Abstract
Mechanistic study of carbohydrate interactions in biological systems calls for the chemical synthesis of these complex structures. Owing to the specific stereo-configuration at each anomeric linkage and diversity in branching, significant breakthroughs in recent years have focused on either stereoselective glycosylation methods or facile assembly of glycan chains. Here, we introduce the unification approach that offers both stereoselective glycosidic bond formation and removal of protection/deprotection steps required for further elongation. Using dialkylboryl triflate as an in situ masking reagent, a wide array of glycosyl donors carrying one to three unprotected hydroxyl groups reacts with various glycosyl acceptors to furnish the desired products with good control over regioselectivity and stereoselectivity. This approach demonstrates the feasibility of straightforward access to important structural scaffolds for complex glycoconjugate synthesis.
Similar content being viewed by others
Introduction
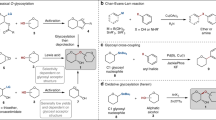
The total synthesis of a seventy-seven nucleotide unit long DNA duplex, coding for the yeast alanine tRNA, by Khorana’s group1 in 1972 is regarded2 as one of the “greatest tour de force organic and biochemists have yet achieved”. This landmark accomplishment at the time prefaced the explosive growth of knowledge in biochemistry research for many decades and the halo effect accelerated many radical innovations in the synthesis of oligonucleotides, oligopeptides and oligosaccharides. The slower pace of breakthroughs in oligosaccharide synthesis is certainly not from limited interest into the field, but rather the inherently complex and diverse structures employed by biological systems3, 4. The remarkable density of information that carbohydrates can carry stemmed from their ability to form branching connections and the unique stereo-configuration between each sugar unit. Compounding the difficulty in isolating homogeneous carbohydrates from living cells is the “micro-heterogeneity”, releasing many variants of the core oligosaccharide scaffold due to minute changes in the environment. At present, classic chemical or hybrid chemoenzymatic approach provide the most reliable access to homogenous form of glycans5,6,7,8,9. Figure 1 highlights the core strategies of a glycosylation reaction. Nucleophilic substitution at the anomeric center is the crucial bottleneck as clean SN2 transformation is almost always accompanied by competing SN1 due to stabilization of C1 cation by the endocyclic oxygen to generate oxocarbenium ion. Controlled outcome of this delicate equilibrium is notoriously difficult since it is easily influenced by many factors, including reactivity of glycosyl donors, temperature, solvents, other additives, etc. (Fig. 1a).
Strategic overview of chemical glycosylation methods. a, General profile of a glycosylation reaction. b, C-2 neighboring group participation for controlled 1,2-cis or 1,2-trans linkages. c, high β-mannosylation and high α-galactosylation employing ring-locked conformation. d, high cis- selectivity for aminoglycosyl and high α-sialylation with cyclic carbamate protection. e, an example of acceptor-controlled stereoselectivity. f, stereoselective and regioselective glycosylation with temporal boron-masking allows flexible switching between donors and acceptors and removal of protection/deprotection step (this work)
To date, specific requirements to deliver a high degree of stereoselectivity have emerged, such as employment of neighboring group participation at C-2 to deliver cis- or trans- selective products10,11,12,13 (Fig. 1b), remote stereo-control with locked conformation14,15,16,17,18,19,20 (Figs. 1c, d) or acceptor-controlled glycosylation21, 22 (Fig. 1e). The successful answers to problems of stereoselective O-glycosylation came with drawbacks in the form of extensive preparative routes or usage of exotic chiral auxiliaries. Different approaches had been reported from other groups towards a protection-free glycosylation, such as glycosyl dithiocarbamates23, oxathiane glycosyl donors24, or boron-mediated glycosylation. The latter mainly focused on employing diol glycosyl acceptors as binding substrates25,26,27,28,29,30,31,32.
In addition to development of better stereo-controlled methodology, notable effort has been directed towards designing a simpler and more efficient assembly of complex glycans from relatively small pool of building blocks. Major breakthroughs included one-pot glycosylation, chemoenzymatic approach and automated synthesis on solid support. Based on early observation made by Paulsen33 and Fraser-Reid34, the concept of armed-disarmed glycosyl donors was further refined by Ley35 and Wong36 into a predictive way to quantify the relative reactivity between donors carrying different electron-withdrawing groups. Careful choice of protective groups and orthogonal activation in an one-pot setup allowed the synthesis of many complex oligosaccharides37, 38, with the advantages of minimal isolation and purifications. Certain limits do exist, such as extensive protective groups tunings, possible self-condensation or crosslinking and unwieldy design when there are more than one branching in the glycan structure. Capitalizing on the knowledge and availability of current glycosyl transferases and bio-engineered glycosyl hydrolases, so-called glycosyl synthases39, a hybrid chemoenzymatic approach saw practice in challenging modifications such as terminal α-2,3- and α-2,6-sialylation40 or core α-fucosylation41, library synthesis of asymmetrically branched, complex N-glycans42, 43, or top-down trimming of high-mannose type N-glycans44. Predicaments to widespread application of this excellent strategy included feedback inhibition, commercial availability and desired regioselectivity. On the other hand, automated glycosylation45 is at its nascent stage now but is fast gaining tractions among the community, with major improvements expected to come. The latest record-holder for the longest chemically prepared carbohydrate structure was a 92-mer polysaccharide arabinogalactan46, whereas the former champion was a 30-mer repeating mannosides47 using automated solid-phase oligosaccharide synthesis. Currently, the most resource-consuming stage is arguably the complete and selective deprotection after every coupling step.
In this work, we aim to provide a solution to the aforementioned challenges with a well-designed temporal group that binds to glycosyl donor in situ, prior to activation, to direct the stereo-outcome but is removed in the work-up phase (Fig. 1f). The result resembles a protection-free glycosylation strategy whereas one, two, or three free hydroxyl groups on donor can be present without detrimental effects on reaction yield and selectivity. This is made possible through combination of temporal boron-protection, stoichiometric activation and sequential addition of glycosyl acceptors. Our approach has the general advantages of employing commonly used thioglycoside donors, flexibility in the number and position of free hydroxyl groups as well as wide compatibility with many activation methods.
Results
Optimizing and control experiments
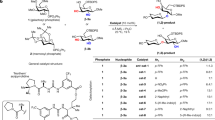
We set out to investigate the use of 2-OH glucosyl donor 1a as the model substrate with various simple alcohol acceptors. Initially, we proposed that usage of different dialkylboryl triflates could have marked effects on the stereo-outcome of reaction. Smaller alkyl groups such as dimethyl may lead to formation of a tetravalent boron intermediate, which facilitate delivery of glycosyl acceptor from the same face to give 1,2-cis product (Fig. 2a). In principle, the mechanism should be similar to the well-studied intramolecular aglycon delivery (IAD)48, 49. A conceptually analogous model was proposed by Toshima28, 29, starting from 1,2-anhydrosugars instead. On the other hand, larger alkyl groups such as di-n-butyl or 9-BBN should provide steric hindrance to force glycosyl acceptor to approach the anomeric center from opposite face, giving 1,2-trans product (Fig. 2b). This time, the boron complex was acting like a directing group through its spatial occupancy.
Optimizing masking effect of boron and several low temperature NMR experiments dismissed the first tetravalent model (Fig. 2a) and suggested the formation of the latter (Fig. 2b). In addition, the boron-masked O-2 exhibited an electron-withdrawing effect on H-2 proton as it was shifted downfield to 4.20 ppm from its previous position at 3.56 ppm (Δ = 0.64 ppm), see Supplementary Discussions. Furthermore, adding sequence of reagents was critical for consistent formation of desired product. The following protocol was established: An equimolar amount of dialkyl boron triflate was transfered to glycosyl donor 1a, followed by addition of thiophilic activator and glycosyl acceptor. At −20 °C, there was little stereoselectivity when methanol was used as acceptor and dimethyl(methylthio) sulfonium tetrafluoroborate (DMTSF) as activator. Nevertheless, product 1b was the only glucoside detected, confirming the free hydroxyl group on 1a was successfully masked and no self-condensation occurred. On the other hand, we noticed an increase in β-product moving from methanol to ethanol, isopropanol and n-butanol (Table 1, entries 1–4), suggested that steric bulk of alkyl chain does play an appreciable role in stereo-outcome of reaction. Using n-butanol as the acceptor, survey of various solvents revealed CH2Cl2 afforded the best yield with moderate stereo-selectivity, amidst DMF, nitroethane, and toluene (Table 1, entries 5–7). No chemical conversion was observed when acetonitrile was used, and all starting materials were recovered (Table 1, entry 8). Lower reaction temperature to −40 °C resulted in pure β-1e (Table 1, entry 9). Within sensitivity limit of NMR instrument the α-product was undetected. It was very encouraging that many commonly used activation conditions, including DMTSF, NIS/TMSOTf, AgOTf/pNO2PhSCl and Ph2SO/Tf2O were compatible with the addition of boron reagent (Table 1, entries 9–12). Entries 1–4 were repeated with other dialkyl boron triflates such as diethyl, 9-BBN or dicyclohexyl but no appreciable change in selectivities were observed. Likewise, these dialkyl boron triflates delivered β-only 1e under optimized conditions (entries 9–12).
A set of control experiments was performed to understand the probable mechanism. Typical glycosylation with fully protected glycosyl donor 1h with Ph2SO/Tf2O yielded product 1f as a mixture of anomers (Fig. 3a). When Bu2BOTf was introduced to the mixture of 1h and Ph2SO, followed by drop-wise addition of Tf2O and then butanol at −60 °C, very little amount of product was observed and most glycosyl donor was recovered (Fig. 3b). Premixing butanol and Bu2BOTf to generate BuO-B(Bu)2 likewise led to very little desired product, with major hydrolysis occurred (Fig. 3c). As expected, Ph2SO/Tf2O activating condition was not compatible with unprotected hydroxyl glycosyl donor50, 51. We reached the conclusion that without a free hydroxyl group to react, Bu2BOTf inhibited the activation process and slowed down the reaction. In addition, hydroxyl group masked by Bu2BOTf were no longer able to act as acceptor. This would explain the necessity to mix donor with equimolar amount of boron reagent before thiophilic activators were introduced (Fig. 3d).
Expanding the substrate scope
With the optimized reaction conditions in hand, the scope of glucosyl donor carrying a single free hydroxyl group at different positions was first evaluated. As seen from Table 2, we observed a strong β-selectivity with 2-OH and 3-OH glucosyl donor. Interestingly, selectivity was reduced when there was an acetyl at adjacent position (Table 2, entry 3 and 6). 11B-NMR experiments revealed an upfield shift to about −20 ppm compared to the chemical shift of −3 ppm with donor 1a, suggesting the formation of a different tetravalent boron intermediate (see Supplementary Discussions). It was probable the acyl group formed a complex with boron center, thus attenuated the steric effect of the auxiliary.
With 4-OH glucosyl donor, selectivity with benzylated 7a was very similar to control experiment with perbenzylated 1h. Still, the masking effect of boron reagent could be used in combination with a stronger directing group, such as 2-O-benzoyl (Table 2, entry 8). Notably, glucosyl donor 9a having an inverted conformation 1C4 resulted in intramolecular displacement of PMB group at O−6 to form 1,6-anhydroglucoside 9e (Table 2, entry 9). If no boron was added and DMTSF was used as activating agent, a complicated mixture was observed and only trace amount of 9e was isolated (<5%). With 6-OH glucosyl donor, significant amount of α-product was formed, raising α/β to1/2. We believed the β-facing of primary 6-OH was responsible for this rise in α-anomers. Again, presence of a directing group such as 2-O-benzoyl restored the selectivity and deliver the desired product in good yield (Table 2, entry 11). As expected, glucosyl donor 12a with inverted conformation 1C4 resulted in formation of 1,6-anhydroglucoside 12e as the only product (Table 2, entry 12).
The substrate scope of glucosyl donors carrying more free hydroxyl groups was examined next. As shown in Table 3, the general trend from Table 2 was noticed. High β-selectivity was observed when there was either 2-OH or 3-OH available (Table 3, entries 1–3, 5) but was strongly reduced when 6-OH was also present (Table 3, entry 4 and 6). As expected, compound 20a equipped with 2-O-benzoyl smoothly produced β-20e as the sole anomer (Table 3, entry 8). The complex interactions between neighboring OHs were more pronounced with glucosyl donor carrying three free hydroxyls (Table 3, entries 9–12) and the selectivity was generally lower, except for 2,4,6-OHs glucosyl donor 22a (Table 3, entry 10). Unexpectedly, protecting of the primary 6-OH in glucosyl donor 24a led to poor selectivity in product 24e (Table 3, entry 12). Still, in all cases the masking effect of boron was complete and only desired product was detected. Trials with glucosyl donor carrying four hydroxyl groups was unfruitful due to low solubility.
The encouraging results prompted us to prepare substrates for disaccharide surveys. To our delight, many commonly encountered glycosidic bonds proceeded smoothly to provide disaccharides in high yield and selectivity (Table 4, entries 1–5). Epimeric forms of glucose were prepared and the reaction under optimized conditions were compared. As expected, both C-2 epimers glucosyl 1a and mannosyl 25a delivered the 1,2-trans product 6ai and 7ai, respectively (Table 4, entries 6–7). With C-3 epimer products glucoside 8ai and alloside 9ai, only β-anomers were detected (Table 4, entries 8–9). For comparison, 2,3,4,6-tetra-O-benzyl glucosyl donor 1h would react to form disaccharide with high β-selectivity (1α/5β) whereas 2,3,4,6-tetra-O-benzyl allosyl donor gave a low selectivity of 1α/1β ratio, consistent with previous reports52. The equatorial 3-O-Boron complex in the case of glucosyl donor 4a was inferred to cause minimal steric crowding in β-face of anomeric C-1 and the enhancement of β-preference was likely from a combination of other factors. On the other hand, the axially positioned 3-O-Boron complex generated prior to activation of allosyl donor 26a should provide a substantial shielding effect on the α-face of anomeric C-1, resulting in higher β-selectivity. Initial trials with C-4 epimers glucosyl 7a and galactosyl 27a returned similar stereoselective results to their perbenzylated versions, indicated the masking effect was successful without appreciable remote directing effect (Table 4, entries 10–11). To our delight, usage of bulkier dicyclohexyl boron triflate resulted in β-10ai as the sole product. Unfortunately, this reagent was unable to improve the selectivity of 11ai (1α/2.5β). Our NMR studies suggested the boron-masked groups exhibited an electron-withdrawing effect similar to acetyl or benzoyl ester. Kim’s group recently published their comprehensive findings on the directing effect by remote electron-withdrawing protecting groups at O-3, O-4 and O-6 positions of donors in glucosylations53, galactosylations53 & mannosylations54. Our stereochemical outcomes closely followed the trend observed by Kim’s group, in particularly the β-directing effect at O-3 and O-4 positions of glucosyl donors. With our current results, we believed the stereoselectivities are largely influenced by the electron-withdrawing effect of boron complex and its spatial occupancy.
We set out to incorporate this chemistry into a streamlined protocol for design and preparation of important structural scaffolds, which were essential for more complex glycoconjugate synthesis. Our early results were demonstrated in Table 5. Activation of boron-masked thiomannoside 25a with stoichiometric amount of p-nitrobenzenesulfenyl chloride and excess silver trifluoromethanesulfonate, followed by introduction of thioglucoside 6i delivered product 12ai (Table 5, entry 1). This compound preserved both thiophenyl on glucose as well as free 2-OH on mannose intact. Since it was possible to use glycosyl donor with unrestricted hydroxyl groups, the same compound could be used as glycosyl acceptor. This presented new opportunities to build diverse library of oligosaccharides in a direct “one step-one glycosylation”, starting from the same building blocks. Herein exemplified, compound 28a and 7i could be interchangeably assigned the role of glycosyl donor or acceptor, or both, to obtain all four disaccharides (Table 5, entries 2–5). Notably, this flexibility was possible even with employment of a single type of thioglycoside as well as generic protective groups at only non-strategic location. To further expand on this concept, synthesis of trisaccharide 17ai was achieved via three possible routes (Fig. 4). Firstly, glucosamine 7i was used as the acceptor to couple with galactosyl donor 28a to provide disaccharide 16ai, which was immediately used as the donor to react with mannosyl acceptor 25a to furnish trisaccharide 17ai (Fig. 4a). Alternatively, glucosamine 7i was used as the donor to couple with mannosyl acceptor 25a to provide disaccharide 18ai, which was immediately used as the acceptor to react with galactosyl donor 28a, furnished the identical trisaccharide 17ai (Fig. 4b). Finally, trisaccharide 17ai was successfully prepared in an one-pot glycosylation protocol. Following complete consumption of donor 28a, a second equivalent of p-NO2PhSCl and freshly dried AgOTf were delivered to reaction mixture, and mannosyl acceptor 25a was added thereafter. Compound 17ai was isolated in 50% overall yield (Fig. 4c). No hydrolysis of acceptors or products as well as any self-condensation such as 13ai and 14ai were found. The moderate yield of 17ai was thought to stem from the less reactive acceptors since the only side product was hydrolysis of donor and employment of molecular sieves only improved the overall yield marginally.
In summary, we report an attractive and practical framework to construct glycosidic bonds with high stereoselectivity and regioselectivity without the need for orthogonal protection/deprotection or specialized leaving groups. While confident control of stereoselectivity has been galvanizing research into the field for generations, streamline of working strategies are increasingly important as the complexity quickly rises with every additional sugar being added into the growing glycan chain. We believe the liberation of protection/deprotection steps through temporal boron-masked chemistry would be of great value to more sophisticated strategies such as reactivity-based glycosylation or orthogonal one-pot activation, which relied heavily on a strict and judicious choice of protective groups to achieve the desired selectivity. Further mechanistic studies as well as refining the method for preparation of various glycoconjugates are ongoing in our laboratory.
Methods
General
The synthesis and characterization of new compounds are provided in the Supplementary Methods. For 1H-NMR and 13C-NMR spectra of the compounds in this article, see Supplementary Figs 1–92. Glycosidic linkage positions of 14ai, 16ai and 17ai were determined based on analysis of 1H, 1H-1H COSY, 1D-TOCSY, 13C-1H HSQC. Anomeric configuration of obtained mannosides 4ai, 7ai, 12ai were determined based on chemical shifts of anomeric protons and coupling constants of 13C1-1H1. For preliminary results, see Supplementary Discussions.
Experimental procedure
All reactions were conducted under an atmosphere of nitrogen, unless otherwise indicated. Anhydrous solvents were transferred via oven-dried syringe. Flasks were flame-dried and cooled under a stream of nitrogen. All reagents and solvents were obtained from commercial suppliers and used without further purification unless otherwise stated. Chromatograms were visualized by fluorescence quenching with UV light at 254 nm or by staining using a basic solution of potassium permanganate. Evaporation of organic solutions was achieved by rotary evaporation with a water bath temperature below 40 °C. Product purification by flash column chromatography was accomplished using silica gel 60 (0.010–0.063 mm). Technical grade solvents were used for chromatography and distilled prior to use. Optical rotations were measured in CHCl3 with a 1 cm cell (c given in g 100 mL−1). Melting points were obtained in open capillary tubes in melting point apparatus. IR spectra were recorded using FTIR and reported in cm−1. High resolution mass spectra (HRMS) were recorded on Q-TOF mass spectrometer. Accurate masses are reported for the molecular ion [M + H]+ or a suitable fragment ion. NMR spectra were recorded at room temperature on a 400 MHz and 500 MHz NMR spectrometer. The residual solvent signals were taken as the reference (726 ppm for 1H-NMR spectroscopy and 77.23 ppm for 13C-NMR spectroscopy). Chemical shifts are reported in delta (δ) units, parts per million (ppm) downfield from trimethylsilane (TMS). Chemical shift (δ) is referred in terms of ppm, coupling constants (J) are given in Hz. Following abbreviations classify the multiplicity: s = singlet, d = doublet, dd = doublet of doublet, t = triplet, q = quartet, m = multiplet, br = broad or unresolved.
Data availability
The authors declare that the data supporting the findings of this study are available within the article and its Supplementary Information files. All data are available from the authors upon reasonable request.
References
Khorana, H. G. et al. Studies on polynucleotides. J. Mol. Biol. 72, 209–475 (1972).
Cell Biology Correspondent. Is enough too much? Nature New Biol. 241, 33 (1973).
Varki, A. et al. Essentials of glycobiology, 2nd edn. (Cold Spring Harbor, 2009).
Dwek, R. A. Glycobiology: toward understanding the function of sugars. Chem. Rev. 96, 683–720 (1996).
Krasnova, L. & Wong, C.-H. Understanding the chemistry and biology of glycosylation with glycan synthesis. Annu. Rev. Biochem. 85, 599–630 (2016).
Wang, L. X. & Davis, B. Realizing the promise of chemical glycobiology. Chem. Sci. 4, 3381–3394 (2013).
Kiessling, L. L. & Splain, R. A. Chemical approaches to glycobiology. Annu. Rev. Biochem. 79, 619–653 (2010).
Boltje, T. J., Buskas, T. & Boons, G.-J. Opportunities and challenges in synthetic oligosaccharide and glycoconjugate research. Nat. Chem. 1, 611–622 (2009).
Wang, C.-C. et al. Regioselective one-pot protection of carbohydrates. Nature. 446, 896–899 (2007).
Cox, D. J. & Fairbanks, A. J. Stereoselective synthesis of α-glucosides by neighbouring group participation via an intermediate thiophenium ion. Tetrahedron: Asymmetry 20, 773–780 (2009).
Smoot, J. T. & Demchenko, A. V. How the arming participating moieties can broaden the scope of chemoselective oligosaccharide synthesis by allowing the inverse armed-disarmed approach. J. Org. Chem. 73, 8838–8850 (2008).
Kim, J. H., Yang, H., Park, J. & Boons, G.-J. A general strategy for stereoselective glycosylations. J. Am. Chem. Soc. 127, 12090–12097 (2005).
Bols, M. & Hansen, H. C. Long range intramolecular glycosidation. Chem. Lett. 6, 1049–1052 (1994).
Huang, M. et al. Dissecting the mechanisms of a class of chemical glycosylation using primary 13C kinetic isotope effects. Nat. Chem. 4, 663–667 (2012).
Crich, D. & Sharma, I. Influence of the O-3 protecting group on stereoselectivity in the preparation of C-mannopyranosides with 4,6-O-benzylidene protected donors. J. Org. Chem. 75, 8383–8391 (2010).
Imamura, A. et al. Di-tert-butylsilylene (DTBS) group-directed α-selective galactosylation unaffected by C-2 participating functionalities. Tetrahedron. Lett. 44, 6725–6728 (2003).
Olsson, J. D. M., Eriksson, L., Lahmann, M. & Oscarson, S. Investigations of glycosylation reactions with 2-N-acetyl-2-N,3-O-oxazolidinone-protected glucosamine donors. J. Org. Chem. 73, 7181–7188 (2008).
Manabe, S., Ishii, K. & Ito, Y. N-benzyl-2,3-oxazolidinone as a glycosyl donor for selective α- glycosylation and one-pot oligosaccharide synthesis involving 1,2-cis-glycosylation. J. Am. Chem. Soc. 128, 10666–10667 (2006).
Benakli, K., Zha, C.-X. & Kerns, R. J. Oxazolidinone protected 2-amino-2-deoxy-D-glucose derivatives as versatile intermediates in stereoselective oligosaccharide synthesis and the formation of α-linked glycosides. J. Am. Chem. Soc. 123, 12933 (2001).
Hsu, C.-H. et al. Highly α-selective sialyl phosphate donors for efficient preparation of natural sialosides. Chem. Eur. J. 16, 1754–1760 (2010).
Le Mai Hoang, K. & Liu, X.-W. The intriguing dual-directing effect of 2-cyanobenzyl ether for a highly stereospecific glycosylation reaction. Nat. Commun. 5, 5051 (2014).
Beaver, M. G. & Woerpel, K. A. Erosion of stereochemical control with increasing nucleophilicity: o-glycosylation at the diffusion limit. J. Org. Chem. 75, 1107–1118 (2010).
Padungros, P., Alberch, L. & Wei, A. Glycosyl dithiocarbamates: β-selective couplings without auxiliary groups. J. Org. Chem. 79, 2611–2624 (2014).
Fascione, M. A. et al. Stereoselective glycosylation using oxathiane glycosyl donors. Chem. Commun. 39, 5841–5843 (2009).
D’Angelo, K. A. & Taylor, M. S. Borinic acid catalyzed stereo- and regioselective couplings of glycosyl methanesulfonates. J. Am. Chem. Soc. 138, 11058–11066 (2016).
Beale, T. M., Moon, P. J. & Taylor, M. S. Organoboron-catalyzed regio- and stereoselective formation of β-2-deoxyglycosidic linkages. Org. Lett. 16, 3604–3607 (2014).
Gouliaras, C., Lee, D., Chan, L. & Taylor, M. S. Regioselective activation of glycosyl acceptors by a diarylborinic acid-derived catalyst. J. Am. Chem. Soc. 133, 13926–13929 (2011).
Tanaka, M., Nashida, J., Takahashi, D. & Toshima, K. Glycosyl-acceptor-derived borinic ester-promoted direct and β-stereoselective mannosylation with a 1,2-anhydromannose donor. Org. Lett. 18, 2288–2291 (2016).
Nakagawa, A., Tanaka, M., Hanamura, S., Takahashi, D. & Toshima, K. Regioselective and 1,2-cis-α-stereoselective glycosylation utilizing glycosyl-acceptor-derived boronic ester catalyst. Angew. Chem. Int. Ed. 54, 10935–10939 (2015).
Kaji, E., Nishino, T., Ishige, K., Ohya, Y. & Shirai, Y. Regioselective glycosylation of fully unprotected methyl hexopyranosides by means of transient masking of hydroxy groups with arylboronic acids. Tetrahedron. Lett. 51, 1570–1573 (2010).
Kaji, E. et al. Thermodynamically controlled regioselective glycosylation of fully unprotected sugars through Bis(boronate) intermediates. Eur. J. Org. Chem. 17, 3536–3539 (2014).
Rocheleau, S., Pottel, J., Huskić, I. & Moitessier, N. Highly regioselective monoacylation of unprotected glucopyranoside using transient directing-protecting groups. Eur. J. Org. Chem. 3, 646–656 (2017).
Paulsen, H. Advances in selective chemical syntheses of complex oligosaccharides. Angew. Chem. Int. Ed. 21, 155–173 (1982).
Mootoo, D. R., Konradsson, P., Udodong, U. & Fraser-Reid, B. Armed and disarmed n-pentenyl glycosides in saccharide couplings leading to oligosaccharides. J. Am. Chem. Soc. 110, 5583–5584 (1988).
Douglas, N. L., Ley, S. V., Lucking, U. & Warriner, S. L. Tuning glycoside reactivity: new tool for efficient oligosaccharide synthesis. J. Chem. Soc., Perkin Trans. 1 1, 51–65 (1998).
Zhang, Z. et al. Programmable one-pot oligosaccharide synthesis. J. Am. Chem. Soc. 121, 734–753 (1999).
Dulaney, S. B., et al. Divergent Synthesis of Heparan Sulfate Oligosaccharides. J. Org. Chem. 80, 12265-12279 (2015).
Burkhart, F., Zhang, Z., Wacowich-Sgarbi, S. & Wong, C.-H. Synthesis of the Globo-H hexasaccharide using the programmable reactivity-based one-pot strategy. Angew. Chem. Int. Ed. 40, 1274–1277 (2001).
Mackenzie, L. F., Wang, Q., Warren, R. A. J. & Withers, S. G. Glycosynthases: mutant glycosidases for oligosaccharide synthesis. J. Am. Chem. Soc. 120, 5583–5584 (1998).
Nycholat, C. M. et al. Synthesis of biologically active N- and O-linked glycans with multisialylated poly-N-acetyllactosamine extensions using p. Damsela α−2,6 sialyltransferase. J. Am. Chem. Soc. 135, 18280–18283 (2013).
Serna, S., Yan, S., Martin-Lomas, M., Wilson, I. B. H. & Reichardt, N.-C. Fucosyltransferases as synthetic tools: glycan array based substrate selection and core fucosylation of synthetic N-glycans. J. Am. Chem. Soc. 133, 16495–16502 (2011).
Shivatare, S. S. et al. Modular synthesis of N-glycans and arrays for the hetero-ligand binding analysis of HIV antibodies. Nat. Chem. 8, 338–346 (2016).
Wang, Z. et al. A general strategy for the chemoenzymatic synthesis of asymmetrically branched N-glycans. Science 341, 379–383 (2013).
Koizumi, A. et al. Top-down chemoenzymatic approach to a high-mannose-type glycan library: synthesis of a common precursor and its enzymatic trimming. Angew. Chem. Int. Ed. 52, 7426–7431 (2013).
Seeberger, P. H. The logic of automated glycan assembly. Acc. Chem. Res. 48, 1450–1463 (2015).
Wu, Y., Xiong, D.-C., Chen, S.-C., Wang, Y.-S. & Ye, X.-S. Total synthesis of mycobacterial arabinogalactan containing 92 monosaccharide units. Nat. Commun. 8, 14851 (2017).
Calin, O., Eller, S. & Seeberger, P. H. Automated polysaccharide synthesis: assembly of a 30-mer mannoside. Angew. Chem. Int. Ed. 52, 5862–5865 (2013).
Ishiwata, A., Lee, Y. J. & Ito, Y. Recent advances in stereoselective glycosylation through intramolecular aglycon delivery. Org. Biomol. Chem. 8, 3596–3608 (2010).
Cumpstey, I. Intramolecular aglycon delivery. Carbohydr. Res. 343, 1553–1573 (2008).
Padungros, P. et al. Synthesis and reactivity of 4’-deoxypentenosyl disaccharides. J. Org. Chem. 79, 4878–4891 (2014).
Codée, J. D. C. et al. Ph2SO/Tf2O: A powerful promotor system in chemoselective glycosylations using thioglycosides. Org. Lett. 5, 1519–1522 (2003).
Dinkelaar, J. et al. Stereodirecting effect of the pyranosyl C-5 substituent in glycosylation reactions. J. Org. Chem. 74, 4982–4991 (2009).
Baek, J. Y. et al. Directing effect by remote electron-withdrawing protecting groups at O−3 or O−4 position of donors in glucosylations and galactosylations. Tetrahedron. 71, 5315–5320 (2015).
Baek, J. Y., Lee, B.-Y., Jo, M. G. & Kim, K. S. β-Directing effect of electron-withdrawing groups at O-3, O-4 and O−6 positions and α-Directing effect by remote participation of 3-O-Acyl and 6-O-Acetyl groups of donors in mannopyranosylations. J. Am. Chem. Soc. 131, 17705–17713 (2009).
Acknowledgements
Financial support from the Ministry of Education (MOE 2013-T3-1-002), National Research Foundation (NRF2016NRF-NSFC002-005) and Nanyang Technological University (RG14/16), Singapore are gratefully acknowledged.
Author information
Authors and Affiliations
Contributions
L.M. H.K. and L.X.-W. designed the project and wrote the manuscript. L.M.H.K. carried out the synthetic work. H.J.X. and B.G. assisted in preparation of several glycosyl donors for the study. All authors discussed the results and commented on the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Le Mai Hoang, K., He, Jx., Báti, G. et al. A minimalist approach to stereoselective glycosylation with unprotected donors. Nat Commun 8, 1146 (2017). https://doi.org/10.1038/s41467-017-01073-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-017-01073-7
- Springer Nature Limited
This article is cited by
-
Stereoselective oxidative glycosylation of anomeric nucleophiles with alcohols and carboxylic acids
Nature Communications (2018)