Abstract
Agricultural industries produce vast amounts of liquid waste, which contains a significant concentration of nutrients. In the context of emphasizing the huge increase in population, climate changes, and pollution which results in depletion of fresh water resources, a sustainable solution for a greener future is needed. Wastewater treatment by the use of microalgae can mitigate a part of the problem by restoring water for irrigating agricultural crops. Little studies give insights on the physiological responses and ultrastructure of the Chlorophyta alga Desmodesmus sp. as it grows in cheese whey (CW). The algal strain was mixotrophically grown in a growth medium composed of CW only and CW supported with Bold’s basal medium (BBM) for 14 days. The potent response was observed with algal cultures fed by 15% CW enriched with 50% BBM. Fifteen percent CW in combination with 50% BBM significantly improved Desmodesmus sp. growth (303%), productivity (325%), and accumulation of cell metabolites, mainly lipids (3.89%), and carbohydrates (1.95%). On the contrary, protein and photosynthetic pigment (chlorophyll a, chlorophyll b, and carotenoids) contents were higher in BBM than in all treatments. Fatty acid composition demonstrated that the predominantly accumulated fatty acids were palmitic (25.86%), oleic (35.31%), and linoleic acid (13.22%). In conclusion, Desmodesmus sp. can be a good candidate for phycoremediation when cultivated on CW, whereas it can reduce the nutrition costs and water demand of algal cultivation by 50% and 15%, respectively. Therefore, it may be an effective strategy for algal mass production in sustainable agricultural systems.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Fresh water availability has a crucial role in sustaining agricultural production. Many countries are suffering from severe water pollution and shortage resulting from the rapid increase in population (Sofy et al., 2022). It is well known that huge amounts of water are consumed to meet household, industrial, and agricultural needs which affects the quantity and quality of available water (El-Mahdy et al., 2021). The planet contains approximately 1400 million km3 of water resources (F.A.O., 2017), of which only 3% is fresh, usable water (Kılıç, 2020).
Contaminated industrial wastewater, overloaded with excessive nitrogen and phosphorus, known as eutrophication, jeopardizes marine ecosystems (Dawood et al., 2023). Cyanobacterial algal blooms degrade water quality in aquatic ecosystems (Bali & Gueddari, 2019; Sonarghare et al., 2020; Su et al., 2022). Low levels of dissolved oxygen resulting from cyanobacterial algal bloom lead to fewer number of fish, which is a significant loss of important food sources for some mammals and birds, leading to the death of some marine animals (Kim et al., 2020; Sonarghare et al., 2020). Furthermore, eutrophication limits sunlight penetration required by plants for photosynthesis, thus resulting in an eventual destruction of sea grass bed (Almanassra et al., 2021; Kim et al., 2020). In addition, excessive growth of algae clogs irrigation and water pipes, thereby affecting transportation and power generation. Also, the increased rate of eutrophication of aquatic ecosystems has been associated with the rise of new diseases (Matei & Racoviteanu, 2021). Moreover, the existence of nitrates in drinking water has been reported to form carcinogenic compounds (Matei & Racoviteanu, 2021).
Nowadays, conserving water resources and reducing wastewater pollutants require highly effective methods that are cost-effective and eco-friendly to maintain climate change and ecological stability (Bhatt et al., 2021). The United Nations Water (UN Water) estimates that the number of those who will suffer from water scarcity will increase to about 1.8 billion people by 2025 (Awad et al., 2019; Baskar et al., 2022). Taking early precautions, including wastewater treatment, may aid in providing clean and safe water for drinking, agriculture, or industrial use. Some of the common wastewater treatment techniques include chemical treatment, physical treatment, the use of biological organisms, and sludge treatment (Kamali et al., 2019).
Recently, global demand and consumption of dairy products is elevating, which led to an enormous growth of dairy industries with the repercussion of dairy waste production including whey, dairy sludges, and wastewater. They are characterized by their high nutrient concentration, biological oxygen demand (BOD), chemical oxygen demand (COD), and organic and inorganic contents (Ahmad et al., 2019).
Dairy wastewaters can be treated by physio-chemical, biological, and biotechnological methods. The physio-chemical treatment (coagulation or flocculation) is used for destruction of milk fat and protein colloids in dairy wastewater, requiring considerable amount of chemicals, energy, and operating costs. Biological treatments including the use of microalgal cultivation in dairy wastewater have been proposed in recent years. Several algal species including Chlorella pyrenoidosa, Anabaena ambigua, Scenedesmus abundans, Chlorella vulgaris, Chlamydomonas, polypyrenoideum, and Acutodesmus dimorphus have been successfully cultivated in dairy wastewater (Brar et al., 2019). Biological treatments are considered a more eco-friendly approach than physio-chemical methods. Moreover, aerobic along with anaerobic process treatments can be applied in order to reach the effluents’ discharge limits for dairy wastewater. Biotechnological processes are implemented recently and can result in the formation of significant products to the industries, such as whey-derived products, bioplastics, biofuels, bioenergy, organic acids, bioactive peptides, and enzymes (Ahmad et al., 2019).
Microalgae are photosynthetic microorganisms that can grow in wastewater as an alternative culturing medium adding to their ability in sequestering carbon from flue gases (Muhammad et al., 2020). When exposed to sunlight, they can carry out photosynthetic processes that turn carbon dioxide into biomass. Microalgae have long been used by humans in the industrial sector; one such use is wastewater treatment, which is effective and energy-efficient in eliminating pollutants (Tan et al., 2023). Wastewaters are typically contaminated with nitrogen, phosphorus, and other trace elements, which microalgae require for their cell growth (Goyal et al., 2023). Additionally, they have high affinity for absorbing heavy metals (Abd El-Hameed et al., 2018; El-Beltagi et al., 2022). Using this strategy, a dual benefit can be obtained by removing pollutants and accumulating algae biomass (Rodríguez-Rángel et al., 2022). Besides bioremediation, the resulting algal biomass can be used to produce biofuel, biofertilizers, and economical bioproducts (Silva et al., 2020), along with fish feed (Ansari et al., 2021). Several microalgal species, such as Desmodesmus, Chlorella, Scenedesmus, Neochloris, Chlamydomonas, Nitzschia, and Cosmarium, have been applied to various types of wastewater treatments coupled with biofuel production under sterilized or non-sterilized conditions (Madakka et al., 2019). More specifically, the green microalga Desmodesmus sp. is adaptable to different growth conditions. It can grow under autotrophic, mixotrophic, and heterotrophic conditions (Ríos et al., 2018). Also, it can survive and grow efficiently under a wide range of temperatures, light intensity, CO2, and pH (Chiu et al., 2016; Ferreira et al., 2021).
There is no doubt that large-scale algal production cultivation requires a huge amount of fresh water for cultivation. Large water consumption is necessary, as microalgae are aquatic species that require a substantial amount of water for survival and cell proliferation (Farooq et al., 2015). According to Nogueira Junior et al. (2018), the production of 1 kg of algae requires 1564 l of water under pond conditions and 372 l when photobioreactors are used. Therefore, recent studies are focusing on cultivating algae using agricultural and industrial wastewater (Abdelfattah et al., 2023). In this regard, agro-industrial wastewater effluents of apple vinegar (Giovanardi et al., 2013), cheese whey (Ribeiro et al., 2017), vinasse (Santana et al., 2017), palm oil mill (Kamyab et al., 2019), and molasses (Yew et al., 2020) were used for cultivating algae. For example, Wang, Wang, Miao, and Tian (2018) evaluated the ability to use tofu whey as an alternative medium for Chlorella pyronoidosa under mixotrophic and heterotrophic conditions.
Consequently, agro-industrial carbon-rich wastes as an alternative, sustainable, and low-cost source of nutrients are an attractive approach for raising algae production, with a particular interest in high-value-added co-products such as vitamins, proteins, and essential amino acids, monounsaturated and polyunsaturated fatty acids, polysaccharides, minerals, and pigments, to improve the economics (Barone et al., 2019; de Medeiros et al., 2020; El-Sayed et al., 2020; El-Sayed et al., 2022; Fetyan et al., 2022; Kamal et al., 2017). For example, Yuan et al. (2021) cultured eight microalgal species of Scenedesmus and Desmodesmus on potato wastewater to purify and produce valuable by-products.
The dairy industry, as an example of the agricultural industry, is usually considered the largest industrial food wastewater source, especially in Europe (Karadag et al., 2015). The main important by-product in milk processing is whey, which accounts for about 85–95% of milk volume and 55% of milk components. Carbohydrates, mostly lactose, represent about 4.8–5% of whey (Carvalho et al., 2013; Pires et al., 2021). Endogenous β-galactosidase activity was exhibited in many species of Chlorophyceae, so it could be a potential candidate for cultivation in a lactose-containing medium (Li et al., 2023). In Egypt, the production of Domiati cheese was 326,000 tons in 2015, and its production increases annually by 2.4% (El Soda & Awad, 2022). According to de Almeida Pires et al. (2022), C. vulgaris can grow on cheese whey and efficiently consume carbon, nitrogen, and phosphorous from this medium. Moreover, Pereira et al. (2019) studied the mixotrophic cultivation of Spirulina platensis in Zarrouk’s medium diluted with different concentrations of mozzarella cheese whey. They found that biomass production, biochemical composition, and antioxidant capacity were positively affected.
In the context of emphasizing the huge increase in population, climate changes, and pollution which results in depletion of fresh water resources, a sustainable solution for a greener future is needed. Wastewater treatment by the use of microalgae can mitigate a part of the problem by restoring water for irrigating agricultural crops. The present study aims for a dual benefit in terms of wastewater treatment and a green biofuel production using an eco-friendly candidate. Using agro-industrial wastewater, specifically cheese whey, as an alternative culturing medium, a green microalga Desmodesmus sp. was cultivated. The physiological performance of Desmodesmus sp. and its ability to accumulate biomass with value-added products were evaluated to confirm the vital role of microalgae in the bioremediation of wastewaters and its potential to produce biofuel as an alternative source of energy.
2 Materials and Methods
2.1 Microalga Isolate and Growth Conditions
The green microalga Desmodesmus sp. was obtained from the Algal Biotechnology Unit, National Research Centre, Dokki, Giza, Egypt. An actively growing culture was maintained in Bold’s basal medium (BBM) according to Bischoff (1963) at pH 7.1, temperature 25 ± 2 °C, under continuous illumination of about 5000 lux provided by white fluorescent lamps, along the incubation period (7 days). Using a batch-mode culturing system for Desmodesmus sp., both experiments were performed using 1-l flasks. Aeration was performed through a sterilized air system using an air pump (power 5 W and max flow 3.5 l min−1) and a disposable syringe filter (0.45 μm).
2.2 Experimental Design
Domiati cheese is one of the most popular soft white pickled cheeses in Egypt. Its production was 326,000 tons in 2015 and increases annually by 2.4% (El Soda & Awad, 2022). Whey is the main by-product of the dairy industry which is produced during cheese manufacturing. Through two experimental stages, Domiati cheese whey was used as an alternative culturing medium in comparison to BBM growth medium as a control. CW was directly collected from the Dairy Technology Unit, Faculty of Agriculture, Cairo University, Egypt, and stored at −20 °C until use. The composition of cheese whey (CW) was determined by measuring the following parameters: color, pH, biological oxygen demand (BOD), chemical oxygen demand (COD), moisture, lactose, protein, fat, nitrogen, sodium, potassium, calcium, iron, magnesium, and phosphate (Table 1).
For stage I, four different concentrations of CW (i.e., 5, 10, 15, and 20 % v/v BBM medium) with a working volume of 700 ml were autoclaved at 121°C, 1.5 bar, for 20 min. Under this condition, all treatments were inoculated with 0.1 g l−1 of pre-washed Desmodesmus sp. then inoculated flasks were incubated for 14 days under optimum growth conditions as described above. At this stage, the best concentration of CW for Desmodesmus sp. growth will be selected.
Stage II: For enhancing Desmodesmus sp. growth, the best growth-promoting concentration of CW for Desmodesmus sp. was 15%, as resulted from stage I. In stage II, a combination of 15% CW and three different concentrations of BBM (25, 50, and 75 % v/v) were examined compared to the control (100% BBM). Autoclaving, inoculation with Desmodesmus sp., and incubation were carried out as previously described.
At a 2-day interval, using 3 replicates for each treatment, dry weight, photosynthetic pigments (Chl a, Chl b, and carotenoids), and pH were determined. Also, total protein, carbohydrates, and lipids were measured at 14 days of the incubation period. The trophic mode, in addition to the initial concentration for both nutrients (N, P, K, Na, Mg, and Fe) and lactose, is listed for all treatments in Table 2.
2.3 Analytical Method
2.3.1 Microalga Growth Determination and Media Reaction Measurement (pH)
The growth of microalga was determined through dry weight (DW) measurement, specific growth rate (μ), and biomass productivity. For dry weight, 10 ml of homogenized Desmodesmus sp. culture was filtered over a pre-weighed (w1) Sartorius cellulose nitrate sterile membrane filter (pore size 0.45 μm), and filters were dried at 105°C in a circulated oven (until getting constant weight). Dried filters were kept over anhydrous calcium chloride until room temperature and re-weighted (w2). The difference between weights (w1 and w2) indicated the net dry weight of the grown alga within a defined sampling time. At the exponential growth phase, Desmodesmus sp.–specific growth rate and biomass productivity were calculated according to Liang et al. (2013) as follows:
where N1 and N2 are the biomass at the initial time (T1) and final time (T2), respectively.
For further biochemical analysis, Desmodesmus sp. biomass was harvested by sedimentation and further centrifuged at 4000 rpm for 10 min. The obtained algal slurry was washed with distilled water to remove salts and then dried in an oven at 70°C overnight.
The growth media reaction (pH) of Desmodesmus sp. cultures was measured against BBM as a blank using a pH meter (HANNA Instruments pH 211).
2.3.2 Determination of Photosynthetic Pigments, Carbohydrates, Lipids, and Protein
Photosynthetic pigment content of Desmodesmus sp. was photometrically determined for chlorophyll a (Chl a), chlorophyll b (Chl b), and total carotenoids. The pigments were extracted using dimethyl sulfoxide (DMOS) according to Wellburn (1994) and calculated (μg ml−1) as the following equations:
For carbohydrate extraction, 0.1 g of dry biomass was hydrolyzed by 20 ml HCl (2.5M) at 100 °C for 30 min then neutralized to pH 7 by using Na2CO3. The volume was adjusted to 100 ml by distilled water and then filtered (Miao et al., 2004). Total carbohydrates were quantified using the phenol-sulfuric acid method (Dubois et al., 1956), using glucose as a standard. The spectrophotometric readings were performed at 490 nm. The extraction of lipids from dried biomass was performed according to the procedure of Bligh and Dyer (1959). The nitrogen (N) content was measured by the elemental CHNS analyzer of dried biomass equipped with a thermal conductivity detector (TCD). Protein contents were calculated from the N content using the conversion factor (4.78), which was used as the nitrogen-to-protein conversion factor instead of 6.25 according to Lourenço et al. (2004).
2.3.3 Fatty Acids Profile and Prediction of Biodiesel Properties
Algal lipids were converted to fatty acid methyl ester (FAME) using the methyl ester boron trifluoride method (AOAC, 1998). This method was implemented by adding sodium methoxide, boron trifluoride solution, and a boiling chip to the produced algal oil and boiled under reflux until the oil droplets disappear (5–10 min) then heptane was added to the mixture. Boiling was continued for 1 min to extract the FAME and then analyzed by using Shimadzu GC-2010, equipped with a flame ionization detector (FID). Calibration curves between peak area and concentration were established by injecting reference FAME samples of known concentrations into the GC-FID. The analysis occurred at the Regional Center for Food and Feed (RCFF), Agricultural Research Center, Giza, Egypt. By using computer software, predicted biodiesel properties based on a fatty acid profile were calculated (Biodiesel analyzer © ver.2.2.) (Talebi et al., 2014).
2.3.4 Ultrastructure Analysis of Desmodesmus sp. Using TEM
The ultrastructure of microalgae (Desmodesmus sp.) was studied using a transmission electron microscope (TEM) to know the effect of CW compared to control (BBM). Cells were harvested after 14 days and then fixed in a 2% (w/w) glutaraldehyde solution in 0.1 M sodium cacodylate buffer at room temperature for 0.5 h, and then post-fixed for 4 h in 1% (w/v) OsO4 in the same buffer. After dehydration through a graded ethanol series, the samples were embedded in an epoxy resin. Ultrathin sections were made with a Leica Ultracut UCT Ultramicrotome, stained with lead citrate and uranyl acetate according to Reynolds (1963), and examined under a Jeol JEM 1400 (JEOL, Tokyo, Japan) microscope in Cairo University Research Park (CURP).
2.4 Statistical Analysis
The mean values of this study, together with the standard deviation (SD) of three replicates, are reported in tables and figures. A two-way ANOVA was used to analyze the effect of CW on growth and photosynthetic pigments of Desmodesmus sp. and the pH of cultures, as well as the effect on biomass productivity, specific growth rate, and biochemical composition, which was analyzed by one-way ANOVA. The differences among means were compared by Duncan’s multiple range test as given by Snedecor and Cochran (1994) by using the Assistat program (Silva & Azevedo, 2009), and figures were generated using Prism version 8.0.2 (GraphPad Software, USA).
3 Results and Discussion
3.1 Effect of CW on Dry Weight, Specific Growth Rate, and Biomass Productivity
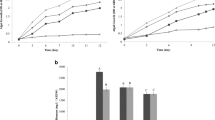
The results obtained from the growth curve of Desmodesmus sp., based on dry weight (Fig. 1), revealed that the mixotrophic mode (CW and CW+BBM) enhanced Desmodesmus sp. growth compared to the autotrophic mode. As shown in Fig. 1a, the increasing concentration of CW as an alternative culturing medium in stage I markedly induced algal growth along the incubation period. The maximum dry weight indicated in 15% CW (3.51g l−1) was 1.87 times higher than the control (1.22g l−1) after 14 days of incubation. Similarly, the specific growth rate (μ) and biomass productivity of Desmodesmus sp. in 15% CW (T4) were higher than in the control (T1) by 38.11% and 201.234%, respectively. Also, it is notable that increasing CW up to 20% caused a slight decrease in growth as compared to 10 and 15% CW, which might return to the high concentration of Na+ (Table 2). Salinity stress might cause growth inhibition because of high salt levels that cause ion toxicity, hyperosmotic stress, and secondary stresses such as oxidative damage (Fal et al., 2022; Qiao et al., 2021). Furthermore, high turbidity limits the amount of light that penetrates the growth medium, which in turn inhibits the growth of the microalgae (Chang et al., 2016).
Regarding the combination between CW and BBM, stage II, it was found that enriching the alternative media (CW) with BBM significantly induced Desmodesmus sp. growth as compared to the control (Fig. 1b). The best growth was achieved using 15% CW + 50% BBM (4.92 g l−1), which was three times higher than the control. Adding BBM to CW with more than 50% started to decrease algal growth significantly as compared to 15% CW + 50% BBM treatment.
It could be concluded that supplying Desmodesmus sp. with lactose as an external carbon source derived from CW positively affected metabolic pathways in such a way as to lead to more biomass accumulation. In this regard, a lot of studies found that the mixotrophic growth of microalgae on wastewater triggered biomass accumulation rather than autotrophic growth (Bonett et al., 2020; Gumbi et al., 2021). In mixotrophic growth, photosynthesis and respiration work together in green microalgae, which avoid the negative effect of oxidative stress resulting from O2 accumulation (Patel et al., 2019), and reuse C, N, P, and Fe, which are produced from respiration (Beardall & Raven, 2016). Girard et al. (2014) reported similar results when adding whey protein to BBM media for cultivating S. obliquus, resulting in 3.6 ± 0.4 g l−1 of dry weight after 13 days.
Calculating the specific growth rate (μ) of Desmodesmus sp. at stages I and II (Table 3) reflected that cheese whey could positively affect the rate of cell divisions and growth phases along the incubation period. Treatment with 15% CW and 50% BBM at stage II recorded the highest growth rate of Desmodesmus sp. In turn, the biomass productivity of Desmodesmus sp. significantly increased (Table 3), as compared to the control, due to the presence of CW in the culturing media. The high content of N, K, and lactose in CW (Table 1) could be a significant factor in inducing Desmodesmus sp. growth and productivity. So, combining CW with BBM, specifically 15% CW plus 50% BBM, causes a synergistic effect leading to vigor algal growth. The synergism arises from the high nutritional load of CW, along with the initial content of P, Mg, and Fe in BBM (Table 2).
3.2 Impact of CW on Media Reaction (pH)
In this study, the initial pH of the media for all treatments was set to 7.0 before inoculation with Desmodesmus sp. and kept uncontrolled during the 14 days of incubation. In autotrophic cultures (control), the pH increased to 10.74, by the fourth day. Then, it gradually levelled off until the 10th day, followed by a slight decrease from the 12th to the 14th day (Fig. 2a).
This trend is expected, because of the fixation of inorganic carbon during algal photosynthesis, which increased pH due to the formation of OH ions resulting from the release of oxygen molecules as a by-product of photosynthesis (Sarwer et al., 2022). In contrast, in mixotrophic cultures of stage I, the pH slightly increased up to 8 days of incubation, while the pH decrement occurred from day 10 until the end of the incubation period, as shown in Fig. 2a. In this regard, we attribute the reduction of pH in mixotrophic cultures to the degradation of soluble organic monomers into organic acids through the processes of acidogenesis and acetogenesis (Deng et al., 2019). In stage II of the experiment, the pH of mixotrophic cultures was increased gradually until the 8th day, followed by a slight decrement until the end of the incubation period (Fig. 2b). A concluding remark is that adding BBM to cheese whey cultures made the pH more stable than cheese whey alone.
3.3 Photosynthetic Pigments
Algal growth requires a healthy chloroplast structure and function; hence, the photosynthetic pigments are significant physiological indicators through which we can examine Desmodesmus sp. adaptation in different treatments of cheese whey as an alternative growth media. The photosynthetic pigment (Chl a, Chl b, and carotenoid) concentrations of Desmodesmus sp. at a 2-day interval during 14 days of growth in different concentrations of cheese whey was illustrated in Fig. 3. The pigment concentrations of all mixotrophic cultures were lower than the photoautotrophic control (BBM) Chl a (18.14 μg ml−1), Chl b (7.72 μg ml−1), and carotenoids (4.07 μg ml−1). Because Chl a is the major pigment for light harvesting (converting light energy to chemical energy) and Chl b is an accessory pigment, the values of Chl a in microalgae were greater than that of Chl b (Tang et al., 2022). Several studies found that acetone was found to be excellent in extracting chlorophyll from microalgae (Tang et al., 2023).
Effect of different concentrations of CW (5, 10, 15, and 20%) as well as control (stage I) on chlorophyll a (a), chlorophyll b (b), and carotenoids (c) of Desmodesmus sp. incubated for different intervals (2 days). T1, BBM (control); T6, 15% CW + 25% BBM; T7, 15% CW + 50% BBM; T8, 15% CW + 75% BBM. Data are presented as means ± SD (n = 3) with significantly different letters (a-t) according to Duncan’s multiple range at P < 0.05
These results indicate that the addition of cheese whey inhibited photosynthesis. Whereas under mixotrophic conditions, algal cells are able to hydrolyze lactose into glucose and galactose by producing extracellular β-galactosidase (Li et al., 2023). Deng et al. (2019) reported that adding glucose to algal culture caused a great reduction in Chl a content which was attributed to its impact on Chl a biosynthesis through inhibition of coproporphyrinogen III-oxidase, which forms protoporphyrinogen IX from coproporphyrinogen III (Stadnichuk et al., 1998). Kong et al. (2020) studied the effects of various carbon sources on Chlorella vulgaris 31 and reported that organic carbon sources inhibited photosynthesis and promoted respiration. de Almeida Pires et al. (2022) found that the cultivation of C. vulgaris on BBM with 1% CW increased chlorophyll value by 100–132% compared to BBM, but high concentrations of CW harm the pigment accumulation.
Not only lactose but also nutrient limitations, especially N, PO4, Mg, and Fe, affect photosynthetic pigment synthesis, according to the data in Table 2. The treatment of 5% CW contains the lowest level of nutrients, negatively affecting chlorophyll synthesis so T2 obtained a yellow culture after 14 days of incubation (Fig. 5). Iron and magnesium are considered essential elements for the photosynthesis pigments in algae (Ermis et al., 2020). In this regard, Xing et al. (2007) reported that Fe+2 limitation causes a simultaneous loss of chlorophyll and degeneration of chlorophyll structure because iron is associated with proteins and enzymes, including photosynthesis (e.g., cytochrome, ferredoxins, NADP+ reductase), cellular respiration (e.g., Aconitase), nitrogen assimilation, and vitamin synthesis (Marchetti & Maldonado, 2016), where magnesium is a vital metal for algal growth because it has a critical position in the porphyrin ring (chlorophyll molecule) and controls the activity of several photosynthetic enzymes (Li et al., 2023).
Carotenoid concentrations are higher in photoautotrophic cultures than in mixotrophic cultures (Figs. 4 and 5c). These findings are consistent with those of Abreu et al. (2012), who also cultivated Chlorella vulgaris using hydrolyzed cheese whey powder solution as mixotrophic cultures in comparison to photoautotrophic ones. The light-harvesting complex of photosynthesis is linked to carotenoids in photosynthetic organisms, which are known for their photoprotective properties (Castillo et al., 2021). The high ratio of carotenoids to total chlorophyll indicates nutritional stress (nutrient limitation) and photo-oxidation, as reviewed by Wong et al. (2017).
Effect of 15% CW supplemented with different concentrations of BBM (25, 50, and 75 %) as well as control (stage II) on chlorophyll a (a), chlorophyll b (b) and carotenoids (c) of Desmodesmus sp. incubated for different intervals (2 days). T1, BBM (control); T6, 15% CW + 25% BBM; T7, 15% CW + 50% BBM; T8, 15% CW + 75% BBM. Data are presented as means ± SD (n = 3) with significantly different letters (a-q) according to Duncan’s multiple range at P < 0.05
Our findings showed that by increasing CW concentrations (stage I) the pigment concentrations were increased significantly (p < 0.05) with increasing CW concentrations to 15% and combining BBM with 15% CW decreases the negative effect of nutrient limitation in CW and enhances algal growth and pigment synthesis, as shown in Figs. 4 and 5.
3.4 Biochemical Composition
Carbohydrate, protein, and lipid concentrations of a stationary phase–harvested algal biomass after 14 days of incubation, as affected by different treatments compared to control, are summarized in Table 4. At stage I, the different concentrations of CW, as compared to control, significantly affected the carbohydrates, proteins, and lipids of Desmodesmus sp., and based on dry weight, in a different manner.
The highest carbohydrates and lipid concentrations were respectively obtained upon cultivating Desmodesmus sp. with 10 and 20% CW, as compared to control (Table 4). On the other hand, protein concentration significantly decreased with all CW concentrations as compared to control. Accordingly, it seems that autotrophic culturing mode enhances protein assimilation in Desmodesmus sp. as compared to mixotrophic mode, which suppresses it. In this regard, Wang, Wang, Tao, et al. (2018) reported that adding an organic carbon source to the culturing medium decreased protein content in Chlorella spp. as compared to autotrophic cultures.
Additionally, Desmodesmus sp. accumulated more lipids at 20% CW, and this could be due to the high content of Na+ in CW as well as protein biosynthesis retardation (Table 2). This result is consistent with Gour et al. (2020) who stated that increasing salinity enhances lipid content and productivity. Furthermore, Wang et al. (2016) reported that the high C/N ratio in growth media increases the lipid content in microalgae.
Since microalgae accumulate more lipids upon exposure to environmental stress such as salinity and nutrient limitation (Sun et al., 2018), therefore, the highest concentration of CW in this study caused the highest lipid accumulation in Desmodesmus sp. This could be explained by a synergistic effect between high Na+ concentrations in CW and nutrient limitation at the harvesting phase (Khatoon et al., 2017).
What can be concluded is that starting nutrient limitation at the stationary phase of Desmodesmus sp. growth led to limited assimilation of protein, although carbohydrates and lipid accumulation increased. In consonance, Zhu et al. (1997) reported that the assimilation of carbohydrates and neutral lipids increased at the early stationary phase of Isochrysis galbana while protein, phospholipids, and glycolipids decreased.
Fortifying CW with different concentrations of BBM in stage II, as indicated in Table 4, revealed that increasing BBM concentration by 25% and 50% significantly increased lipids and carbohydrates in Desmodesmus sp., respectively, as compared to control. On the other hand, 75% BBM significantly decreased carbohydrate concentration and non-significantly affected protein and lipids, all compared to control. In general, protein concentrations in Desmodesmus sp. gradually increased with increasing BBM concentrations. In contrast, lipid concentration gradually decreased by increasing BBM concentration, both as compared to control.
These obtained results were consistent with the transmission electron micrographs, as indicated in Fig. 6. Supplying 15% CW with 50% BBM affected the ultrastructure of Desmodesmus sp. in terms of starch granules and oil bodies. It is obvious that starch granules occupied most of the Desmodesmus sp. cells as compared to the control. Also, oil bodies were larger and denser as compared to controls. Peng et al. (2019) reported that mixotrophic cultivation by adding glucose and sodium acetate to wastewater enhanced the accumulation of lipids and carbohydrates in C. vulgaris. As shown in Fig. 6, the pyrenoid of Desmodesmus sp. grown on BBM is larger than that grown on 15% CW + 50% BBM (T7). This finding is similar to that of Yang et al. (2018), who found that Scenedesmus sp. pyrenoid became smaller under phosphorus stress, which improved lipid accumulation.
3.5 Fatty Acids Profiling and Biodiesel Parameters Calculation
The fatty acid composition of Desmodesmus sp. grown in 15% CW + 50% BBM (T7) for 14 days is listed in Table 5, and the data were expressed as relative percentages. The obtained results showed that Desmodesmus sp. mainly produced high amounts of palmitic acid (25.86%), oleic acid (35.31%), and linoleic acid (13.22%), as saturated, monounsaturated, and polyunsaturated fatty acids, respectively. The highest fatty acid was oleic acid (C18:1ω9) which agrees with the results of Chen et al. (2020) and Hu et al. (2013). Gogna et al. (2020) correlated the increase of oleic acid and reduction of linoleic acid to the negative effect of toxic ions (Na+ and Cl−) on Δ12 desaturase (oleate desaturase). Δ12 desaturase is an enzyme that synthesizes linoleic acid from oleic acid by adding a double bond at position 12 (Ghassemi-Golezani & Farhangi-Abriz, 2018; Gogna et al., 2020).
Fatty acid compositions highly affect biodiesel properties (Khethiwe et al., 2020; Salah et al., 2023). As shown in Table 5, Desmodesmus sp. contents of saturated, monounsaturated, and polyunsaturated were 39.01, 39.23, and 21.76%, respectively. The majority of fatty acids in Desmodesmus sp. are short-chain fatty acids (C14–C18), which are the dominant components of biodiesel (Choi, 2016). Data in Table 6 shows the predicted biodiesel properties resulting from Desmodesmus sp. grown in 15% CW + 50% BBM (T7) as culture media, with comparison to the American Society of Testing and Materials (ASTM) D6751 and the European (EN) 14214 standards. The characterization of biodiesel produced from Desmodesmus sp. was in the normal range of the ASTM-D6751 and EN 14214 standards, which revealed excellent suitability for biodiesel production.
4 Conclusion
The growth curve of Desmodesmus sp. based on dry weight during 14 days of incubation revealed that the mixotrophic mode (CW and CW+BBM) enhanced algal growth compared to the autotrophic mode (BBM). The best growth was achieved using 15% CW + 50% BBM and was three times higher than BBM (control). In mixotrophic growth, photosynthesis and respiration function together in green microalgae which avoid the negative effect of oxidative stress resulting from O2 accumulation and reuse of C, N, P, and Fe that are produced from respiration. Microalgal cultures grown on CW and CW+BBM accumulated fewer concentrations of chlorophyll a, chlorophyll b, and carotenoids compared to cultures grown on BBM. The highest carbohydrate and lipid concentrations were respectively obtained upon cultivating Desmodesmus sp. with 10 and 20% CW, as compared to BBM (control). On the other hand, protein concentration significantly decreased with all CW concentrations as compared to BBM (control). Protein concentrations in Desmodesmus sp. gradually increased with increasing BBM concentrations. In contrast, lipid concentration gradually decreased by increasing BBM concentration compared to BBM (control). Transmission electron micrographs of Desmodesmus sp. show that cultivation of alga on 15% CW + 50% BBM enhanced the accumulation of starch granules and oil bodies in algal cells more than control. Based on fatty acid composition of Desmodesmus sp. grown on 15% CW combined with 50% BBM, palmitic (25.86%), oleic (35.31%), and linoleic acid (13.22%) were the most abundant fatty acids. So, the produced algae have a significant amount of short-chain fatty acids (C14–C18) which make up the majority of biodiesel. In conclusion, the highly accumulated biomass of Desmodesmus sp., along with the high contents of carbohydrates and lipids, makes this strategy a pioneer for phycoremediation and biofuel production and also, the potential application of CW can help reduce the dairy industry’s negative impacts on the environment.
Data Availability
All data generated or analyzed during this study are included in this published article.
References
Abd El-Hameed, M. M., Abuarab, M. E., Mottaleb, S. A., El-Bahbohy, R. M., & Bakeer, G. A. (2018). Comparative studies on growth and Pb (II) removal from aqueous solution by Nostoc muscorum and Anabaena variabilis. Ecotoxicology and Environmental Safety, 165, 637–644.
Abdelfattah, A., Ali, S. S., Ramadan, H., El-Aswar, E. I., Eltawab, R., Ho, S. H., Elsamahy, T., Li, S., El-Sheekh, M. M., Schagerl, M., & Kornaros, M. (2023). Microalgae-based wastewater treatment: Mechanisms, challenges, recent advances, and future prospects. Environmental Science and Ecotechnology, 13, 100205. https://doi.org/10.1016/j.ese.2022.100205
Abreu, A. P., Fernandes, B., Vicente, A. A., Teixeira, J., & Dragone, G. (2012). Mixotrophic cultivation of Chlorella vulgaris using industrial dairy waste as organic carbon source. Bioresource Technology, 118, 61–66.
Ahmad, T., Aadil, R. M., Ahmed, H., Rahman, U. U., Soares, B. C. V., Souza, S. L. Q., Pimentel, T. C., Scudino, H., Guimarães, J. T., Esmerino, E. A., Freitas, M. Q., Almada, R. B., Vendramel, S. M. R., Silva, M. C., & Cruz, A. G. (2019). Treatment and utilization of dairy industrial waste: A review. Trends in Food Science and Technology, 88, 361–372. https://doi.org/10.1016/j.tifs.2019.04.003
Almanassra, I. W., Mckay, G., Kochkodan, V., Ali Atieh, M., & Al-Ansari, T. (2021). A state of the art review on phosphate removal from water by biochars. Chemical Engineering Journal, 409, 128211. https://doi.org/10.1016/j.cej.2020.128211
Ansari, F. A., Guldhe, A., Gupta, S. K., Rawat, I., & Bux, F. (2021). Improving the feasibility of aquaculture feed by using microalgae. Environmental Science and Pollution Research, 28(32), 43234–43257.
AOAC, A., (1998). Fatty acids in oils and fats. Preparation of methyl esters. Boron trifluoride method. Official Method 969.33. Rockville: AOAC International.
Awad, H., Gar Alalm, M., & El-Etriby, H. K. (2019). Environmental and cost life cycle assessment of different alternatives for improvement of wastewater treatment plants in developing countries. Science of The Total Environment, 660, 57–68. https://doi.org/10.1016/j.scitotenv.2018.12.386
Bali, M., & Gueddari, M. (2019). Removal of phosphorus from secondary effluents using infiltration–percolation process. Applied Water Science, 9(3), 54. https://doi.org/10.1007/s13201-019-0945-5
Barone, V., Puglisi, I., Fragalà, F., Lo Piero, A. R., Giuffrida, F., & Baglieri, A. (2019). Novel bioprocess for the cultivation of microalgae in hydroponic growing system of tomato plants. Journal of Applied Phycology, 31(1), 465–470.
Baskar, A. V., Bolan, N., Hoang, S. A., Sooriyakumar, P., Kumar, M., Singh, L., Jasemizad, T., Padhye, L. P., Singh, G., Vinu, A., Sarkar, B., Kirkham, M. B., Rinklebe, J., Wang, S., Wang, H., Balasubramanian, R., & Siddique, K. H. M. (2022). Recovery, regeneration and sustainable management of spent adsorbents from wastewater treatment streams: A review. Science of The Total Environment, 822, 153555. https://doi.org/10.1016/j.scitotenv.2022.153555
Beardall, J., & Raven, J. A. (2016). Carbon acquisition by microalgae. In The physiology of microalgae (pp. 89–99). Springer.
Bhatt, P., Gangola, S., Bhandari, G., Zhang, W., Maithani, D., Mishra, S., & Chen, S. (2021). New insights into the degradation of synthetic pollutants in contaminated environments. Chemosphere, 268, 128827.
Bischoff, H. W. (1963). Phycological studies. IV. Some algae from enchanted rock and related algal species (Vol. 6318, p. 95). University of Texas Publication.
Bligh, E. G., & Dyer, W. J. (1959). A rapid method of total lipid extraction and purification. Canadian Journal of Biochemistry and Physiology, 37(8), 911–917.
Bonett, J. E. A., de Sousa Geraldino, P., Cardoso, P. G., de Freitas Coelho, F., & Duarte, W. F. (2020). Isolation of freshwater microalgae and outdoor cultivation using cheese whey as substrate. Biocatalysis and Agricultural Biotechnology, 29, 101799.
Brar, A., Kumar, M., & Pareek, N. (2019). Comparative appraisal of biomass production, remediation, and bioenergy generation potential of microalgae in dairy wastewater. Frontiers in Microbiology, 10, 443920. https://doi.org/10.3389/fmicb.2019.00678
Carvalho, F., Prazeres, A. R., & Rivas, J. (2013). Cheese whey wastewater: Characterization and treatment. Science of the Total Environment, 445–446, 385–396. https://doi.org/10.1016/j.scitotenv.2012.12.038
Castillo, T., Ramos, D., García-Beltrán, T., Brito-Bazan, M., & Galindo, E. (2021). Mixotrophic cultivation of microalgae: An alternative to produce high-value metabolites. Biochemical Engineering Journal, 176). Elsevier B.V. https://doi.org/10.1016/j.bej.2021.108183
Chang, H. X., Fu, Q., Huang, Y., Xia, A., Liao, Q., Zhu, X., Zheng, Y. P., & Sun, C. H. (2016). An annular photobioreactor with ion-exchange-membrane for non-touch microalgae cultivation with wastewater. Bioresource Technology, 219, 668–676.
Chen, Z., Shao, S., He, Y., Luo, Q., Zheng, M., Zheng, M., Chen, B., & Wang, M. (2020). Nutrients removal from piggery wastewater coupled to lipid production by a newly isolated self-flocculating microalga Desmodesmus sp. PW1. Bioresource Technology, 302, 122806. https://doi.org/10.1016/j.biortech.2020.122806
Chiu, P. H., Soong, K., & Chen, C. N. N. (2016). Cultivation of two thermotolerant microalgae under tropical conditions: Influences of carbon sources and light duration on biomass and lutein productivity in four seasons. Bioresource Technology, 212, 190–198.
Choi, H. J. (2016). Dairy wastewater treatment using microalgae for potential biodiesel application. Environmental Engineering Research, 21(4), 393–400.
Dawood, M. F. A., Sofy, M. R., Mohamed, H. I., Sofy, A. R., & Abdelkader, H. A. A. (2023). N- or/and P-deprived Coccomyxa chodatii SAG 216–2 extracts instigated mercury tolerance of germinated wheat seedlings. Plant Soil, 483, 225–253. https://doi.org/10.1007/s11104-022-05732-7
de Almeida Pires, T., Cardoso, V. L., & Batista, F. R. X. (2022). Feasibility of Chlorella vulgaris to waste products removal from cheese whey. International Journal of Environmental Science and Technology, 19, 4713–4722. https://doi.org/10.1007/s13762-021-03423-x
de Medeiros, V. P. B., Pimentel, T. C., Varandas, R. C. R., Dos Santos, S. A., de Souza Pedrosa, G. T., da Costa Sassi, C. F., da Conceição, M. M., & Magnani, M. (2020). Exploiting the use of agro-industrial residues from fruit and vegetables as alternative microalgae culture medium. Food Research International, 137, 109722.
Deng, X., Xue, C., Chen, B., Amoah, P. K., Li, D., Hu, X., & Gao, K. (2019). Glucose addition-induced changes in the growth and chemical compositions of a freshwater microalga Chlorella kessleri. Journal of Chemical Technology & Biotechnology, 94(4), 1202–1209.
Dubois, M., Gilles, K. A., Hamilton, J. K., Rebers, P. A. T., & Smith, F. (1956). Colorimetric method for determination of sugars and related substances. Analytical Chemistry, 28(3), 350–356.
El-Beltagi, H. S., Mohamed, A. A., Mohamed, H. I., Ramadan, K. M. A., Barqawi, A. A., & Mansour, A. T. (2022). Phytochemical and potential properties of seaweeds and their recent applications: A review. Marine Drugs, 20(6), 342. https://doi.org/10.3390/md20060342
El-Mahdy, O. M., Mohamed, H. I., & Mogazy, A. M. (2021). Biosorption effect of Aspergillus niger and Penicillium chrysosporium for Cd- and Pb-contaminated soil and their physiological effects on Vicia faba L. Environmental Science Pollution Research, 28, 67608–67631. https://doi.org/10.1007/s11356-021-15382-4
El-Sayed, A. B., Fetyan, N. A. H., Ibrahim, F., Fayed, A., & S., & Sadik, M. W. (2020). Application of bagasse extract in economic Nannochloropsisoculata mass production. Egyptian Journal of Chemistry, 63(12), 5183–5192.
El-Sayed, A. E.-K. B., Fetyan, N. A., Moghanm, F. S., Elbagory, M., Ibrahim, F. M., Sadik, M. W., & Shokr, M. S. (2022). Biomass fatty acid profile and fuel property prediction of bagasse waste grown Nannochloropsis oculata. Agriculture, 12(8), 1201.
El Soda, M., & Awad, S. (2022). Cheeses matured in brine. In P. L. H. McSweeney & J. P. McNamara (Eds.), Encyclopedia of dairy sciences ((third edition) ed., pp. 131–136). Academic Press. https://doi.org/10.1016/B978-0-12-818766-1.00156-2
Ermis, H., Guven-Gulhan, U., Cakir, T., & Altinbas, M. (2020). Effect of iron and magnesium addition on population dynamics and high value product of microalgae grown in anaerobic liquid digestate. Scientific Reports, 10(1), 1–12.
Food and Agriculture Organization of the United Nations (F.A.O.) (2017) Water for sustainable food and agriculture—A report produced for the G20 presidency of Germany. http://www.fao.org/3/i7959e/i7959e.pdf
Fal, S., Aasfar, A., Rabie, R., Smouni, A., & Arroussi, H. E. L. (2022). Salt induced oxidative stress alters physiological, biochemical and metabolomic responses of green microalga Chlamydomonas reinhardtii. Heliyon, 8(1), e08811.
Farooq, W., Suh, W. I., Park, M. S., & Yang, J. W. (2015). Water use and its recycling in microalgae cultivation for biofuel application. Bioresource Technology, 184, 73–81. https://doi.org/10.1016/j.biortech.2014.10.140
Ferreira, G. F., Ríos Pinto, L. F., Maciel Filho, R., & Fregolente, L. V. (2021). Effects of cultivation conditions on Chlorella vulgaris and Desmodesmus sp. grown in sugarcane agro-industry residues. Bioresource Technology, 342, 125949. https://doi.org/10.1016/j.biortech.2021.125949
Fetyan, N. A. H., El-Sayed, A. E.-K. B., Ibrahim, F. M., Attia, Y. A., & Sadik, M. W. (2022). Bioethanol production from defatted biomass of Nannochloropsis oculata microalgae grown under mixotrophic conditions. Environmental Science and Pollution Research, 29(2), 2588–2597.
Ghassemi-Golezani, K., & Farhangi-Abriz, S. (2018). Changes in oil accumulation and fatty acid composition of soybean seeds under salt stress in response to salicylic acid and jasmonic acid. Russian Journal of Plant Physiology, 65(2), 229–236.
Giovanardi, M., Ferroni, L., Baldisserotto, C., Tedeschi, P., Maietti, A., Pantaleoni, L., & Pancaldi, S. (2013). Morphophysiological analyses of Neochloris oleoabundans (Chlorophyta) grown mixotrophically in a carbon-rich waste product. Protoplasma, 250(1), 161–174.
Girard, J. M., Roy, M. L., Hafsa, M. B., Gagnon, J., Faucheux, N., Heitz, M., Tremblay, R., & Deschênes, J. S. (2014). Mixotrophic cultivation of green microalgae Scenedesmus obliquus on cheese whey permeate for biodiesel production. Algal Research, 5, 241–248.
Gogna, M., Choudhary, A., Mishra, G., Kapoor, R., & Bhatla, S. C. (2020). Changes in lipid composition in response to salt stress and its possible interaction with intracellular Na+-K+ ratio in sunflower (Helianthus annuus L.). Environmental and Experimental Botany, 178, 104147.
Gour, R. S., Garlapati, V. K., & Kant, A. (2020). Effect of salinity stress on lipid accumulation in Scenedesmus sp. and Chlorella sp.: Feasibility of stepwise culturing. Current Microbiology, 77(5), 779–785.
Goyal, S., Dhanker, R., Hussain, T., Ferreira, A., Gouveia, L., Kumar, K., & Mohamed, H. I. (2023). Modern advancement in biotechnological applications for wastewater treatment through microalgae: A review. Water, Air, Soil Pollution, 234(7), 417. https://doi.org/10.1007/s11270-023-06409-2
Gumbi, S. T., Mutanda, T., & Olaniran, A. O. (2021). Nutrient removal from dairy and poultry wastewater with simultaneous biomass and biodiesel production by Chlorella sp. T4 isolated from a freshwater stream in South Africa. Waste and Biomass Valorization, 12(12), 6931–6943.
Hu, G., Fan, Y., Zhang, L., Yuan, C., Wang, J., Li, W., Hu, Q., & Li, F. (2013). Enhanced lipid productivity and photosynthesis efficiency in a Desmodesmus sp. mutant induced by heavy carbon ions. PloS One, 8(4), e60700.
Kamal, S., El-Sayed, A. B., Hassan, A. A., El-Shazly, H. A. M., & Ibrahim, M. T. (2017). Use of okara waste for algae nutrition. Arab Universities Journal of Agricultural Sciences, 25(2), 271–279.
Kamali, M., Persson, K. M., Costa, M. E., & Capela, I. (2019). Sustainability criteria for assessing nanotechnology applicability in industrial wastewater treatment: Current status and future outlook. Environment International, 125, 261–276. https://doi.org/10.1016/j.envint.2019.01.055
Kamyab, H., Chelliapan, S., Lee, C. T., Khademi, T., Kumar, A., Yadav, K. K., Rezania, S., Kumar, S., & Ebrahimi, S. S. (2019). Improved production of lipid contents by cultivating Chlorella pyrenoidosa in heterogeneous organic substrates. Clean Technologies and Environmental Policy, 21(10), 1969–1978.
Karadag, D., Köroğlu, O. E., Ozkaya, B., & Cakmakci, M. (2015). A review on anaerobic biofilm reactors for the treatment of dairy industry wastewater. Process Biochemistry, 50(2), 262–271.
Khatoon, H., Haris, N., Banerjee, S., Rahman, N. A., Begum, H., Mian, S., Abol-Munafi, A. B., & Endut, A. (2017). Effects of different salinities on the growth and proximate composition of Dunaliella sp. isolated from South China Sea at different growth phases. Process Safety and Environmental Protection, 112, 280–287.
Khethiwe, E., Clever, K., & Jerekias, G. (2020). Effects of fatty acids composition on fuel properties of Jatropha curcas biodiesel. Smart Grid and Renewable Energy, 11(10), 165.
Kılıç, Z. (2020). The importance of water and conscious use of water. International Journal of Hydrology, 4(5), 239–241.
Kim, K., Yoon, Y., Cho, H., & Hwang, S. J. (2020). Molecular probes to evaluate the synthesis and production potential of an odorous compound (2-methylisoborneol) in cyanobacteria. International Journal of Environmental Research and Public Health, 17(6), 1933.
Kong, W., Yang, S., Wang, H., Huo, H., Guo, B., Liu, N., Zhang, A., & Niu, S. (2020). Regulation of biomass, pigments, and lipid production by Chlorella vulgaris 31 through controlling trophic modes and carbon sources. Journal of Applied Phycology, 32(3), 1569–1579. https://doi.org/10.1007/s10811-020-02089-1
Li, Y., Miros, S., Kiani, H., Eckhardt, H. G., Blanco, A., Mulcahy, S., McDonnell, H., Tiwari, B. K., & Halim, R. (2023). Mechanism of lactose assimilation in microalgae for the bioremediation of dairy processing side-streams and co-production of valuable food products. Journal of Applied Phycology, 35(4), 1649–1661. https://doi.org/10.1007/s10811-023-03002-2
Liang, F., Wen, X., Geng, Y., Ouyang, Z., Luo, L., & Li, Y. (2013). Growth rate and biomass productivity of Chlorella as affected by culture depth and cell density in an open circular photobioreactor. Journal of Microbiology and Biotechnology, 23(4), 539–544.
Lourenço, S. O., Barbarino, E., Lavín, P. L., Lanfer Marquez, U. M., & Aidar, E. (2004). Distribution of intracellular nitrogen in marine microalgae: Calculation of new nitrogen-to-protein conversion factors. European Journal of Phycology, 39(1), 17–32.
Madakka, M., Jayaraju, N., Rajesh, N., & Chandra, M. R. G. S. (2019). Development in the treatment of municipal and industrial wastewater by microorganism. In Recent developments in applied microbiology and biochemistry (pp. 263–273). Elsevier.
Marchetti, A., & Maldonado, M. T. (2016). Iron. In M. A. Borowitzka, J. Beardall, & J. A. Raven (Eds.), The physiology of microalgae (pp. 233–279). Springer International Publishing. https://doi.org/10.1007/978-3-319-24945-2_11
Matei, A., & Racoviteanu, G. (2021). Review of the technologies for nitrates removal from water intended for human consumption. IOP Conference Series: Earth and Environmental Science, 664(1), 012024. https://doi.org/10.1088/1755-1315/664/1/012024
Miao, X., Wu, Q., & Yang, C. (2004). Fast pyrolysis of microalgae to produce renewable fuels. Journal of Analytical and Applied Pyrolysis, 71(2), 855–863. https://doi.org/10.1016/j.jaap.2003.11.004
Muhammad, G., Alam, M. A., Xiong, W., Lv, Y., & Xu, J. L. (2020). Microalgae biomass production: An overview of dynamic operational methods. In M. A. Alam, J.-L. Xu, & Z. Wang (Eds.), Microalgae biotechnology for food, health and high value products (pp. 415–432). https://doi.org/10.1007/978-981-15-0169-2_13
Nogueira Junior, E., Kumar, M., Pankratz, S., Oyedun, A. O., & Kumar, A. (2018). Development of life cycle water footprints for the production of fuels and chemicals from algae biomass. Water Research, 140, 311–322. https://doi.org/10.1016/j.watres.2018.04.046
Patel, A. K., Joun, J. M., Hong, M. E., & Sim, S. J. (2019). Effect of light conditions on mixotrophic cultivation of green microalgae. Bioresource Technology, 282, 245–253.
Peng, Y., Gao, F., Hang, W. W., Yang, H., Jin, W., & Li, C. (2019). Effects of organic matters in domestic wastewater on lipid/carbohydrate production and nutrient removal of Chlorella vulgaris cultivated under mixotrophic growth conditions. Journal of Chemical Technology & Biotechnology, 94(11), 3578–3584.
Pereira, M. I. B., Chagas, B. M. E., Sassi, R., Medeiros, G. F., Aguiar, E. M., Borba, L. H. F., Silva, E. P. E., Neto, J. C. A., & Rangel, A. H. N. (2019). Mixotrophic cultivation of Spirulina platensis in dairy wastewater: Effects on the production of biomass, biochemical composition and antioxidant capacity. PloS One, 14(10), e0224294.
Pires, A. F., Marnotes, N. G., Rubio, O. D., Garcia, A. C., & Pereira, C. D. (2021). Dairy by-products: A review on the valorization of whey and second cheese whey. Foods, 10(5), 1067. https://doi.org/10.3390/foods10051067
Qiao, T., Zhao, Y., Zhong, D., & Yu, X. (2021). Hydrogen peroxide and salinity stress act synergistically to enhance lipids production in microalga by regulating reactive oxygen species and calcium. Algal Research, 53, 102017.
Reynolds, E. S. (1963). The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. The Journal of Cell Biology, 17(1), 208.
Ribeiro, J. E. S., Martini, M., Altomonte, I., Salari, F., Nardoni, S., Sorce, C., da Silva, F. L. H., & Andreucci, A. (2017). Production of Chlorella protothecoides biomass, chlorophyll and carotenoids using the dairy industry by-product scotta as a substrate. Biocatalysis and Agricultural Biotechnology, 11, 207–213.
Ríos, L. F., Martinez, A., Klein, B. C., Maciel, M. R. W., & Filho, R. M. (2018). Comparison of growth and lipid accumulation at three different growth regimes with Desmodesmus sp. Waste and Biomass Valorization, 9, 421–427.
Rodríguez-Rángel, H., Arias, D. M., Morales-Rosales, L. A., Gonzalez-Huitron, V., Valenzuela Partida, M., & García, J. (2022). Machine learning methods modeling carbohydrate-enriched cyanobacteria biomass production in wastewater treatment systems. Energies, 15(7), 2500.
Salah, A., Sany, H., El-Khair B. El-Sayed A., M El-Bahbohy, R., I. Mohamed H., & Amin, A., (2023). The impact of inoculum preparation media on pollutant removal through phycoremediation of agricultural drainage water by Desmodesmus sp. Phyton, 0(0), 1–16. https://doi.org/10.32604/phyton.2023.031064
Santana, H., Cereijo, C. R., Teles, V. C., Nascimento, R. C., Fernandes, M. S., Brunale, P., Campanha, R. C., Soares, I. P., Silva, F. C. P., & Sabaini, P. S. (2017). Microalgae cultivation in sugarcane vinasse: Selection, growth and biochemical characterization. Bioresource Technology, 228, 133–140.
Sarwer, A., Hamed, S. M., Osman, A. I., Jamil, F., Al-Muhtaseb, A. H., Alhajeri, N. S., & Rooney, D. W., (2022). Algal biomass valorization for biofuel production and carbon sequestration: A review. In Environmental Chemistry Letters (20, 5,. 2797–2851). Springer Science and Business Media Deutschland GmbH. https://doi.org/10.1007/s10311-022-01458-1
Silva, F. A. S., & Azevedo, C. A. V. (2009). Principal components analysis in the software Assistat-statistical attendance. World Congress on Computers in Agriculture, 7, 22–24.
Silva, S. C., Ferreira, I. C. F. R., Dias, M. M., & Barreiro, M. F. (2020). Microalgae-derived pigments: A 10-year bibliometric review and industry and market trend analysis. Molecules, 25(15), 3406.
Snedecor, G. W., & Cochran, W. G. (1994). Statistical methods (8th ed.). IOWA State University Press.
Sofy, M., Mohamed, H., Dawood, M., Abu Elsaoud, A., & Soliman, M. (2022). Integrated usage of Trichoderma harzianum and biochar to ameliorate salt stress on spinach plants. Archives of Agronomy and Soil Science, 68(14), 2005–2026. https://doi.org/10.1080/03650340.2021.1949709
Sonarghare, P. C., Masram, S. C., Sonparote, U. R., Khaparde, K. P., & Kharkate, S. K. (2020). Causes and effects of eutrophication on aquatic life (a review). International Journal for Environmental Rehabilitation and Conservation, XI(SP2), 213–218.
Stadnichuk, I. N., Rakhimberdieva, M. G., Bolychevtseva, Y., & v, Yurina, N. P., Karapetyan, N. v, & Selyakh, I. O. (1998). Inhibition by glucose of chlorophyll a and phycocyanobilin biosynthesis in the unicellular red alga Galdieria partita at the stage of coproporphyrinogen III formation. Plant Science, 136(1), 11–23.
Su, M., Dell’Orto, M., Scaglia, B., D’Imporzano, G., & Adani, F. (2022). Growth performance and biochemical composition of waste-isolated microalgae consortia grown on nano-filtered pig slurry and cheese whey under mixotrophic conditions. Fermentation, 8, 474. https://doi.org/10.3390/fermentation8100474
Sun, X. M., Ren, L. J., Zhao, Q. Y., Ji, X. J., & Huang, H. (2018). Microalgae for the production of lipid and carotenoids: A review with focus on stress regulation and adaptation. Biotechnology for Biofuels, 11, 272. https://doi.org/10.1186/s13068-018-1275-9
Talebi, A. F., Tabatabaei, M., & Chisti, Y. (2014). BiodieselAnalyzer: A user-friendly software for predicting the properties of prospective biodiesel. Biofuel Research Journal, 1(2), 55–57.
Tan, K. Y., Low, S. S., Manickam, S., Ma, Z., Banat, F., Munawaroh, H. S. H., & Show, P. L. (2023). Prospects of microalgae in nutraceuticals production with nanotechnology applications. Food Research International, 169, 112870. https://doi.org/10.1016/j.foodres.2023.112870
Tang, D. Y. Y., Chew, K. W., Chia, S. R., Ting, H. Y., Sia, Y. H., Gentili, F. G., Ma, Z., Awasthi, M. K., & Show, P. L. (2022). Triphasic partitioning of mixed Scenedesmus and Desmodesmus for nutrients’ extraction and chlorophyll composition prediction for algae bloom. Environmental Technology (United Kingdom). https://doi.org/10.1080/09593330.2022.2150094
Tang, D. Y. Y., Chew, K. W., Ting, H. Y., Sia, Y. H., Gentili, F. G., Park, Y. K., Banat, F., Culaba, A. B., Ma, Z., & Show, P. L. (2023). Application of regression and artificial neural network analysis of red-green-blue image components in prediction of chlorophyll content in microalgae. Bioresource Technology, 370, 128503. https://doi.org/10.1016/j.biortech.2022.128503
Wang, S.-K., Wang, X., Miao, J., & Tian, Y.-T. (2018). Tofu whey wastewater is a promising basal medium for microalgae culture. Bioresource Technology, 253, 79–84.
Wang, S. K., Wang, X., Tao, H. H., Sun, X. S., & Tian, Y. T. (2018). Heterotrophic culture of Chlorella pyrenoidosa using sucrose as the sole carbon source by co-culture with immobilized yeast. Bioresource Technology, 249, 425–430.
Wang, Y. Z., Hallenbeck, P. C., Leite, G. B., Paranjape, K., & Huo, D. Q. (2016). Growth and lipid accumulation of indigenous algal strains under photoautotrophic and mixotrophic modes at low temperature. Algal Research, 16, 195–200.
Wellburn, A. R. (1994). The spectral determination of chlorophylls a and b, as well as total carotenoids, using various solvents with spectrophotometers of different resolution. Journal of Plant Physiology, 144(3), 307–313.
Wong, Y., Ho, Y. H., Ho, K. C., Leung, H. M., & Yung, K. K. L. (2017). Growth medium screening for Chlorella vulgaris growth and lipid production. Journal of Marine Biology and Aquaculture, 6(1), 00143.
Xing, W., Huang, W., Li, D., & Liu, Y. (2007). Effects of iron on growth, pigment content, photosystem II efficiency, and siderophores production of Microcystis aeruginosa and Microcystis wesenbergii. Current Microbiology, 55(2), 94–98.
Yang, F., Xiang, W., Li, T., & Long, L. (2018). Transcriptome analysis for phosphorus starvation-induced lipid accumulation in Scenedesmus sp. Scientific Reports, 8(1), 16420. https://doi.org/10.1038/s41598-018-34650-x
Yew, G. Y., Puah, B. K., Chew, K. W., Teng, S. Y., Show, P. L., & Nguyen, T. H. P. (2020). Chlorella vulgaris FSP-E cultivation in waste molasses: Photo-to-property estimation by artificial intelligence. Chemical Engineering Journal, 402, 126230.
Yuan, S., Ye, S., Yang, S., & Luo, G. (2021). Purification of potato wastewater and production of byproducts using microalgae Scenedesmus and Desmodesmus. Journal of Water Process Engineering, 43, 102237.
Zhu, C. J., Lee, Y. K., & Chao, T. M. (1997). Effects of temperature and growth phase on lipid and biochemical composition of Isochrysis galbana TK1. Journal of Applied Phycology, 9(5), 451–457.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
The authors confirm their contribution to the paper as follows: study conception and design: A.S., H.S., A.B.E., R.M.E., and A.A; data collection: A.S.; analysis and interpretation of results: A.S., H.S., A.B.E., R.M.E., H.I.M., and A.A.; draft manuscript preparation: A.S., H.S., A.B.E., R.M.E., H.I.M., and A.A. All authors reviewed the results and approved the final version of the manuscript.
Corresponding authors
Ethics declarations
Ethics Approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Consent to Participate
Not applicable
Consent for Publication
The manuscript is original. It has not been published previously by any of the authors and even not under the consideration in any other journal at the time of submission.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Salah, A., Sany, H., El-Sayed, A.EK.B. et al. Growth Performance and Biochemical Composition of Desmodesmus sp. Green Alga Grown on Agricultural Industries Waste (Cheese Whey). Water Air Soil Pollut 234, 770 (2023). https://doi.org/10.1007/s11270-023-06780-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11270-023-06780-0