Abstract
Background
In this study, wheat bran, an agricultural waste, was utilized as a low-cost carbon source for algal cultivation.
Results
Treatment of lignocellulosic waste by two fungal species (Pleurotus ostreatus or Trichoderma viride) caused the accumulation of reducing sugar at a relatively high concentration (50.58 and 54.30 mg/g wheat bran) after 7 days of incubation, respectively. The soluble products of treated wheat bran increased the growth, carbohydrate, and protein contents of both Chlorella vulgaris and Scenedesmus obliquus under mixotrophic and heterotrophic conditions.
Conclusions
The obtained data suggest that soluble product of treated wheat bran could be used as an efficient medium for the mixotrophic and heterotrophic growth of both algal species.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cellulosic materials were commonly used as carbon/energy substrate and source of inducers in fermentation ([Lee and Fan 1983]). Lignocellulosic residues can be classified into two main groups: those in which the lignocellulose is the major carbon source and those in which, in addition to the lignocellulose, there are significant concentrations of simple carbohydrates, such as mono- and disaccharides (Villas-Bôas et al. [2002]); vitamins and minerals, due to their organic nature, are easily assimilated for microorganisms (Rosales et al. [2005]). Wheat bran is a very important dietary fiber source which is widely used in the food industry in order to produce fiber-enriched foods (Wang et al. [2012]). Wheat bran is the major lignocellulosic waste which is mainly disposed by burning, causing air pollution and thus could not be considered as an animal feed due to its low digestibility, low protein content, and high lignin and silica contents. The conversion of lignocellulosic material to fermentable sugars requires three basic processes, i.e., enzyme (cellulase) production, pretreatment of raw material, and hydrolysis and cellulolysis of microorganisms. The ability of fungi to degrade lignocellulosic materials is due to their highly efficient enzymatic system. It is known that fungi have two types of extracellular enzymatic systems: the hydrolytic system, which produces hydrolases that are responsible for polysaccharide degradation, and a unique oxidative and extracellular ligninolytic system, which degrades lignin and opens phenyl rings (Sánchez [2009]). The obtained reducing sugar can be used as carbon source for algal production. Many species of microalgae take up organic compounds from the medium; for example, glycerol, glucose, and acetate are taken up by a number of chlorophytes ([Ukeles and Rose 1976]). Aqueous extracts of tea leaves and soybean residues have been used as culture media for the cultivation of a unicellular green alga, Chlorella pyrenoidosa ([Wong and Lay 1980]). Chlorella vulgaris and Scenedesmus obliquus have the ability to utilize organic substrates under both light and dark conditions (Combres et al. [1994]), C. vulgaris possesses an inducible active hexose transport system ([Tanner 1969]), and this system is constitutive in S. obliquus ([Abeliovich and Weisman 1978]).
In general, and in most cases, heterotrophic cultivation is far cheaper, simpler to construct in facilities, and easier than autotrophic cultivation to maintain on a large scale (Perez-Garcia et al. [2011]). Glucose can be utilized under heterotrophic conditions by Chlorella protothecoides, which can grow under heterotrophic conditions using acetate, glucose, or other organic compounds as carbon source (Wu et al. [1994]). Micractinium pusillum grows in the presence of organic substrates, i.e., glucose and acetate, in the light (mixotrophic growth) as well as in the dark (heterotrophic growth) (Bouarab et al. [2004]). This work aims to study the effect of the soluble product of fermented wheat bran on the growth of some green algae under mixotrophic and heterotrophic conditions.
Methods
Lignocellulosic waste
Wheat bran was collected from fields in Mahelet Roh near Tanta City in El Gharbia Governorate, Egypt.
Microorganisms
C. vulgaris and S. obliquus were collected from different channels and sewage in Gharbia Governorate and then purified and identified according to [Fott (1969]) and [Prescott (1978]). The axenic culture was maintained on Kühl agar slants (Kühl [1962]) at 4°C. Subcultures were made regularly, nearly every month.
Pleurotus ostreatus (HARI7 Hungary) and Trichoderma viride were obtained from the Laboratory of Mycology, Botany Department, Faculty of Science, Tanta University and were grown and maintained on malt extract Czapek-Dox agar medium (Starr et al. [1981]) slants and plates, respectively, at 5°C.
Pretreatment of lignocellulosic wastes by fungi
Wheat bran was moistened by distilled water; then about 50 g of dry waste was added in 250-mL flasks and sterilized for 20 min at 121°C and 1.5 atm. The flasks were cooled, inoculated with two blocks of P. ostreatus and T. viride separately, and incubated at 26 ± 2°C. During mycelial growth, water-soluble reducing sugars and total carbohydrates (Dubois et al. [1956]) were measured after 1, 3, 5, 7, 9, and 11 days.
Preparation of lignocellulosic wastes for analysis
Three replicates (1 g) were removed every 2 days from each of the flask containing the mycelia-colonized wheat bran (each was inoculated by P. ostreatus and T. viride). Each sample was mixed with 10 mL of hot water (70°C) for 2 h; the mixture was squeezed through several layers of cheesecloth and was filtered. The filtrate was cleared by centrifugation at 6,000 × g for 20 min at 4°C. The resulting supernatants were used for all subsequent assays ([Wood and Goodenough 1977]).
Cultivation of algae
The inoculum was prepared by dispensing 250 mL of Kühl medium in 500-mL Erlenmeyer flasks and then sterilized in an autoclave at 121°C and 1.5 atm. for 20 min. After cooling, the Erlenmeyer flasks were inoculated by one loop of 7-day-old cultures (160 mg/100 mL algal suspension for both tested green algae and chlorophyll (a + b) 6.33 and 6.45 μg/mL for C. vulgaris and S. obliquus suspension, respectively). The culture flasks were aerated by air pumps and incubated at 25 ± 1°C under continuous illumination provided from daylight fluorescent tubes (80 μmol photon·m−2·s−1). The growth of the inoculum was assessed at 560 nm to adjust the number of cells to 2 × 105 cell·mL−1. One-liter flasks were used as growth vessels, in which 600 mL of medium was poured and inoculated with different concentrations of treated soluble product of wheat bran (v/v) or pure glucose with the same concentration of soluble product, and then pH of the medium was adjusted at 6.8. The obtained culture medium was sterilized and then inoculated with 20% (v/v) of exponentially growing inocula. Growth vessels were bubbled with air pumps and incubated at 25 ± 1°C for 10 days under continuous illumination for mixotrophic conditions, and another set of test flasks was wrapped with aluminum foil to shade from the light for heterotrophic conditions. In each experiment, the growth curve, total protein, pigment, carbohydrate, and total lipid content were determined.
Determination of algal growth parameters
Algal growth including optical density ([Wetherell 1961]), dry weight, pigment content ([Steinman and Lamberti 1996]), total carbohydrate content (Dubois et al. [1956]), total soluble proteins (Lowry et al. [1951]), and total lipids ([Varma and Tiwari 1967]) for 10 days of incubation period was determined.
Statistical analysis
All reported results are the means of the three replicates. One way analysis of variance and Pearson correlation coefficient were carried out using the SPSS (version 1999) computer program of biostatistics ([Ghonam and Sabre 2000]).
Results and discussion
Effect of P. ostreatus and T. viride on pretreated wheat bran
The results obtained revealed that there is a negative correlation between the reducing sugars and carbohydrate contents of fermented lignocellulosic waste by action of fungal growth (Figure 1a,b). Our results are in agreement with those obtained by Rabinovich et al. ([2002]) who found that Trichoderma, Penicillium, Fusarium, and mushroom produced large amounts of polysaccharide hydrolases which act to depolymerize lignocellulosic polymers into compounds of lower molecular weight which can be assimilated by algal cells. Fungal enzymes have been suggested as a feasible alternative for the conversion of lignocelluloses into fermentable sugars and fuel ethanol (Szengyel et al. [2000]). The decrease in carbohydrate contents of lignocellulosic waste was coupled with the increase in reducing sugar content (glucose) due to the effect of hydrolytic enzymes which were secreted during fungal growth. In early studies, [Toyama and Ogawa (1975]) found that inoculation of rice straw or wheat bran by T. viride resulted in the formation of 5% to 10% of sugar solution after 48 h of incubation.
The results indicate that carbohydrate contents of wheat bran increased again as the incubation time increased for more than 7 days. These results are more or less similar to that reported by [Montant and Thomas (1977]) who stated that growing fungi might secrete different polysaccharides in the medium at different times of the incubation period and that reducing sugar content of fermented lignocellulosic wastes also decreased by the increase in the incubation period. This reduction in reducing sugar content may be due to the assimilation of monosaccharide released from the hydrolysis of fungi themselves. Hence, the compounds of lower molecular weight released by depolymerization of lignocellulose can be assimilated by fungus ([Wood 1985]).
Growth response
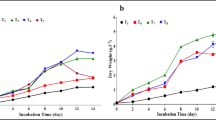
The results show that the effect of soluble product of wheat bran on growth of both tested algae is a similar to the effect of glucose on algal growth under both conditions (mixotrophic and heterotrophic) if added in the same concentration (Figures 2 and 3). This indicates that both tested algae can utilize the reducing sugar (glucose) produced from wheat bran. Our results are in agreement with Shamala et al. ([1982a]) who stated that the addition of 0.05% (w/v) glucose in mixotrophic culture of Scenedesmus acutus increased the biomass threefold. The biomass concentrations of C. protothecoides CS-41 increased from 4.9 to 31.2 g·L−1 of dry cells with increasing initial glucose concentration from 10 to 80 g·L−1 under heterotrophic conditions (Shi et al. [1999]).
Carbohydrate contents
The carbohydrate contents of both tested algae were significantly increased according to the concentration of soluble product of wheat bran or glucose, algal species, and culture conditions (light or dark). This observation is similar to that observed by Shamala et al. ([1982b]) who stated that S. acutus produced high amounts of carbohydrates even at lower glucose concentrations under mixotrophic conditions. Inclusion of glucose in the culture of Ankistrodesmus convolutus induced the increase in carbohydrate contents under mixotrophic conditions (Chu et al. [1995]).
On the other hand, the carbohydrate contents of both tested organisms increased with the increase of the reducing sugar (soluble product of wheat bran or glucose) under heterotrophic conditions (Tables 1 and 2). This result is more or less similar to that of [Griffiths (1965]) who observed that heterotrophically grown cells were diverted toward carbohydrate synthesis rather than toward the synthesis of other cellular constituents. Heterotrophic growth leads to the accumulation of carbohydrate (Shamala et al. [1982b]).
Total soluble proteins
The effect of soluble product of treated wheat bran or glucose on the protein contents of both algal species showed an increase in the total soluble protein under mixotrophic conditions (Tables 1 and 2). Our results are in agreement with those of Shamala et al. ([1982a]) who stated that mixotrophic cultivation appears to be advantageous for the mass production of algal protein. [Hu and Gao (2003]) found that mixotrophic cultures of Nannochloropsis sp. gave a greater protein content. However, our results are in contrast with those of Orus et al. ([1991]) who noted that the culture growth of C. vulgaris UAM 101 grown on glucose reduced in protein content.
Under heterotrophic conditions, the protein contents of both the tested organisms were decreased gradually by increasing the concentrations of soluble product of treated wheat and glucose, but still more than the control; this means that the algal protein production depended on the concentration of the used carbon source and light. These findings are in accordance with those of [Ogbonna and Tanaka (2000]) who found that protein contents of Chlorella decreased during the heterotrophic phase.
Pigments contents
The results show that under mixotrophic and heterotrophic conditions, the different pigment fractions of C. vulgaris were increased as the concentration of the soluble product of wheat bran or glucose was increased. On the other hand, the pigment fractions of S. obliquus were reduced by the increase of soluble product of wheat bran under mixotrophic conditions, whereas no significant effect was observed in the content of the pigment fractions under heterotrophic conditions (Tables 1 and 2).
The pigment fractions of treated S. obliquus with soluble product of wheat bran or glucose showed lower values as compared with those in the control under mixotrophic condition. The effect of glucose on the pigment fractions of S. obliquus showed different changes as compared with that in soluble product of wheat bran under heterotrophic conditions. However, they increased as the concentration of glucose increase, but still lower than the control (Table 2). Our results are in good agreement with those of [Ogawa and Aiba (1981]) who observed that the effect of glucose in the culture medium depends on its concentration. Chlorophyll and carotenoid content of A. convolutus decreased with the increase of glucose concentrations (Chu et al. [1995]).
Under heterotrophic conditions, the reduction in chlorophyll and carotenoids and the decrease in chlorophyll a/b ratio are also characteristic of dark adaptation, which is commonly observed in higher plants ([Young 1993]). The reduction in pigment content of heterotrophic conditions may be attributed to the reduction in the number of chloroplasts ([Scheerer and Parthier 1982]). The disappearance of pigment content of Chlorella protothecoides during the heterotrophic growth was due to the alteration of photosynthetic membrane proteins ([Sasidharan and Gnanam 1990]). Furthermore, the biosynthesis of chlorophyll a is completed through light-dependent reaction; through this reaction, the protochlorophyll can be reduced to form chlorophyll a ([Devlin and Barker 1971]).
Lipid contents
Our results revealed that the soluble product of wheat and glucose were stimulatory for lipid production of both the tested algae under mixotrophic conditions (Figure 4). The stimulatory responses were extremely variable in the quantity according to the applied concentrations. Our results are in conformity with those of Orus et al. ([1991]) who recorded that the cultures of C. vulgaris UAM 101 grown on glucose produced high amounts of lipid. Our results also conform to those of Liu et al. ([2010]) who stated that glucose is the best carbon source for growth and lipid production.
Under heterotrophic conditions, the treated soluble product of wheat bran and glucose induced nonsignificant reduction in lipid content of both the tested algae (Figure 4). In this context, [Day and Tsavalos (1996]) detected that the lipid contents (unsaturated fatty acid) in heterotrophically grown algal cells might increase or decrease depending on the algal species used. The heterotrophic culture of Tetraselmis had low lipid levels (especially fatty acids) compared with the phototrophically cultured cells ([Becker 1994]). Our results are in contrast with that reported by Gao et al. ([2010]) who detected that C. protothecoides can grow heterotrophically with glucose as the carbon source and can accumulate high proportion of lipids. Furthermore, they indicated that sweet sorghum juice could effectively enhance algal lipid production, and its application may reduce the cost of algae-based biodiesel. This result suggests that the production of lipid by both tested algae depends upon algal species, culture condition, and concentration of the reducing sugar.
Conclusions
This work is an endeavor for the cultivation of some green algae using cheap carbon source (wheat bran) when treated with some fungi for increasing soluble-reducing content. The soluble product of wheat bran has a similar effect with glucose as carbon source under both mixotrophic and heterotrophic conditions. Our study suggests that the efficiency of wheat bran soluble product as carbon source for algal growth depend on the reducing sugar produced, algal species, and culture condition (light or dark).
References
Abeliovich A, Weisman D: Role of heterotrophic nutrition in growth of the alga Scenedesmus obliquus in high-rate oxidation ponds. Appl Environ Microbiol 1978,35(1):32–37.
Becker EW: Microalgae: biotechnology and microbiology. Cambridge University Press, Cambridge; 1994:293.
Bouarab L, Dautab A, Loudikia M: Heterotrophic and mixotrophic growth of Micractinium pusillum Fresenius in the presence of acetate and glucose: effect of light and acetate gradient concentration. Water Res 2004,38(11):2706–2712. 10.1016/j.watres.2004.03.021
Chu WL, Phang SM, Hock GS: Influence of carbon source on growth biochemical composition and pigmentation of Ankistrodesmus convolutus. J Appl Phycol 1995, 7: 59–64. 10.1007/BF00003551
Combres C, Laliberte G, Reyssac JS, de LaNoue J: Effect of acetate on growth and ammonium uptake in the microalga Scenedesmus obliquus. Physiol Plant 1994, 91: 729–734. 10.1111/j.1399-3054.1994.tb03012.x
Day JG, Tsavalos AJ: An investigation of heterotrophic culture of the green alga Tetraselmis. J Appl Phycol 1996, 8: 73–77. 10.1007/BF02186225
Devlin RM, Barker AV: Photosynthesis. Van Nostrand Raeinbold Co., New York; 1971.
Dubois M, Gilles KA, Hamilton JK, Rebers PA, Smith F: Colorimetric method for determination of sugars and related substances. Anal Chem 1956, 28: 350–356. 10.1021/ac60111a017
Fott B: Studies in phycology. Academic Publishing House of Czechoslovak Academy of Science, Prague; 1969.
Gao C, Zhai Y, Ding Y, Wu Q: Application of sweet sorghum for biodiesel production by heterotrophic microalga Chlorella protothecoides. Appl Energy 2010,87(3):756–761. 10.1016/j.apenergy.2009.09.006
Ghonam AA, Sabre NM: Statistical analysis of data by using SPSS (1999). Dar Kappa Publishing, Cairo; 2000.
Griffiths DJ: The accumulation of carbohydrate in Chlorella vulgaris under heterotrophic conditions. Ann Bot 1965, 115: 347–357.
Hu H, Gao K: Optimization of growth and fatty acid composition of unicellular marine picoplankton, Nannochloropsis sp., with enriched carbon sources. Biotechnol Lett 2003,25(5):421–425. 10.1023/A:1022489108980
Kühl A: Zur physiologie der speicherung kondersierter anorganischer phosphate in Chlorella. Vortr Botan Hrsg Deut Botan Ges (NF) 1962, 1: 157–166.
Lee YH, Fan LT: Kinetic studies of enzymatic hydrolysis of insoluble cellulose II. Analysis of extended hydrolysis times. Biotechnol Bioeng 1983, 25: 939–966. 10.1002/bit.260250406
Lowry OH, Roserbrough NJ, Farr LA, Randall RJ: Protein measurements with the folin phenol reagent. J Biol Chem 1951, 193: 265–275.
Liu J, Huang J, Fan KW, Jiang Y, Zhong Y, Sun Z, Chen F: Production potential of Chlorella zofingienesis as a feedstock for biodiesel. Bioresource Technol 2010,101(2):8658–8663. 10.1016/j.biortech.2010.05.082
Montant C, Thomas L: Structure d’un glucane exocellulaire produit par le Botryti cinerea (Pers). Ann Sci Naturelles Bot 1977, 18: 185–192.
Ogawa T, Aiba S: Bioenergetic analysis of mixotrophic growth in Chlorella vulgaris and Scenedesmus acutus. Biotechnol Bioengng 1981, 23: 1121–1132. 10.1002/bit.260230519
Ogbonna JC, Tanaka H: Production of pure photosynthetic cell biomass for environmental biosensors. Materials Sci Eng 2000, 12: 9–15. 10.1016/S0928-4931(00)00150-8
Orus MI, Marco E, Martinez F: Suitability of Chlorella vulgaris UAM 101 for heterotrophic biomass production. Biores Technol 1991, 38: 179–184. 10.1016/0960-8524(91)90151-9
Perez-Garcia O, Escalante FME, de-Bashan LE, Bashan Y: Heterotrophic cultures of microalgae: metabolism and potential products. Water Res 2011, 45: 11–36. 10.1016/j.watres.2010.08.037
Prescott GW: How to know the fresh water algae. WC Brown Company, Dubuque; 1978.
Rabinovich ML, Mel’nik MS, Bolobova AV: Microbial cellulases. Prikl Biokhim Mikrobiol 2002,38(4):355–373.
Rosales E, Couto SR, Sanromán MÁ: Reutilization of food processing wastes for production of relevant metabolites: application to laccase production by Trametes hirsuta. J Food Eng 2005, 66: 419–423. 10.1016/j.jfoodeng.2004.04.010
Sánchez C: Lignocellulosic residues: biodegradation and bioconversion by fungi. Biotechnol Adv 2009,27(2):185–194. 10.1016/j.biotechadv.2008.11.001
Sasidharan A, Gnanam A: Immunological identification and quantification of light harvesting chlorophyll a/b protein of Chlorella protothecoides. Indian J Biochem Biophys 1990,27(1):1–4.
Scheerer A, Parthier B: Dark induced chloroplast dedifferentiation in Euglena gracilis. Planta 1982, 156: 274–281. 10.1007/BF00393736
Shamala TR, Drawert F, Leupold G: Studies on Scenedesmus acutus growth. I. Effect of autotrophic and mixotrophic conditions on the growth of Scenedesmus acutus. Biotech Bioeng 1982, 24: 1287–1299. 10.1002/bit.260240605
Shamala TR, Drawert F, Leupold G: Studies on Scenedesmus acutus growth. II. Effect of autotrophic and mixotrophic growth on the amino acid and carbohydrate composition of Scenedesmus acutus. Biotech Bioeng 1982, 24: 1301–1317. 10.1002/bit.260240606
Shi XM, Zhang XW, Liu HJ, Chen F: Production of biomass and lutein by Chlorella protothecoides at various glucose concentrations in heterotrophic cultures. Process Biochem 1999, 34: 341–347. 10.1016/S0032-9592(98)00101-0
Starr MP, Stolp HG, Schlegel HG, Schlegel A: The prokaryotes: a handbook of habitats, isolation, and identification of bacteria, volume 1. Springer-Verlag, Berlin; 1981.
Steinman AD, Lamberti GA: Biomass and pigments of benthic algae. In Methods in stream ecology. Edited by: Hauer FR, Lamberti GA. Academic Press, San Diego; 1996.
Szengyel Z, Zacchi G, Varga A, Reczey K: Cellulase production of Trichoderma reesei RUT C30 using steam-pretreated spruce: hydrolytic potential of cellulases on different substrate. Appl Biochem Biotechnol 2000, 84–86: 679–691.
Tanner W: Light-driven active uptake of 3-O-methylglucose via an inducible hexose uptake system of Chlorella. Biochem Biophys Res Commun 1969, 36: 278–283. 10.1016/0006-291X(69)90326-X
Toyama N, Ogawa K: Sugar production from agricultural woody wastes by saccharification with Trichoderma viride cellulase. Biotechnol Bioeng Symp 1975, 5: 225–244.
Ukeles R, Rose WE: Observations on organic carbon utilization by photosynthetic marine microalgae. Mar Biol 1976, 37: 11–28. 10.1007/BF00386774
Varma AK, Tiwari PN: Rhizobium inoculation and oil content of soy bean seeds (Glycine max). Curr Sci 1967, 20: 275.
Villas-Bôas SG, Elisa E, David AM: Microbial conversion of lignocellulosic residues for production of animal feeds. Animal Feed Sci Technol 2002,98(1–2):1–12.
Wang T, Sun X, Zhou Z, Chen G: Effects of microfluidization process on physicochemical properties of wheat bran. Food Res Internat 2012,48(2):742–747. 10.1016/j.foodres.2012.06.015
Wetherell DF: Culture of fresh water algae in enriched natural seawater. Plant Physiol (Copenh) 1961, 14: 1–6. 10.1111/j.1399-3054.1961.tb08131.x
Wood DA: Production and roles of extracellular enzymes during morphogenesis of basidiomycete fungi. In Developmental biology of higher fungi. Edited by: Moore D, Casselton LA, Wood DA, Frankland JC. Cambridge University Press, Cambridge; 1985:387.
Wood DA, Goodenough PW: Fruiting of Agaricus bisporus. Arch Microbiol 1977, 114: 161–165. 10.1007/BF00410778
Wong MH, Lay CC: The comparison of soy-bean wastes, used tea-leaves and sewage sludge for growing Chlorella pyrenoidosa. Environmental Pollution Series A. Ecol Biol 1980,23(4):247–259.
Wu Q, Yin S, Sheng G, Fu J: New discoveries in study on liquid hydrocarbons from thermal degradation of heterotrophically yellowing algae. Science in China (B) 1994, 37: 326–335.
Young AJ: Factors that affect the carotenoid composition of higher plants and algae. In Carotenoids and photosynthesis. Edited by: Young AJ, Britton G. Chapman and Hall, London; 1993:161–205.
Acknowledgments
The authors would like to acknowledge the fellowship for Mona M Ismail from Tanta University and the Faculty of Science to carry out this work.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
This work is part of the Mater thesis of MMI where MME, MYB, and MEO supervised the theses, and MME wrote the paper that was read by the other authors. All authors read and approved the final manuscript.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 2.0 International License (https://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
EL-Sheekh, M.M., Bedaiwy, M.Y., Osman, M.E. et al. Mixotrophic and heterotrophic growth of some microalgae using extract of fungal-treated wheat bran. Int J Recycl Org Waste Agricult 1, 12 (2012). https://doi.org/10.1186/2251-7715-1-12
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/2251-7715-1-12