschke UK, Eikenberry EF, Hulmes DJS, Galla HJ, Bruckner P. Collagen XI nucleates self-assembly and limits lateral growth of cartilage fibrils. J Biol Chem. 2000;275:10370–8.
Gregory KE, Oxford JT, Chen Y, Gambee JE, Gygi SP, Aebersold R, et al. Structural organization of distinct domains within the non-collagenous N-terminal region of collagen type XI. J Biol Chem. 2000;275:11498–506.
Fernandes RJ, Weis MA, Scott MA, Seegmiller RE, Eyre DR. Collagen XI chain misassembly in cartilage of the chondrodysplasia (cho) mouse. Matrix Biol. 2007;26:597–603.
Li Y, Lacerda DA, Warman ML, Beier DR, Yoshioka H, Ninomiya Y, et al. A Fibrillar Collagen Gene , Collla 1 , Is Essential for Skeletal Morphogenesis. Cell. 1995;80:423–30.
Valdes AM. Osteoarthritis – Genetic Studies of Monogenic and Complex Forms. In: Eisman J, Thakker RV, Igarashi T, Whyte MP, editors. Genetics of Bone Biology and Skeletal Disease: Academic Press; 2013. p. 275–93. https://www.sciencedirect.com/science/article/pii/B9780123878298000378?via%3Dihub.
Posey K, Hecht J. The role of cartilage Oligomeric matrix protein (COMP) in skeletal disease. Curr Drug Targets. 2008;9:869–77 https://doi.org/10.2174/138945008785909293.
Roman-Blas J, Dion AS, Seghatoleslami MR, Giunta K, Oca P, Jimenez SA, et al. MED and PSACH COMP mutations affect chondrogenesis in chicken limb bud micromass cultures. J Cell Physiol. 2010;224:817–26.
Saghafi M, Khodashahi M, Saadati N, Azarian A, Rezaieyazdi Z, Salehi M, et al. Relationship between cartilage oligomeric matrix protein (COMP) and rheumatoid arthritis severity. Electron Physician. 2017;9:5940–7.
Firner S, Willwacher S, de Marées M, Bleuel J, Zaucke F, Brüggemann GP, et al. Effect of increased mechanical knee joint loading during running on the serum concentration of cartilage oligomeric matrix protein (COMP). J Orthop Res. 2018;36(7):1937–46.
Gotoh M, Sato T, Akashima T, Iwasaki H, Kameyama A, Mochizuki H, et al. Enzymatic synthesis of chondroitin with a novel chondroitin sulfate N-acetylgalactosaminyltransferase that transfers N-acetylgalactosamine to glucuronic acid in initiation and elongation of chondroitin sulfate synthesis. J Biol Chem. 2002;277:38189–96 https://doi.org/10.1074/jbc. M203619200.
Vodopiutz J, Mizumoto S, Lausch E, Rossi A, Unger S, Janocha N, et al. Chondroitin sulfate N -acetylgalactosaminyltransferase-1 (CSGalNAcT-1) deficiency results in a mild skeletal dysplasia and joint laxity. Hum Mutat. 2017;38:34–8 https://doi.org/10.1002/humu.23070.
Shimbo M, Suzuki R, Fuseya S, Sato T, Kiyohara K, Hagiwara K, et al. Postnatal lethality and chondrodysplasia in mice lacking both chondroitin sulfate N-acetylgalactosaminyltransferase-1 and -2. PLoS One. 2017;12:e0190333 https://doi.org/10.1371/journal.pone.0190333.
Bornstein P. Diversity of function is inherent in matricellular proteins: an appraisal of thrombospondin 1. J Cell Biol. 1995;130:503–6.
Yang GP, Lau LF. Cyr61, product of a growth factor-inducible immediate early gene, is associated with the extracellular matrix and the cell surface. Cell Growth Differ. 1991;2:351–7.
Jun J, Lau LF. The matricellular protein CCN1 induces fibroblast senescence and restricts fibrosis in cutaneous wound healing. Nat Cell Biol. 2010;12:676–85.
Kireeva ML, Mo F-E, Yang GP, Lau LF. Cyr61, a product of a growth factor-inducible immediate-early gene, promotes cell proliferation, migration, and adhesion. Mol Cell Biol. 1996;16:1326–34.
Kim S, Park J, Chung S, Hwang H, Chung K, Jo I, et al. Coxsackievirus B3 Infection Induces cyr61 Activation via JNK To Mediate Cell Death Coxsackievirus B3 Infection Induces cyr61 Activation via JNK To Mediate Cell Death. J Virol. 2004;78:13479–88.
Wiedmaier N, Müller S, Köberle M, Manncke B, Krejci J, Autenrieth IB, et al. Bacteria induce CTGF and CYR61 expression in epithelial cells in a lysophosphatidic acid receptor-dependent manner. Int J Med Microbiol. 2008;298:231–43.
Bovolenta P, Esteve P, Ruiz JM, Cisneros E, Lopez-Rios J. Beyond Wnt inhibition: new functions of secreted frizzled-related proteins in development and disease. J Cell Sci. 2008;121:737–46 https://doi.org/10.1242/jcs.026096.
Lories RJ, Corr M, Lane NE. To Wnt or not to Wnt: the bone and joint health dilemma. Nat Rev Rheumatol. 2013;9:328–39 https://doi.org/10.1038/nrrheum.2013.25.
Lories RJU, Peeters J, Bakker A, Tylzanowski P, Derese I, Schrooten J, et al. Articular cartilage and biomechanical properties of the long bones in Frzb -knockout mice. Arthritis Rheum. 2007;56:4095–103.
Thysen S, Luyten FP, Lories RJ. Loss of Frzb and Sfrp1 differentially affects joint homeostasis in instability-induced osteoarthritis. Osteoarthr Cartil. 2015;23:275–9 https://doi.org/10.1016/j.joca.2014.10.010.
Carrillo-López N, Panizo S, Alonso-Montes C, Román-García P, Rodríguez I, Martínez-Salgado C, et al. Direct inhibition of osteoblastic Wnt pathway by fibroblast growth factor 23 contributes to bone loss in chronic kidney disease. Kidney Int. 2016;90:77–89 https://doi.org/10.1016/j.kint.2016.01.024.
Shaw AT, Maeda Y, Gravallese EM. IL-17A deficiency promotes periosteal bone formation in a model of inflammatory arthritis. Arthritis Res Ther. 2016;18:104 https://doi.org/10.1186/s13075-016-0998-x.
Fraser JRE, Laurent TC, Laurent UBG. Hyaluronan: its nature, distribution, functions and turnover. J Intern Med. 1997;242:27–33.
Oohashi T, Edamatsu M, Bekku Y, Carulli D. The hyaluronan and proteoglycan link proteins: organizers of the brain extracellular matrix and key molecules for neuronal function and plasticity. Exp Neurol. 2015;274:134–44 https://doi.org/10.1016/j.expneurol.2015.09.010.
Dicker KT, Gurski LA, Pradhan-Bhatt S, Witt RL, Farach-Carson MC, Jia X. Hyaluronan: a simple polysaccharide with diverse biological functions. Acta Biomater. 2014;10:1558–70 https://doi.org/10.1016/j.actbio.2013.12.019.
Wang W, Yu Y, Hao J, Wen Y, Han J, Hou W, et al. Genome-wide DNA methylation profiling of articular cartilage reveals significant epigenetic alterations in Kashin-Beck disease and osteoarthritis. Osteoarthr Cartil. 2017;25:2127–33 https://doi.org/10.1016/j.joca.2017.08.002.
Lin Y, Lewallen EA, Camilleri ET, Bonin CA, Jones DL, Dudakovic A, et al. RNA-seq analysis of clinical-grade osteochondral allografts reveals activation of early response genes. J Orthop Res. 2016;34:1950–9 https://doi.org/10.1002/jor.23209.
Bitgood MJ, McMahon AP. Hedgehog and bmp genes are Coexpressed at many diverse sites of cell–cell interaction in the mouse embryo. Dev Biol. 1995;172:126–38 https://doi.org/10.1006/dbio.1995.0010.
de Crombrugghe B, Lefebvre V, Nakashima K. Regulatory mechanisms in the pathways of cartilage and bone formation. Curr Opin Cell Biol. 2001;13:721–8 https://doi.org/10.1016/S0955-0674(00)00276-3.
Maeda Y, Nakamura E, Nguyen M-T, Suva LJ, Swain FL, Razzaque MS, et al. Indian hedgehog produced by postnatal chondrocytes is essential for maintaining a growth plate and trabecular bone. Proc Natl Acad Sci U S A. 2007;104:6382–7.
Kurio N, Saunders C, Bechtold TE, Salhab I, Nah HD, Sinha S, et al. Roles of Ihh signaling in chondroprogenitor function in postnatal condylar cartilage. Matrix Biol. 2018;67:15–31 https://doi.org/10.1016/j.matbio.2018.02.011.
Jin S, Zhu F, Wang Y, Yi G, Li J, Lian L, et al. Deletion of Indian hedgehog gene causes dominant semi-lethal Creeper trait in chicken. Sci Rep. 2016;6:1–10 https://doi.org/10.1038/srep30172.
Ma G, Yu J, Xiao Y, Chan D, Gao B, Hu J, et al. Indian hedgehog mutations causing brachydactyly type A1 impair hedgehog signal transduction at multiple levels. Cell Res. 2011;21:1343–57 https://doi.org/10.1038/cr.2011.76.
Hiraki Y, Mitsui K, Endo N, Takahashi K, Hayami T, Inoue H, et al. Molecular cloning of human chondromodulin-I, a cartilage-derived growth modulating factor, and its expression in Chinese hamster ovary cells. Eur J Biochem. 1999;260:869–78 https://doi.org/10.1046/j.1432-1327.1999.00227.x.
Chun XS, Xin DL, Zhou W, Qiang HY, Feng Y, Feng LH, et al. Proteomic analysis of chondromodulin-I-induced differentiation of mesenchymal stem cells into chondrocytes. J Proteome. 2017;159:1–18 https://doi.org/10.1016/j.jprot.2017.02.017.
Zhang X, Prasadam I, Fang W, Crawford R, Xiao Y. Chondromodulin-1 ameliorates osteoarthritis progression by inhibiting HIF-2α activity. Osteoarthr Cartil. 2016;24:1970–80 https://doi.org/10.1016/j.joca.2016.06.005.
Peterson PA. Studies on the interaction between Prealbumin, retinol-binding protein, and vitamin a. J Biol Chem. 1971;246:44–9.
Berry DC, Byrne SMO, Vreeland AC, Blaner WS, Noy N. Cross Talk between Signaling and Vitamin A Transport by the the Retinol-Binding Protein Receptor STRA6. Mol Cell Biol. 2012;32:3164–75.
Leitch VD, Dwivedi PP, Anderson PJ, Powell BC. Retinol-binding protein 4 downregulation during osteogenesis and its localization to non-endocytic vesicles in human cranial suture mesenchymal cells suggest a novel tissue function. Histochem Cell Biol. 2013;139:75–87.
Hatfield JT, Anderson PJ, Powell BC. Retinol-binding protein 4 is expressed in chondrocytes of developing mouse long bones: implications for a local role in formation of the secondary ossification center. Histochem Cell Biol. 2013;139:727–34.
NIH NI of H. Vitamin A and Bone Health National. 2015.
Johansson S, Lind P, Hakansson H, Oxlund H, Rberg J, Melhus H. Subclinical hypervitaminosis a causes fragile bones in rats. Bone. 2002;31:685–9 https://doi.org/10.1016/S8756-3282(02)00910-9.
Joo N-S, Yang S-W, Song B, Yeum K-J. Vitamin a intake, serum vitamin D and bone mineral density: analysis of the Korea National Health and nutrition examination survey (KNHANES, 2008–2011). Nutrients. 2015;7:1716–27 https://doi.org/10.3390/nu7031716.
WOLBACH SB. Vitamin-a deficiency and excess in relation to skeletal growth. J Bone Joint Surg Am. 1947;29:171–92 http://www.ncbi.nlm.nih.gov/pubmed/20284696.
Binkley N, Krueger D. Hypervitaminosis a and bone. Nutr Rev. 2000;58:138–44 http://www.ncbi.nlm.nih.gov/pubmed/10860393.
De Luca F, Uyeda JA, Mericq V, Mancilla EE, Yanovski JA, Barnes KM, Zile MH, Baron J. Retinoic acid is a potent regulator of growth plate chondrogenesis. Endocrinology. 2000;141:346–53.
Cohen AJ, Lassová L, Golden EB, Niu Z, Adams SL. Retinoids directly activate the collagen X promoter in prehypertrophic chondrocytes through a distal retinoic acid response element. J Cell Biochem. 2006;99:269–78 https://doi.org/10.1002/jcb.20937.
Huang N, Zhou J, Wang W, Wang Q, Tang Y, Sun Y, et al. Retinol-binding protein 4 is positively associated with bone mineral density in patients with type 2 diabetes and osteopenia or osteoporosis. Clin Endocrinol. 2018;2017:659–64.
Kvansakul M, Adams JC, Hohenester E. Structure of a thrombospondin C-terminal fragment reveals a novel calcium core in the type 3 repeats. EMBO J. 2004;23:1223–33.
Maumus M, Manferdini C, Toupet K, Chuchana P, Casteilla L, Gachet M, et al. Thrombospondin-1 partly mediates the cartilage protective effect of adipose-derived mesenchymal stem cells in osteoarthritis. Front Immunol. 2017;8.
Amend SR, Uluckan O, Hurchla M, Leib D, Veis Novack D, Silva M, et al. Thrombospondin-1 regulates bone homeostasis through effects on bone matrix integrity and nitric oxide signaling in osteoclasts. J Bone Miner Res. 2015;30:106–15 https://doi.org/10.1002/jbmr.2308.
Kim RY, Seong Y, Cho TH, Lee B, Kim IS, Hwang SJ. Local administration of nuclear factor of activated T cells (NFAT) c1 inhibitor to suppress early resorption and inflammation induced by bone morphogenetic protein-2. J Biomed Mater Res Part A. 2018;106:1299–310 https://doi.org/10.1002/jbm.a.36332.
Zanotti S, Canalis E. Activation of Nfatc2 in osteoblasts causes osteopenia. J Cell Physiol. 2015;230:1689–95 https://doi.org/10.1002/jcp.24928.
Lei Z, Sabine A, Daniel A, M VP, Beat T, et al. Swiss Med Wkly. 2017;147 https://doi.org/10.4414/smw.2017.14529.
Nishikomori R, Kawai T, Toshiyuki K, Oda H, Yasumi T, Izawa K, et al. Remarkable improvement of articular pain by biologics in a Multicentric carpotarsal osteolysis patient with a mutation of MAFB gene. Pediatr Rheumatol. 2015;13(Suppl 1):152 https://doi.org/10.1186/1546-0096-13-S1-P152.
Kim K, Kim JH, Lee J, Jin HM, Kook H, Kim KK, et al. MafB negatively regulates RANKL-mediated osteoclast differentiation. Blood. 2007;109:3253–9 https://doi.org/10.1182/blood-2006-09-048249.
Chen X, Gu S, Chen B-F, Shen W-L, Yin Z, Xu G-W, et al. Nanoparticle delivery of stable miR-199a-5p agomir improves the osteogenesis of human mesenchymal stem cells via the HIF1a pathway. Biomaterials. 2015;53:239–50 https://doi.org/10.1016/J.BIOMATERIALS.2015.02.071.
Fernández-Torres J, Hernández-Díaz C, Espinosa-Morales R, Camacho-Galindo J, del Carmen G-SN, López-Macay Á, et al. Olymorphic variation of hypoxia inducible factor-1 a (HIF1A) gene might contribute to the development of knee osteoarthritis: a pilot study. BMC Musculoskelet Disord. 2015;16:218 https://doi.org/10.1186/s12891-015-0678-z.
Sato Y, Miyauchi Y, Yoshida S, Morita M, Kobayashi T, Kanagawa H, et al. The vitamin D analogue ED71 but not 1,25(OH) 2 D 3 targets HIF1a protein in osteoclasts; 2014. https://doi.org/10.1371/journal.pone.0111845.
Zanotti S, Smerdel-Ramoya A, Canalis E. Nuclear factor of activated T-cells (NFAT)C2 inhibits notch receptor signaling in osteoblasts. J Biol Chem. 2013;288:624–32 https://doi.org/10.1074/jbc.M112.340455.
Sieweke MH, Tekotte H, Frampton J, Graf T. MafB is an interaction partner and repressor of Ets-1 that inhibits Erythroid differentiation. Cell. 1996;85:49–60 https://doi.org/10.1016/S0092-8674(00)81081-8.
Park JG, Tischfield MA, Nugent AA, Cheng L, Di Gioia SA, Chan W-M, et al. Loss of MAFB function in humans and mice causes Duane syndrome, aberrant Extraocular muscle innervation, and inner-ear defects. Am J Hum Genet. 2016;98:1220–7 https://doi.org/10.1016/j.ajhg.2016.03.023.
Grimmer C, Pfander D, Swoboda B, Aigner T, Mueller L, Hennig FF, et al. Hypoxia-inducible factor 1α is involved in the prostaglandin metabolism of osteoarthritic cartilage through up-regulation of microsomal prostaglandin E synthase 1 in articular chondrocytes. Arthritis Rheum. 2007;56:4084–94 https://doi.org/10.1002/art.23136.
Grohar PJ, Janeway KA, Mase LD, Schiffman JD. Advances in the Treatment of Pediatric Bone Sarcomas. Am Soc Clin Oncol Educ B. 2017;37:725–35 https://doi.org/10.14694/EDBK_175378.
Zhang H, Mai Q, Chen J. MicroRNA-210 is increased and it is required for dedifferentiation of osteosarcoma cell line. Cell Biol Int. 2017;41:267–75 https://doi.org/10.1002/cbin.10721.
Kim CH, Oda T, Itoh M, Jiang D, Artinger KB, Chandrasekharappa SC, et al. Repressor activity of headless/Tcf3 is essential for vertebrate head formation. Nature. 2000;407:913–6.
Al-Huseini I, Ashida N, Nakao T, Ono K, Kimura T. P672Inhibition of NF-kappaB in smooth muscle cells promotes calcification. Eur Heart J. 2017;38(suppl_1) https://doi.org/10.1093/eurheartj/ehx501.P672.
Olivotto E, Otero M, Marcu KB, Goldring MB. Pathophysiology of osteoarthritis: canonical NF-κB/IKKβ-dependent and kinase-independent effects of IKKα in cartilage degradation and chondrocyte differentiation. RMD Open. 2015;1(Suppl 1):e000061 https://doi.org/10.1136/rmdopen-2015-000061.
Li H, Durbin R. Fast and accurate short read alignment with burrows-wheeler transform. Bioinformatics. 2009;25:1754–60.
Anders S, Pyl PT, Huber W. HTSeq-A Python framework to work with high-throughput sequencing data. Bioinformatics. 2015;31:166–9.
Robinson MD, McCarthy DJ, Smyth GK. edgeR: a bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics. 2009;26:139–40.
R Core Team. A language and environment for statistical computing: R Foundation for Statistical Computing; 2018. http://www.r-project.org/.
Sandelin A, Alkema W, Engström P, Wasserman WW, Lenhard B. JASPAR: an open-access database for eukaryotic transcription factor binding profiles. Nucleic Acids Res. 2004;32(Database issue):D91–4 https://doi.org/10.1093/nar/gkh012.
Acknowledgments
The authors thank to Alexandre L. Tessmann for technical assistance. HCO received a scholarship from the National Council for Scientific and Technological Development (CNPq) at the Universidade Federal de Viçosa. MCL, SEFG and LLC are CNPq fellows.
Funding
This study was funded by the Brazilian Agricultural Research Corporation (Embrapa), project number 01.11.07.002.04.03, from the Brazilian Government, which provided financial support to generate the experiment and data. Our study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nivel Superior-Brasil (CAPES)-Finance Code 001, which provided access to the journals for the literature review. However, they do not participate in the design of the study, sample collection, analysis, data interpretation and in writing the manuscript.
Author information
Authors and Affiliations
Contributions
Conceived and designed the experiment: MCL, JOP, AMGI.
Performed the experiment: HCO, AMGI, JOP, LLC, MEC, MCL.
Data analysis and curation: HCO, AMGI, JOP, LLC, MEC, MCL, SEFG.
Writing – Original Draft preparation: HCO, AMGI, JOP, LLC, MEC, MCL, SEFG.
Funding Acquisition and supervision of the research: JOP, MCL.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was performed with the approval of the Embrapa Swine and Poultry Ethical Committee of Animal Use (CEUA) under protocol number 012/2012.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Additional file 1.
Detailed expressed values of all Differentially Expressed Genes, showing the name and description of each annotated gene, LogFoldChange, logCPM, FDR and gene Ensembl ID.
Additional file 2.
Detailed table showing genes among all Biological Process.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
de Oliveira, H.C., Ibelli, A.M.G., Guimarães, S.E.F. et al. RNA-seq reveals downregulated osteochondral genes potentially related to tibia bacterial chondronecrosis with osteomyelitis in broilers. BMC Genet 21, 58 (2020). https://doi.org/10.1186/s12863-020-00862-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12863-020-00862-2