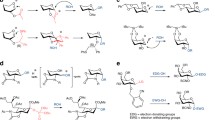
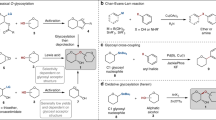
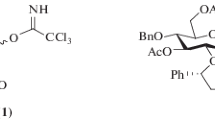Abstract
The identification of general and efficient methods for the construction of oligosaccharides stands as one of the great challenges for the field of synthetic chemistry1,2. Selective glycosylation of unprotected sugars and other polyhydroxylated nucleophiles is a particularly significant goal, requiring not only control over the stereochemistry of the forming bond but also differentiation between similarly reactive nucleophilic sites in stereochemically complex contexts3,4. Chemists have generally relied on multi-step protecting-group strategies to achieve site control in glycosylations, but practical inefficiencies arise directly from the application of such approaches5,6,7. Here we describe a strategy for small-molecule-catalyst-controlled, highly stereo- and site-selective glycosylations of unprotected or minimally protected mono- and disaccharides using precisely designed bis-thiourea small-molecule catalysts. Stereo- and site-selective galactosylations and mannosylations of a wide assortment of polyfunctional nucleophiles is thereby achieved. Kinetic and computational studies provide evidence that site-selectivity arises from stabilizing C–H/π interactions between the catalyst and the nucleophile, analogous to those documented in sugar-binding proteins. This work demonstrates that highly selective glycosylation reactions can be achieved through control of stabilizing non-covalent interactions, a potentially general strategy for selective functionalization of carbohydrates.




Similar content being viewed by others
Data availability
The data that support the findings in this work are available within the paper and its Supplementary Information.
References
Boltje, T. J., Buskas, T. & Boons, G.-J. Opportunities and challenges in synthetic oligosaccharide and glycoconjugate research. Nat. Chem. 1, 611–622 (2009).
Krasnova, L. & Wong, C.-H. Oligosaccharide synthesis and translational innovation. J. Am. Chem. Soc. 141, 3735–3754 (2019).
Dimakos, V. & Taylor, M. S. Site-selective functionalization of hydroxyl groups in carbohydrate derivatives. Chem. Rev. 118, 11457–11517 (2018).
Bati, G., He, J. X., Pal, K. B. & Liu, X. W. Stereo- and regioselective glycosylation with protection-less sugar derivatives: an alluring strategy to access glycans and natural products. Chem. Soc. Rev. 48, 4006–4018 (2019).
Green, L. G. & Ley, S. V. in Carbohydrates in Chemistry and Biology (eds Ernst, B. et al.) Ch. 17, 427–448 (Wiley-VCH, 2000).
Volbeda, A. G., van der Marel, G. A. & Codée, J. D. C. in Protecting Groups Strategies and Applications in Carbohydrate Chemistry (ed. Vidal, S.) Ch. 1, 1–24 (Wiley-VCH, 2019).
Young, I. & Baran, P. Protecting-group-free synthesis as an opportunity for invention. Nat. Chem. 1, 193–205 (2009).
Huang, Z. & Dong, G. Site-selectivity control in organic reactions: a quest to differentiate reactivity among the same kind of functional groups. Acc. Chem. Res. 50, 465–471 (2007).
Hartwig, J. F. Catalyst-controlled site-selective bond activation. Acc. Chem. Res. 50, 549–555 (2007).
Trincone, A. & Giordano, A. Glycosyl hydrolases and glycosyltransferases in the synthesis of oligosaccharides. Curr. Org. Chem. 10, 1163–1193 (2006).
Lairson, L. L., Henrissat, B., Davies, G. J. & Withers, S. G. Glycosyltransferases: structures, functions, and mechanisms. Annu. Rev. Biochem. 77, 521–555 (2008).
Guo, J. & Ye, X. S. Protecting groups in carbohydrate chemistry: influence on stereoselectivity of glycosylations. Molecules 15, 7235–7265 (2010).
van der Vorm, S. et al. Acceptor reactivity in glycosylation reactions. Chem. Soc. Rev. 48, 4688–4706 (2019).
van der Vorm, S. et al. Mapping the relationship between glycosyl acceptor reactivity and glycosylation stereoselectivity. Angew. Chem. Int. Ed. 57, 8240–8244 (2018).
Cordero-Vargas, A. & Sartillo-Piscil, F. in Protecting‐Group‐Free Organic Synthesis: Improving Economy and Efficiency (ed. Fernandes, R. A.) Ch. 7, 183–200 (Wiley, 2018).
Muramatsu, W. & Yoshimatsu, H. Regio- and stereochemical controlled Koenigs−Knorr-type monoglycosylation of secondary hydroxy groups in carbohydrates utilizing the high site recognition ability of organotin catalysts. Adv. Synth. Catal. 355, 2518–2524 (2013).
Gouliaras, C., Lee, D., Chan, L. & Taylor, M. S. Regioselective activation of glycosyl acceptors by a diarylborinic acid-derived catalyst. J. Am. Chem. Soc. 133, 13926–13929 (2011).
D’Angelo, K. A. & Taylor, M. S. Borinic acid catalyzed stereo- and regioselective couplings of glycosyl methanesulfonates. J. Am. Chem. Soc. 138, 11058–11066 (2016).
Nishi, N., Nashida, J., Kaji, E., Takahashi, D. & Toshima, K. Regio- and stereoselective beta-mannosylation using a boronic acid catalyst and its application in the synthesis of a tetrasaccharide repeating unit of lipopolysaccharide derived from E. coli O75. Chem. Commun. 53, 3018–3021 (2017).
Nishi, N. et al. Stereospecific β‐l‐rhamnopyranosylation through an SNi‐type mechanism by using organoboron reagents. Angew. Chem. Int. Ed. 57, 13858–13862 (2018).
Tomita, S. et al. Diboron-catalyzed regio- and 1,2-cis-β-stereoselective glycosylation of trans-1,2-diols. J. Org. Chem. 85, 16254–16262 (2020).
Hudson, K. L. et al. Carbohydrate–aromatic interactions in proteins. J. Am. Chem. Soc. 137, 15152–15160 (2015).
Asensio, J. L., Arda, A., Canada, F. J. & Jimenez-Barbero, J. Carbohydrate–aromatic interactions. Acc. Chem. Res. 46, 946–954 (2013).
Screen, J. et al. IR-spectral signatures of aromatic-sugar complexes: probing carbohydrate-protein interactions. Angew. Chem. Int. Ed. 46, 3644–3648 (2017).
Davis, A. P. Biomimetic carbohydrate recognition. Chem. Soc. Rev. 49, 2531–2545 (2020).
Arnaud, J., Audfray, A. & Imberty, A. Binding sugars: from natural lectins to synthetic receptors and engineered neolectins. Chem. Soc. Rev. 42, 4798–4813 (2013).
Kawabata, T., Muramatsu, W., Nishio, T., Shibata, T. & Schedel, H. A catalytic one-step process for the chemo- and regioselective acylation of monosaccharides. J. Am. Chem. Soc. 129, 12890–12895 (2007).
Griswold, K. S. & Miller, S. J. A peptide-based catalyst approach to the regioselective functionalization of carbohydrates. Tetrahedron 59, 8869–8875 (2003).
Sun, X., Lee, H., Lee, S. & Tan, K. L. Catalyst recognition of cis-1,2-diols enables site-selective functionalization of complex molecules. Nat. Chem. 5, 790–795 (2013).
Xiao, G. et al. Catalytic site-selective acylation of carbohydrates directed by cation–n interaction. J. Am. Chem. Soc. 139, 4346–4349 (2017).
Lee, J., Borovika, A., Khomutnyk, Y. & Nagorny, P. Chiral phosphoric acid-catalyzed desymmetrizative glycosylation of 2-deoxystreptamine and its application to aminoglycoside synthesis. Chem. Commun. 53, 8976–8979 (2017).
Tay, J.-H. et al. Regiodivergent glycosylations of 6-deoxy-erythronolide B and oleandomycin-derived macrolactones enabled by chiral acid catalysis. J. Am. Chem. Soc. 139, 8570–8578 (2017).
Park, Y. et al. Macrocyclic bis-thioureas catalyze stereospecific glycosylation reactions. Science 355, 162–166 (2017).
Levi, S. M., Li, Q., Rötheli, A. R. & Jacobsen, E. N. Catalytic activation of glycosyl phosphates for stereoselective coupling reactions. Proc. Natl Acad. Sci. USA 116, 35–39 (2019).
Mayfield, A. B., Metternich, J. B., Trotta, A. H. & Jacobsen, E. N. Stereospecific furanosylations catalyzed by bis-thiourea hydrogen-bond donors. J. Am. Chem. Soc. 142, 4061–4069 (2020).
Li, Q., Levi, S. M. & Jacobsen, E. N. Highly selective β-mannosylations and β-rhamnosylations catalyzed by bis-thiourea. J. Am. Chem. Soc. 142, 11865–11872 (2020).
Tsuzuki, S., Uchimaru, T. & Mikami, M. Magnitude and nature of carbohydrate-aromatic interactions in fucose-phenol and fucose-indole complexes: CCSD(T) level interaction energy calculations. J. Phys. Chem. A 115, 11256–11262 (2011).
Raju, R. K., Ramraj, A., Vincent, M. A., Hillier, I. H. & Burton, N. A. Carbohydrate–protein recognition probed by density functional theory and ab initio calculations including dispersive interactions. Phys. Chem. Chem. Phys. 10, 6500–6508 (2008).
Ryu, H. et al. Pitfalls in computational modelling of chemical reactions and how to avoid them. Organometallics 37, 3228–3239 (2018).
Plata, R. E. & Singleton, D. A. A case study of the mechanism of alcohol-mediated Morita Baylis− Hillman reactions. The importance of experimental observations. J. Am. Chem. Soc. 137, 3811–3826 (2015).
Acknowledgements
This work was supported by the NIH through GM132571 and the Common Fund Glycoscience Program (U01 GM116249) and a postdoctoral fellowship to A.E.W., and by NSF pre-doctoral fellowships to S.M.L. and C.W.
Author information
Authors and Affiliations
Contributions
Q.L., S.M.L., A.E.W. and E.N.J. conceived the work, S.M.L., Q.L., A.E.W. and C.C.W. conducted the experiments, E.N.J. directed the research, and Q.L., S.M.L., C.C.W. and E.N.J. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature thanks Steven Townsend and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
This Supplementary Information file contains Materials and Methods, Supplementary Text, Figs. 1–22 and Tables 1–11.
Rights and permissions
About this article
Cite this article
Li, Q., Levi, S.M., Wagen, C.C. et al. Site-selective, stereocontrolled glycosylation of minimally protected sugars. Nature 608, 74–79 (2022). https://doi.org/10.1038/s41586-022-04958-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-022-04958-w
- Springer Nature Limited
This article is cited by
-
Enantioselective transition-metal catalysis via an anion-binding approach
Nature (2023)
-
A synergistic Rh(I)/organoboron-catalysed site-selective carbohydrate functionalization that involves multiple stereocontrol
Nature Chemistry (2023)
-
Direct synthesis of unprotected aryl C-glycosides by photoredox Ni-catalysed cross-coupling
Nature Synthesis (2023)
-
Efficient and versatile formation of glycosidic bonds via catalytic strain-release glycosylation with glycosyl ortho−2,2-dimethoxycarbonylcyclopropylbenzoate donors
Nature Communications (2023)