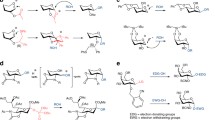
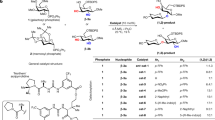
Abstract—
Synthetic oligosaccharides are objects of interest as model compounds in studies on the biological activity of natural compounds, but also as components for new drugs, glycoconjugate vaccines, carbohydrate diagnostic agents and various other products. The key stage in oligosaccharide synthesis is the glycosylation reaction, which leads to the formation of a linkage between carbohydrate fragments. This review highlights a current problem in modern glycochemistry – the methods of stereocontrol in the glycosylation reaction. Protecting groups within the structure of glycosyl donors are considered as stereocontrolling factors, affecting the reaction mechanism by means of (1) participation or anchimeric assistance, (2) deactivation, (3) intramolecular aglycone delivery. The well-established mechanism of neighboring group participation at O-2 for the synthesis of 1,2- trans glycosides is shown, as well as its modern modifications, including activating ethers, chiral protecting groups and achiral bicyclic glycosyl donors. The mechanisms of remote participation from O-3, O-4 and O-6 of the glycosyl donor are discussed in detail. In addition, approaches to stereocontrol using deactivating protection (conformation constraining and electron withdrawing groups) are described. Finally, synthetic approaches based on intramolecular aglycone delivery are considered. Both classical and novel protecting groups used to control the steric outcome of glycosylation are presented, the mechanisms underlying the presented approaches are discussed in detail, while also showing how the described strategies apply to the synthesis of complex structures.

Similar content being viewed by others
REFERENCES
Essentials of Glycobiology, 3rd ed., Varki, A., Cummings, R.D., Esko, J.D., Stanley, P., Hart, G.W., Aebi, M., Darvill, A.G., Kinoshita, T., Packer, N.H., Prestegard, J.H., Schnaar, R.L., and Seeberger, P.H., Eds., Cold Spring Harbor, New York: Cold Spring Harbor Laboratory Press, 2017.
Varki, A., Cold Spring Harb. Perspect. Biol., 2011, vol. 3. https://doi.org/10.1101/cshperspect.a005462
Moremen, K.W., Tiemeyer, M., and Nairn, A.V., Nat. Rev. Mol. Cell Biol., 2012, vol. 13, pp. 448–462. https://doi.org/10.1038/nrm3383
Varki, A., Glycobiology, 2016, vol. 27, pp. 3–49. https://doi.org/10.1093/glycob/cww086
Bochkov, A.F., Afanas’ev, V.A., and Zaikov, G.E., Obrazovanie i rasshcheplenie glikozidnykh svyazei (Formation and Cleavage of Glycosidic Bonds), Moscow: Nauka, 1978.
Nukada, T., Bereces, A., Zgierski, M.Z., and Whitfield, D., J. Am. Chem. Soc., 1998, vol. 120, pp. 13291–13295. https://doi.org/10.1021/ja981041m
Chatterjee, S., Moon, S., Hentschel, F., Gilmore, K., and Seeberger, P.H., J. Am. Chem. Soc., 2018, vol. 140 no. 38, pp. 11942–11953. https://doi.org/10.1021/jacs.8b04525
Kim, J.-H., Yang, H., Khot, V., Whitfield, D., and Boons, G.-J., Eur. J. Org. Chem., 2006, pp. 5007–5028. https://doi.org/10.1002/ejoc.200600440
Yang, B., Yang, W., Ramadan, S., and Huang, X., Eur. J. Org. Chem., 2018, vol. 2018, pp. 1075–1096. https://doi.org/10.1002/ejoc.201701579
Leng, W.-L.YaoH., He, J.-X., and Liu, X.-W., Acc. Chem. Res., 2018, vol. 51, pp. 628–639. https://doi.org/10.1021/acs.accounts.7b00449
Tipson, S., J. Biol. Chem., 1939, vol. 130, pp. 55–59.
Isbell, H.S., Annu. Rev. Biochem., 1940, vol. 9, pp. 65–92.
Paulsen, H. and Herold, C.-P., Chem. Ber., 1970, vol. 103, pp. 2450–2462. https://doi.org/10.1002/cber.19701030817
Crich, D., Dain, Z., and Gastaldi, S., Org. Chem., 1999, vol. 64, pp. 5224–5229. https://doi.org/10.1021/jo990424f
Mucha, E., Marianski, M., Xu, F.-F., Thomas, D.A., Meijer, G., von Helden, G., Seeberger, P.H., and Pagel, K., Nat. Commun., 2018, vol. 9, p. 4174. https://doi.org/10.1038/s41467-018-06764-3
Boltje, T.J., Liu, L., and Boons, G.-J., in Glycochemical Synthesis, Hung, S.-C. and Zulueta, M. M. L., Eds., Hoboken, New Jersey: Wiley, 2016, pp. 97–130. https://doi.org/10.1002/9781119006435.ch4
Yu, H., Williams, D.L., and Ensley, H.E., Tetrahedron Lett., 2005, vol. 46, pp. 3417–3421. https://doi.org/10.1016/j.tetlet.2005.03.099
Liu, H., Hansen, T., Zhou, S.-Y., Wen, G.-E., Liu, X.-X., Zhang, Q.-J., Codee, J.D.C., Schmidt, R.R., and Sun, J.-S., Org. Lett., 2019, vol. 21, pp. 8713–8717. https://doi.org/10.1021/acs.orglett.9b03321
Smoot, J.T., Pornsuriyasak, P., and Demchenko, A.V., Angew. Chem., Int. Ed. Engl., 2005, vol. 44, pp. 7123–7126. https://doi.org/10.1002/anie.200502694
Buda, S., Gołębiowska, P., and Mlynarski, J., Eur. J. Org. Chem., 2013, pp. 3988–3991. https://doi.org/10.1002/ejoc.201300123
Le Mai, HoangK. and Liu, X.-W., Nat. Commun., 2014, vol. 5, p. 5051. https://doi.org/10.1038/ncomms6051
Karak, M., Suenaga, M., Oishi, T., and Torikai, K., Org. Lett., 2019, vol. 21 , no. 4, pp. 1221–1225. https://doi.org/10.1021/acs.orglett.9b00220
Kim, J.-H., Yang, H., and Boons, G.-J., Angew. Chem., Int. Ed. Engl., 2005, vol. 44, pp. 947–949. https://doi.org/10.1002/anie.200461745
Kim, J.-H., Yang, H., Park, J., and Boons, G.-J., J. Am. Chem. Soc., 2005, vol. 127, pp. 12090–12097. https://doi.org/10.1021/ja052548h
Cox, D.A. and Fairbanks, A.J., Tetrahedron: Asymmetry, 2009, vol. 20, pp. 773–780. https://doi.org/10.1016/j.tetasy.2009.02.018
Fascione, M.A. and Turnbull, W.B., Beilstein J. Org. Chem., 2010, vol. 6. https://doi.org/10.3762/bjoc.6.19
Fang, T., Mo, K.-F., and Boons, G.-J., J. Am. Chem. Soc., 2012, vol. 134 P, p. 7545. https://doi.org/10.1021/ja3018187
Huang, W., Gao, Q., and Boons, G.-J., Chem.-Eur. J., 2015, vol. 21, pp. 12920–12926. https://doi.org/10.1002/chem.201501844
Komarova, B.S., Tsvetkov, Y.E., and Nifantiev, N.E., Chem. Res., 2016, vol. 16, pp. 488–506. https://doi.org/10.1002/tcr.201500245
Khatuntseva, E.A., Ustuzhanina, N.E., Zatonskii, G.V., Shashkov, A.S., Usov, A.I., and Nifant’ev, N.E., J. Carbohydr. Chem., 2000, vol. 19, pp. 1151–1173. https://doi.org/10.1080/07328300008544140
Gerbst, A.G., Ustuzhanina, N.E., Grachev, A.A., Zlotina, N.S., Khatuntseva, E.A., Tsvetkov, D.E., Shashkov, A.S., Usov, A.I., and Nifantiev, N.E., J. Carbohydr. Chem., 2002, vol. 21, pp. 313–324. https://doi.org/10.1081/CAR-120013500
Gerbst, A.G., Ustuzhanina, N.E., Grachev, A.A., Tsvetkov, D.E., Khatuntseva, E.A., Shashkov, A.S., Usov, A.I., Preobrazhenskaya, M.E., Ushakova, N.A., and Nifantiev, N.E., J. Carbohydr. Chem., 2003, vol. 22 P, pp. 109–122. https://doi.org/10.1081/CAR-120020481
Ustyuzhanina, N., Krylov, V., Grachev, A., Gerbst, A., and Nifantiev, N., Synthesis, 2006, vol. 23, pp. 4017–4031. https://doi.org/10.1055/s-2006-950333
Krylov, V.B., Kaskova, Z.M., Vinnitskiy, D.Z., Ustyuzhanina, N.E., Grachev, A.A., Chizhov, A.O., and Nifantiev, N.E., Carbohydr. Res., 2011, vol. 346, pp. 540–550. https://doi.org/10.1016/j.carres.2011.01.005
Gerbst, A.G., Grachev, A.A., Ustyuzhanina, N.E., Khatuntseva, E.A., Tsvetkov, D.E., Usov, A.I., Shashkov, A.S., Preobrazhenskaya, M.E., Ushakova, N.A., and Nifantiev, N.E., Russ. J. Bioorg. Chem., 2004, vol. 30, pp. 137–147. https://doi.org/10.1023/B:RUBI.0000023099.48598.9a
Vinnitsky, D.Z., Krylov, V.B., Ustyuzhanina, N.E., Dmitrenok, A.S., and Nifantiev, N.E., Org. Biomol. Chem., 2016, vol. 14, pp. 598–611. https://doi.org/10.1039/c5ob02040a
Komarova, B.S., Tsvetkov, Y.E., Knirel, Y.A., Zahringer, U., Pier, G.B., and Nifantiev, N.E., Tetrahedron Lett., 2006, vol. 47, pp. 3583–3587. https://doi.org/10.1016/j.tetlet.2006.03.045
Komarova, B.S., Tsvetkov, Y.E., Pier, G.B., and Nifantiev, N.E., Org. Chem., 2008, vol. 73, pp. 8411–8421. https://doi.org/10.1021/jo801561p
Komarova, B.S., Tsvetkov, Y.E., Pier, G.B., and Nifantiev, N.E., Carbohydr. Res., 2012, vol. 360, pp. 56–68. https://doi.org/10.1016/j.carres.2012.07.019
Komarova, B.S., Orekhova, M.V., Tsvetkov, Y.E., Beau, R., Aimanianda, V., Latgé, J.-P., and Nifantiev, N.E., Chem.-Eur. J., 2015, vol. 20, pp. 1029–1035. https://doi.org/10.1002/chem.201404770
Komarova, B.S., Wong, S.S., Orekhova, M.V., Tsvetkov, Y.E., Krylov, V.B., Beauvais, A., Bouchara, J.-P., Kearney, J., Aimanianda, V., Latgé, J.-P., and Nifantiev, N.E., Org. Chem., 2018, vol. 83, pp. 12965–12976. https://doi.org/10.1021/acs.joc.8b01142
Komarova, B.S., Dorokhova, V.S., Tsvetkov, Y.E., and Nifantiev, N.E., Org. Chem. Front., 2018, vol. 5, pp. 909–928. https://doi.org/10.1039/c7qo01007a
Kazakova, E.D., Yashunsky, D.V., Krylov, V.B., Bouchara, J.P., Cornet, M., Valsecchi, I., Fontaine, T., Latge, J.-P., and Nifantiev, N.E., J. Am. Chem. Soc., 2020, vol. 142, pp. 1175–1179. https://doi.org/10.1021/jacs.9b11703
Kazakova, E.D., Yashunsky, D.V., Khatuntseva, E.A., and Nifantiev, N.E., Pure Appl. Chem., 2020, vol. 92, pp. 1047–1056. https://doi.org/10.1515/pac-2020-0105
Hahm, H.S., Hurevich, M., and Seeberger, P.H., Nat. Commun, 2016, vol. 7, p. 12482. https://doi.org/10.1038/ncomms12482
Krylov, V., Ustyuzhanina, N., Bakker, H., and Nifantiev, N., Synthesis, 2007, pp. 3147–3154. https://doi.org/10.1055/s-2007-990784
Sethi, M.K., Buettner, F.F.R., Krylov, V., Takeuchi, H., Nifantiev, N., Haltiwanger, R.S., and Gerardy-Schahn, R., J. Biol. Chem., 2010, vol. 285, pp. 1582–1586. https://doi.org/10.1074/jbc.C109.065409
Sethi, M.K., Buettner, F.F.R., Ashikov, A., Krylov, V.B., Takeuchi, H., Nifantiev, N.E., Haltiwanger, R.S., Gerardy-Schahn, R., and Bakker, H., J. Biol. Chem., 2012, vol. 287, pp. 2739–2748. https://doi.org/10.1074/jbc.M111.302406
Mukaiyama, T., Suenaga, M., Chiba, H., and Jona, H., Chem. Lett., 2002, pp. 56–57. https://doi.org/10.1246/cl.2002.56
Li, Z., Zhu, L., and Kalikanda, J., Tetrahedron Lett., 2011, vol. 52, pp. 5629–5632. https://doi.org/10.1016/j.tetlet.2011.08.091
Komarova, B.S., Gerbst, A.G., Finogenova, A.M., Dmitrenok, A.S., Tsvetkov, Y.E., and Nifantiev, N.E., Org. Chem., 2017, vol. 82, pp. 8897–8908. https://doi.org/10.1021/acs.joc.7b01167
Komarova, B.S., Orekhova, M.V., Tsvetkov, Y.E., and Nifantiev, N.E., Carbohydr. Res., 2014, vol. 384, pp. 70–86. https://doi.org/10.1016/j.carres.2013.11.016
Crich, D., Hu, T., and Cai, F., Org. Chem., 2008, vol. 73, pp. 8942–8953. https://doi.org/10.1021/jo801630m
Baek, J.Y., Lee, B.-Y., Jo, M.G., and Kim, K.S., J. Am. Chem. Soc., 2009, vol. 131, pp. 17705–17713. https://doi.org/10.1021/ja907252u
Marianski, M., Mucha, E., Greis, K., Moon, S., Pardo, A., Kirschbaum, K., Thomas, D.A., Meijer, G., von Helden, G., Gilmore, K., Seeberger, P.H., and Pagel, K., Angew. Chem., Int. Ed. Engl., 2020, vol. 59, pp. 6166–6171. https://doi.org/10.1002/anie.201916245
Gerbst, A.G., Ustuzhanina, N.E., Grachev, A.A., Khatuntseva, E.A., Tsvetkov, D.E., Whitfield, D.M., Bereces, A., and Nifantiev, N.E., J. Carbohydr. Chem., 2001, vol. 20, pp. 821–831. https://doi.org/10.1081/CAR-100108659
Gerbst, A.G., Krylov, V.B., and Nifantiev, N.E., in Carbohydrate Chemistry—A Specialist Periodical Reports, Rauter, A.P., Lindhorst, T., and Queneau, Y., Eds., 2021, vol. 44, pp. 151–169. https://doi.org/10.1039/9781788013864-00151
Hansen, T., Elferink, H., van Hengst, J.M.A., Houthuijs, K.J., Remmerswaal, W.A., Kromm, A., Berden, G., van der Vorm, S., Rijs, A.M., Overkleeft, H.S., Filippov, D.V., Rutjes, F.P.J., van der Marel, G., Martens, J., Oomens, J., Codée, J.D.C., and Boltje, T.J., Nat. Commun., 2020, vol. 11. https://doi.org/10.1038/s41467-020-16362-x
Mootoo, D.R., Konradsson, P., Udodong, U., and Fraser-Reid, B., J. Am. Chem. Soc., 1988, vol. 110, pp. 5583–5584. https://doi.org/10.1021/ja00224a060
Yang, B., Yoshida, K., and Huang, X., in Glycochemical Synthesis, Hung, S.-C. and Zulueta, M.M.L., Eds., Hoboken, New Jersey: Wiley, 2016, pp. 155–188. https://doi.org/10.1002/9781119006435.ch6
Crich, D. and Sun, S., J. Am. Chem. Soc., 1997, vol. 119, pp. 11217–11223. https://doi.org/10.1021/ja971239r
Kahne, D., Walker, S., Cheng, Y., and Van Engen, D., J. Am. Chem. Soc., 1989, vol. 111, pp. 6881–6882. https://doi.org/10.1021/ja00199a081
Fraser-Reid, B., Wu, Z., Andrews, C.W., and Skowronski, E., J. Am. Chem. Soc., 1991, vol. 113, pp. 1434–1435. https://doi.org/10.1021/ja00004a066
Jensen, H.H., Nordstrom, L.U., and Bols, M., J. Am. Chem. Soc., 2004, vol. 126, pp. 9205–9213. https://doi.org/10.1021/ja047578j
Crich, D., Acc. Chem. Res., 2010, vol. 43 I, pp. 1144–1153. https://doi.org/10.1021/ar100035r
Crich, D., de la Mora, M., and Vinod, A.U., Org. Chem., 2003, vol. 68, pp. 8142–8148. https://doi.org/10.1021/jo0349882
Kumagai, D., Miyazaki, M., and Nishimura, S.-I., Tetrahedron Lett., 2001, vol. 42, pp. 1953–1956. https://doi.org/10.1016/S0040-4039(01)00044-2
Yun, M., Shin, Y., Chun, K.H., and Jen, S., Bull. Korean Chem. Soc., 2000, vol. 21, pp. 562–566.
Weingart, R. and Schmidt, R.R., Tetrahedron Lett., 2000, vol. 41, pp. 8753–8758. https://doi.org/10.1016/S0040-4039(00)01497-0
Baek, J.Y., Choi, T.J., Jeon, H.B., and Kim, K.S., Angew. Chem., Int. Ed. Engl., 2006, vol. 45, pp. 7436–7440. https://doi.org/10.1002/anie.200602642
Tsuda, T., Sato, S., Nakamura, S., and Hashimoto, S., Heterocycles, 2003, vol. 59, pp. 509–515. https://doi.org/10.3987/COM-02-S80
Karelin, A.A., Tsvetkov, Y.E., Paulovicova, E., Paulovicova, L., and Nifantiev, N.E., Eur. J. Org. Chem., 2016, pp. 1173–1181. https://doi.org/10.1002/ejoc.201501464
Crich, D. and Vinogradova, O., J. Am. Chem. Soc., 2007, vol. 129, pp. 11756–11765. https://doi.org/10.1021/ja0730258
Baek, J.Y., Kwon, H.-W., Myug, S.J., Park, J.J., Kim, M.Y., Rathwell, D.C.K., Jeon, H.B., Seeberger, P.H., and Kim, K.S., Tetrahedron, 2015, vol. 71, pp. 5315–5320. https://doi.org/10.1016/j.tet.2015.06.014
Bos, L.J., Dinkelaar, J., Overkleeft, H.S., and van der Marel, G., J. Am. Chem. Soc., 2006, vol. 128, pp. 13066–13067. https://doi.org/10.1021/ja064787q
Tang, S.-L. and Pohl, N.L.B., Org. Lett., 2015, vol. 17, pp. 2642–2645. https://doi.org/10.1021/acs.orglett.5b01013
Behrendt, M.E. and Schmidt, R.R., Tetrahedron Lett., 1993, vol. 34, pp. 6733–6736. https://doi.org/10.1016/S0040-4039(00)61687-8
Xiao, G.J. and Demchenko, A.V., Beilstein J. Org. Chem., 2017, vol. 13, pp. 2028–2048. https://doi.org/10.3762/bjoc.13.201
Kusomoto, S., Imoto, M., Ogiku, T., and Shiba, T., Bull. Chem. Soc. Jpn., 1986, vol. 59, pp. 1419–1423. https://doi.org/10.1246/bcsj.59.1419
Barresi, F. and Hindsgau, O., J. Am. Chem. Soc., 1991, vol. 113, pp. 9376–9377. https://doi.org/10.1021/ja00024a057
Stork, G. and Kim, G., J. Am. Chem. Soc., 1992, vol. 114, pp. 1087–1088. https://doi.org/10.1021/ja00029a047
Bols, M., Tetrahedron, 1993, vol. 49, pp. 10049–10060. https://doi.org/10.1016/S0040-4020(01)80200-3
Ito, Y. and Ogawa, T., Angew. Chem., Int. Ed. Engl., 1994, vol. 33, pp. 1765–1767. https://doi.org/10.1002/anie.199417651
Ishiwata, A.ItoY., in Selective Glycosylations: Synthetic Methods and Catalysts, Bennet, C.S., Ed., Weinheim: Wiley-VCH Verlag GmbH & Co. KgaA, 2017, pp. 81–96.
Yasomanee, J.P. and Demchenko, A.V., J. Am. Chem. Soc., 2012, vol. 134, pp. 20097–20102. https://doi.org/10.1021/ja307355n
Zhang, Y., Zhou, S., Wang, X., Zhan, H., Guo, Z., and Gao, J., Org. Chem. Front., 2019, vol. 6, pp. 762–772.
Shchegravina, E.S., Sachkova, A.A., Usova, S.D., Nyuchev, A.V., Gracheva, Yu.A., and Fedorov, A.Yu., Russ. J. Bioorg. Chem., 2021, vol. 47, pp. 69–96. https://doi.org/10.31857/S013234232101022X.
Gening, M.L., Kurbatova, E.A., and Nifantiev, N.E., Russ. J. Bioorg. Chem., 2021, vol. 47, pp. 1–24. https://doi.org/10.31857/S0132342321010073.
Khatuntseva, E.A. and Nifantiev, N.E., Russ. J. Bioorg. Chem., 2021, vol. 47. 25–51. https://doi.org/10.31857/S0132342321010103.
Adamo, R., Nilo, A., Castagner, B., Boutureira, O., Berti, F., and Bernardes, G.J.L., Chem. Sci., 2013, vol. 4, pp. 2995–3008. https://doi.org/10.1039/C3SC50862E
Hu, Q.-Y., Berti, F., and Adamo, R., Chem. Soc. Rev., 2016, vol. 45, pp. 1691–1719. https://doi.org/10.1039/c4cs00388h
Phillips-Jones, M.K. and Harding, S.E., Biotechnol. Genet. Eng. Rev., 2019, vol. 35, pp. 93–125. https://doi.org/10.1080/02648725.2019.1703614
Seeberger, P.H., Acc. Chem. Res., 2015, vol. 48, p. 1450. https://doi.org/10.1021/ar5004362
Pardo-Vargas, A., Delbianco, M., and Seeberger, P.H., Curr. Opin. Chem. Biol., 2018, vol. 46, pp. 48–55. https://doi.org/10.1016/j.cbpa.2018.04.007
Funding
This work was supported by the Russian Scientific Foundation (Grant no. 19-73-20240).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
COMPLIANCE WITH ETHICAL STANDARDS
This publication does not contain the description of any research with the participation of humans or animals as research objects.
Conflict of Interests
The authors declare no conflict of interests.
Additional information
Abbreviations: CIP, contact ion pair; SSIP, solvent-separated ion pair.
Corresponding author: phone: +7 (499) 135-87-84.
Rights and permissions
About this article
Cite this article
Tokatly, A.I., Vinnitskiy, D.Z., Ustuzhanina, N.E. et al. Protecting Groups as a Factor of Stereocontrol in Glycosylation Reactions. Russ J Bioorg Chem 47, 53–70 (2021). https://doi.org/10.1134/S1068162021010258
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1068162021010258