Abstract
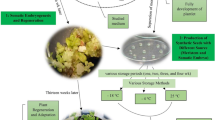
Enhancing of the efficient tissue culture protocol for somatic embryos would facilitate the engineered breeding plants program. In this report, we describe the reproducible protocol of Malaysian rice (Oryza sativa L.) cultivar MR219 through somatic embryogenesis. Effect of a wide spectrum of exogenesis materials was assessed in three phases, namely callogenesis, proliferation and regeneration. Initially, rice seeds were subjected under various auxin treatments. Secondly, the effect of different concentrations of 2,4-D on callus induction was evaluated. In the next step, the efficiency of different explants was identified. Subsequently, the effects of different auxins, cytokinins, l-proline, casein hydrolysate and potassium metasilicate concentrations on the callus proliferation and regeneration were considered. For the callogenesis phase, 2 mg L−1of 2,4-D and roots were chosen as the best auxin and explant. In the callus proliferation stage, the highest efficiency was observed at week eight in the MS media supplemented with 2 mg L−1 of 2,4-D, 2 mg L−1 of kinetin, 50 mg L−1 of l-proline, 100 mg L−1 of casein hydrolysate and 30 mg L−1 of potassium metasilicate. In the last phase of the research, the MS media added with 3 mg L−1 of kinetin, 30 mg L−1of potassium metasilicate and 2 mg L−1 of NAA were selected. Meanwhile, to promote the roots of regenerated explants, 0.4 mg L−1 of IBA has shown potential as an appropriate activator.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The production of high productive engineered plants needs appropriate strategies. The genetic transformation mechanism involves pivotal steps such as preparation of the initial conditions, usage of an efficient DNA delivery method, establishment of effective growth and selection of medium as well as maintenance of transformants (Abiri et al. 2015). Notwithstanding improvement of gene transformation efficiency in some plant species, the pre- and post-transformation conditions of Indica rice have been a matter of concern (Visarada and Sarma 2002). The efficiency ratio of engineered plants is severely genotype dependent (Yinxia and Te-chato 2012). On the other hand, presenting protocols of pre-transformation phases for the same species have revealed the vast effects of other factors on the plant’s adaptation to in vitro experiments. In this regard, size, source and age of explants, seasonal variation, oxygen gradient, intensity as well as quality of light, temperature and ploidy level are effective endogenous or exogenous factors which may change the genotype feedback in different experiments (Aggarwal et al. 2012). Therefore, the evaluation of useful endogenous or exogenous factors for differentiation and regeneration of Indica rice in vitro are pre-requirements in genetic transformation programs (Haque et al. 2003).
Plant tissue culture is a symphony of art and science, which develops genetic diversity, produces virus-free plants and improves micropropagation under aseptic conditions in the short term (Birch 1997). Plant cells possess high plasticity potential for cell differentiation. Stresses such as pathogen infection or wounding may lead to the production of tumours or callus. The history of the first callus growth traced back to 1979 when Neely described a massive and disorganized cell mass in debarked trees (Neely 1979). Embryogenic calli, rather than direct tissues such as immature inflorescences, shoot spices, leaves and roots, is an effective and safe tool for regeneration of wild and modified plants in vitro conditions (Benlioglu et al. 2015). Interestingly, calli are divided to various subgroups according to their microscopic traits. For instance, calli with some organ regeneration are named embryonic, shooty or rooty calli, whereas calli without organ regeneration are called compact or friable callus (Ikeuchi et al. 2013). Callogenesis and growth highly depend on genotype, basal salt mediums, plant growth regulators (PGRs), organic components, carbohydrate, explants and adjuvant materials (Pawar et al. 2015). Additionally, media strength is another essential factor to regulate callus’ growth and regeneration (Din et al. 2016).
A single or combination of PGRs plays a pivotal role in tissue culture growth, cell division and morphogenesis (Dahot 2007). Auxins are involved in several developmental pathways of crops, including rooting, abscission, internodes and stem elongation, apical dominance and tropisms (Visarada and Sarma 2002; Roy et al. 2015; Din et al. 2016). Auxins in tissue culture induce embryogenic and organogenic differentiation, cyto-differentiation and cell division. Natural auxins such as indole-3-acetic acid (IAA), indole-3-butyric acid (IBA), a-naphthaleneacetic acid (NAA), para-chlorophenoxyacetic acid (p-CPA), 4-amino-3, 5,6-tricholoropyridinecarboxylic acid (picloram), 2,4-dicholorophenoxyacetic acid (2,4-D), 2,4,5-tricholorophenoxyacetic acid (2,4,5-T), naphthoxyacetic acid (NOA) and 3,6-dichloro-o-anisic acid (dicamba) are examples of synthetic auxins in plants. IAA, IBA and NAA mainly interact with cytokinin to proliferate shoots and induce roots. The three auxins have been implicated in tracheid differentiation of callus and cell culture (Bhojwani and Dantu 2013). 2,4,-D and 2,4,5-T (systematic herbicides) promote callogenesis, differentiation and growth. Noticeably, 2,4-D, dicamba and picloram are the pivotal auxins for somatic embryogenesis. Although 2,4-D is used for the majority of plants, dicamba and picloram are particularly used for monocots and legumes, respectively (Konaté et al. 2013; Egan et al. 2014).
Cytokinins are N6-substituted adenine derivatives which naturally affect shoot differentiation, apical dominance modification and cell division. BAP [(benzylamino) purine, kinetin (6-furfurylamino) purine (KIN), 6-(c,c-dimethylallyl amino) purine)], Thidiazuron (TDZ), 2iP [(2- isopentenyl)-adenine, a dipheny1-substituted urea and zeatin [6-(4-hydroxy-3- methyl but-2-enyl amino)-purine)] are common sorts of cytokinins (Bhojwani and Dantu 2013). Cytokinins decrease the apical meristem dominance and induce adventitious shoots formation and auxiliary from meristematic explants (Ngomuo and Ndakidemi 2013). The ratio of auxin/cytokinin in the medium may lead to somatic embryo’s development as well as root and shoot induction (Azizi et al. 2015).
The explant’s type and age, physiological stage and differentiation degree of tissues have been recognized as the most important factors influencing regeneration via somatic embryogenesis. Generally speaking, meristematic tissues and immature organs which possess undifferentiated cells are appropriate explants for plant regeneration. Reportedly, immature embryos are the suitable responsive explants for rice tissue culture (Haque et al. 2003; Konaté et al. 2013; Vennapusa et al. 2015). Due to some restriction factors such as a shorter period of time for the immature embryos’ cycle, seasonal limitations and dormancy, other sorts of explants which are available during the year may be the appropriate choice (Hoque and Mansfield 2004; Wu et al. 2013). In comparison with immature seeds, mature seeds are not appropriate explants for embryogenic callus for recalcitrant plants in tissue culture. Seeds of various recalcitrant plant species like Indica rice may possess restrictions in promoting callus in vitro culture (Thokozani et al. 2013). Efforts have been made to find appropriate rice explants to induce embryogenic calli under suitable in vitro conditions (Zuraida et al. 2011).
Artificial media are not a fully autotrophic method. For the plant to survive, it needs energy and the osmotic potential needs a carbohydrate source in the culture media itself (Yaseen et al. 2013). Water movement and potential and mineral movements in the media are the most important factors that are affected by carbon sources (Buah et al. 2011). Based on plants spices’ genotype and explants, many carbon sources have been used to develop somatic embryogenesis (Buah et al. 2011; Yaseen et al. 2013). Growth, development and morphogenesis of plant organs, tissues and cells in vitro culture are importantly affected by the composition of mineral nutrients (Sivanesan and Park 2014). Nutritional supplements including proline and casein hydrolysate have been reported to increase callus induction (Lin and Zhang 2005). Proline is an α-amino acid that is essential for embryogenic callus growth, formation and primary metabolism (Szabados and Savoure 2010; Che Radziah et al. 2012; Pawar et al. 2015). Interaction with nitrogen sources such as ammonium and nitrate is the main function of proline in plant tissue culture (Holme et al. 1997). Silicon (Si), the most abundant mineral and organic nutrient in soil, is beneficial for growth, improvement and production of plant spices (Sahebi et al. 2015). Additionally, Si in tissue culture may improve embryogenesis, organogenesis, and the physiological and anatomical characteristics of explants. Accordingly, Si may participate in various crop tissue cultures including secondary metabolites production, micropropagation, organogenesis, somatic embryogenesis and cryopreservation (Sivanesan and Park 2014). Although silicon is not an essential nutrient for all plants, many plants accumulate higher silicon levels than essential macronutrients (Abro et al. 2009).
Rice (Oryza sativa L.) is the main staple food source for more than half of the world’s population (Zuraida et al. 2010). Asian cultivated rice comprise of two main classes, the Japonica (Oryza sativa ssp. Japonica) and Indica (Oryza sativa ssp.Indica). In South-East and South Asia countries, the Indica species is the most cultivated rice (Zhang et al. 2005). Some Malaysian rice cultivars have been shown to have poor responses to callus induction, growth, proliferation and regeneration. Diverse tissue culture protocols in different rice cultivars were mostly because of different developmental stages of explants and genotypes potential (Saharan et al. 2004; Islam et al. 2005; Khaleda and Al-Forkan 2006; Zuraida et al. 2010; Islam et al. 2014).
Azizi et al. (2015) showed that two Malaysian cultivars, namely MARDI’s Quality rice 74 (MRQ74) and MRQ50, also had the lowest number of callus induction from seed, proliferation and regeneration frequency. Among the four Malaysian upland rice cultivars, Kusan and Siam cultivars showed lowest response in terms of callus induction frequency and regeneration capability of the embryogenic callus (Shahsavari 2010). MR219 cultivar is an Indica rice developed via a cross between MR151 and MR137 by the Malaysian Agricultural Research and Development Institute (MARDI), in 2001 (Talei et al. 2013). The cultivar has been considered as a high production rice with an appropriate quality in taste and shape, but sensitive to environmental changes (Panjaitan et al. 2009). In spite of some agricultural advantages of MR219, the rice cultivar is regarded as a recalcitrant variety. Although various protocols have been reported for micropropagation of MR219, its callogenesis and regeneration ratio are still limited. However, it seems that by evaluating the effect of other factors such as 2,4,5-T, other explants and the suitable time to add adjuvant materials as well as using specific PGRs in the relevant stages, researchers can find new insights into the tissue culture of recalcitrant plant species such as MR219.
Keeping in view the above facts, in the present investigation we attempted to develop a robust protocol for callogenesis, proliferation and plant regeneration of MR219 by using different concentrations of PGRs, interactions of auxin/cytokinin, explants and adjuvant materials.
Materials and methods
Plant materials and culture conditions
MR219 seeds were obtained from the Malaysian Agricultural Research and Development Institute (MARDI), Seberang Perai, Malaysia. The mature seeds were dehusked and rinsed with running water for 1 h. The dehulled seeds were surface sterilized with 70% (v/v) ethanol for 3 min and rinsed with double distilled water to remove ethanol traces. Then, the dehusked seeds were shaken in 40% (v/v) sodium hypochlorite for 20 min. The seeds were rinsed thoroughly with double distilled water before being dried on sterilized filter papers in a petri dish. The Murashige and Skoog (1962) medium supplemented with 2.75 g L−1gelrite, 30 g L−1 sucrose and modified B5 vitamin (Nwe et al. 2011) was used as the basal medium. The medium’s pH was adjusted to 5.8, then autoclaved and cooled under a laminar flow cabinet.
Callus frequency players
Optimization of auxins application on callus frequency
MS basal media supplemented with 1 mg L−1 of different types of auxins, 2,4-D, NAA, IBA, picloram, dicamba and 2,4,5-T were used for rice callogenesis. In the experiment, callus induction frequency (Azizi et al. 2015), days to callus induction and rooty or shooty callus induction percentages (R/S CI %) were evaluated after four weeks. Then, to optimize the concentration of auxin used, MS media were supplemented with different concentrations of 2,4-D (control, 0.5, 1, 1.5, 2, 2.5, 3, 5, 10, 15 and 20 mg L−1). Parameters recorded were days to callus induction and dead explants/no callus induction.
Effect of different explants on callus frequency
To find out the effects of explants on callus frequency, different explants involving seeds, roots, shoots and leave were used. To obtain the roots, shoots and leaves explants, the rice seeds were cultured in MSO (MS media-free hormone) media. Two weeks after germination of seeds in the media, the explants were excised and transferred to MS media supplemented with the best 2,4-D concentration (2 mg L−1) achieved form the previous step. In the current experiment, various traits such as callus induction frequency, days to callus induction and dead explants were evaluated after 4 weeks. For callus frequency experiments, three replicates with 40 samples were implemented.
Callus growth orchestration
To find out the most effective adjuvant materials on callus growth, MS media supplemented with 2 mg L−1 of 2,4-D were used as selection media. In the current step, various cytokinins, casein hydrolysate, proline and silicon concentrations were evaluated.
Effect of various cytokinin concentrations on callus growth
To optimize the callus growth pattern, 200 mg callus achieved from the previous steps was cultured in the selected MS media supplemented with 0, 1, 2 and 3 mg L−1 of BAP, KIN, TDZ and Zeatin. MS media added with 2 mg L−1 of 2,4-D were used as control. After culturing calli in the selection media added with cytokinins, the petri dishes were kept in the dark place in the chamber room at 25 °C. For callus growth curve identification, the calluses’ fresh and dry weight was measured at weekly intervals for 7 weeks.
Effect of various adjuvant materials and concentrations on callus growth
To find out the effect of different adjuvant materials and concentrations, experiments were implemented using casein hydrolysate, l-proline and potassium metasilicate. To run the investigation, MS proliferation media (MS selection media added with 2 mg L−1 of 2,4-D and 2 mg L−1 of KIN) were applied simultaneously in three separate steps. To this end, the four-week-old clump callus (200 mg) was transferred to MS proliferation media supplemented with 0, 50, 100, 150 and 200 mg L−1 of casein hydrolysate, l-proline and 0, 10, 20, 30, 40 mg L−1 of potassium metasilicate (K2SiO3). To identify the effect of each treatment on callus growth, the fresh and dry weights of calli were evaluated in week 4 (the highest fresh and dry weight callus achieved from the last step).
Plant regeneration symphony
To find out the optimum regeneration media, regeneration experiments were implemented by using different concentrations of cytokinins, auxins, l- proline, casein hydrolysate and potassium metasilicate.
Effect of various cytokinin concentrations on plant regeneration
Two grams of seven-week-old clump calluses was transferred to the initial MS regeneration media supplemented with 2 mg L−1 of 2,4-D, 0, 1, 2, 3, 4 and 5 mg L−1 of BAP, KIN, TDZ and Zeatin. The culture media were placed under light conditions at 27 °C with a 16-h photo period (110 mmol/m2/s) for 20 days.
Effect of various auxin concentrations on plant regeneration
Two grams of seven-week-old clump calluses was transferred to the MS regeneration media supplemented with 2 mg L−1 of 2,4-D, 3 mg L−1 of KIN, 0, 1, 2 and 3 mg L−1 of IAA, ABA, NAA. As a control treatment, a MS medium added with 2 mg L−1 of 2,4-D, 3 mg L−1of KIN was used. The culture media were placed under light conditions at 27 °C with a 16-h photo period (110 mmol/m2/s) for 20 days.
Effect of various adjuvant material concentrations on plant regeneration
Two grams of seven-week-old clump calli was transferred to MS regeneration media supplemented with 2 mg L−1 of 2,4-D, 3 mg L−1 of KIN, 2 mg L−1 NAA, 0, 50, 100, 150 and 200 mg L−1 of casein hydrolysate and l-proline and 0, 10, 20, 30, 40 mg L−1 of potassium metasilicate (K2SiO3) simultaneously. The culture media were placed under light conditions at 27 °C with a 16-h photo period (110 mmol/m2/s) for 20 days.
For all the regeneration experiments, the green plantlet differentiation rate was measured as follows.
Regenerated plantlets’ protection art
One-month-old regenerated plants were transferred to various root induction media. To this end, MS media added with diverse concentrations of IAA, IBA and ABA, 0, 0.1, 0.2, 0.3 and 0.4 were used. Before transferring the regenerated plants to the new media, the mean of root length was measured. After one month, the root length mean was calculated again and the percentage of increasing root length was evaluated. Root segments above 2 mm were assumed as induced roots. After root and shoot induction, the explants were transferred to Yoshida media and then transferred to the soil.
Localization of potassium metasilicate in treated samples
Quantitative analysis and images of samples under potassium metasilicate (K2SiO3) were obtained using energy-dispersive X-ray spectroscopy (EDX) (LEO 1455 VPSEM, New England) and scanning electron microscopy (SEM).
To evaluate the amount of silicon by EDX, energy to wavelengths were measured as follows
Three independent replicates of control and treated samples were used to display the effect of potassium metasilicate accumulation in calli and roots of the individual samples. To evaluate the silicon content of samples, three different spectrums of roots were randomly measured for each image. The magnification and accelerating voltage of images are 350 × and 20.00 kV, respectively.
Statistical analysis method
To analyse the data, the SAS software version 9.4 was used. The level of significance was evaluated from the analysis of variance (ANOVA). Duncan’s multiple range was used to compare the mean values, and interpretations were made accordingly.
Results and discussion
Callus induction frequency
Effect of different auxins on callus frequency
The initial step of this experiment was designed to identify the best auxin effect on callus frequency, days to callus induction and R/S CI %. The results showed significant differences between the average of callus frequency and R/S CI % for treatments. In spite of R/S CI %, days to callus induction for auxins which produced calli were not significant (Fig. 1). Meanwhile, seeds in the control treatments germinated without callogenesis (Fig. 2a). Accordingly, among all the auxins, 2,4-D produced the highest callus frequencies, 61% without rooty or shooty callus (Fig. 2b), whereas lower percentage of callogenesis was observed in picloram (16%) (Fig. 2d), dicamba (19%) (Fig. 2e) and 2,4,5-T (14%) (Fig. 2f) treatments. On the other hand, NAA and IBA treatments induced roots and shoots only in seeds (Fig. 2c).
Interestingly, structural cell wall materials’ deposition such as pectin, hemicellulose and cellulose is essential for inducing, maintaining or/and establishing cellular differentiation and proliferation steps. Therefore, calli are induced under the loss-of-function mutations in cell wall (Iwai et al. 2002). Callogenesis is usually accompanied by tissue reunion or cork formation and is occasionally followed by organogenesis (Blackman and Matthaei 1901; Walles and Buvat 1989; Sugiyama 2015). Three dependent pathways are assumed for the organogenesis process in vitro conditions. In the initial step, cells are able to response to organogenesis induction signals such as PGRs (competence acquisition). During the second step, competent cells are determined and canalized by a suitable growth regulator balance for a specific organogenesis (organogenesis induction). Finally, organ development happens independently of external PGRs (morphological differentiation) (Wani et al. 2011; Sugiyama 2015). While in the current study, the most effective auxin to initiate callogenesis is 2,4-D, some researchers prefer to use IAA or NAA (Wani et al. 2011; Ngomuo and Ndakidemi 2013). Based on the evaluated traits in the present phase, plant cells under 2,4-D are able to produce higher percentages of callus. To achieve the second phase where the competent cells should be canalized to perform specific pathways of organogenesis, the experiment stage was implemented to release the best 2,4-D concentration on the somatic callus induction. Rahman et al. (2007) reported that 2,4-D possess the capacity to remove actin and slow down cytoplasmatic streaming. Reportedly, the actin cytoskeleton shows an active role in the elaboration of cell division, expansion, differentiation and organ initiation by specific changes in cell morphology and cytoarchitecture (Kandasamy et al. 2001). Actin cytoskeleton structures are affected by 2,4-D, which leads to crop epinasty, the mobility alteration of mitochondria and peroxisomes into the cell environments, and an impression on their metabolism, as they share many metabolites with each other and with chloroplasts. Lastly, reactive oxygen species (ROS) production is blocked by the antioxidant activity of peroxisomes in different cell parts. A reduced peroxisomes antioxidative function was observed after affection of actin polymerization and bundling by auxinic herbicides. Hence, the cell enters a state of severe oxidative stress because of the loss of peroxisomes and the mitochondria actions (Rodríguez-Serrano et al. 2014).
Effect of 2,4-D concentrations on callus frequency
In the second step of this experiment, the effects of various concentrations of 2,4-D on the callus frequency percentage, days to callus induction and dead seeds/no callus were evaluated. Analysis of different concentrations of 2,4-D showed significant differences between all the traits. Interestingly, the highest percentage of callus frequency was observed in 2 mg L−1 of 2,4-D (75%), whereas the lowest percentage of callus frequency was found in the higher levels of 2,4-D in 10, 15 and 5 mg L−1 with 44, 48 and 49%, respectively. On the other hand, the lowest and highest numbers of days to callus induction were observed at 2, 15 and 20 mg L−1 of 2,4-D at 13, 21 and 20 days, respectively. Finally, 2 mg L−1 of 2,4-D produced less dead seeds or seeds without callus (22%) but regulated the promotion of germinated seeds (Fig. 3).
The embryogenic calli produced in 2 mg L−1 of 2,4-D were compact, nodular, dry, iso-diametric, big and rather regular in size and were tightly held together, relatively smooth and yellowish to whitish (Fig. 4a). In contrast, non-embryogenic calli were elongated, soft and loosely held cells on the surface (Fig. 4b), which turned brown and died (Fig. 4c). In parallel with the above observations, Christianson and Warnick (1985) and Rashid et al. (2009) reported that nodular and compact type of calli showed the unorganized cell division with highly viable cells for additional culture growth. Based on Jila et al. (2014), the calli colour is the suitable selection criterion for classifying embryogenic calli. Reportedly, the inhibitory impact of 2,4-D on chlorophyll formation may be cause of the whitish or yellowish calli (Shukla et al. 2014). According to the results of the present experiment, the frequency of callus induction between different treatments was significant, and the quality had improved in the MS media supplemented with 2 mg L−1 of 2,4-D. Nonetheless, due to the low rate of callus induction in the mentioned media, the next step was designed to find out the efficiency of explants in response to the optimized media.
Effect of various explants on callus frequency
The mean comparison of different explant treatments revealed that there are significant differences (p ≤ 0.01) among the four rice explants in terms of callus frequency, days to callus induction and dead explants/no callus. In comparisons with other explants, root possesses the highest callus frequency, lowest days to callus induction and lowest percentage of dead explants/no callus. Although the highest callus frequency was observed in roots and seeds with 82 and 76%, respectively, the days to callus induction between roots and seeds was not significant. On the other hand, the percentage of callus inductions was low and the days to callus induction % was higher than both root and seed explants. These results confirmed that root was the best explants, whereas the lowest amount of the measured traits was found in leaf explants (Fig. 5).
Single totipotent cells and the fertilized egg are the initial sources of all cell sorts which compose multi-cellular tissues and organisms via successive cell division as well as cell differentiation procedures. In plant, most cells possess the totipotency potential, since they are able to regenerate the complete array of plant tissues via already differentiated organs. A wide spectrum of crop tissues has been shown the ability to regenerate whole plants under suitable culture conditions (Stefanello et al. 2005; Thokozani et al. 2013; Rodríguez-Serrano et al. 2014; Ahmad et al. 2015; Vennapusa et al. 2015; Elhiti and Stasolla 2016).
In plants kingdom, pericycle is a vital tissue which enables plants development through various cell types activity at all stages of life. Reportedly, root or shoot explants of Arabidopsis culture in callus-inducing medium (CIM) added with cytokinin and auxin induced callus from pericycle cells adjacent to the xylem poles. Additionally, shoot and root regeneration seemed to partly resemble the lateral root meristem formation (Jia et al. 2014; Ji et al. 2015). Interestingly, the root or shoot calli are not unorganized cells mass, as they were organized structures similar to the primordia of lateral roots (Jia et al. 2014). Furthermore, the transcriptome analysis showed that the gene expression profile of these calli types is similar to the root meristems (Atta et al. 2009). Sugimoto et al. (2010) revealed that aerial organs calli such as petals and cotyledons possess organized structures resembling lateral root primordia. In the current step, using excised roots has been established as a suitable tentative model for the callogenesis step.
Callus growth orchestration
Effect of cytokinins concentrations on callus growth
Fresh and dry callus growth rates were significant between all the treatments. The lowest and highest growth rates belonged to control (no cytokinin) and 2 mg L−1 of KIN. Meanwhile, the highest fresh and dry weight (Fig. 6) rates belonged to week 4 for lots of cytokinins treatments. This results show that the combination of cytokines with auxins were more effective in the induction and proliferation of embryogenic callus. Interestingly, while auxin (2,4-D) increases the callus’ quality, cytokinins like KIN increase the callus growth rate. In this step, the differences between PGR combinations may be due to the physiological activity of the cytokinins. The experiment results showed that using 2,4-D alone is not suitable to proliferate and increase callus. Meanwhile, the maintenance of callus in the media for long periods of time releases inhibitory effects on callus proliferation. In the weekly analysis, the highest rate of compact, yellowish, iso-diametric cells and smooth callus were observed in week four of 2 mg L−1 of KIN (Fig. 7a). After four weeks, growth weight was decreased and leads to change the callus colour to brown (Fig. 7b). Reportedly, the positive effect of auxins and cytokinins concentrations was seen in embryogenic types of Indica rice and maize callus (Valvekens et al. 1988; Li et al. 2009; Sugimoto et al. 2010).
Effect of various adjuvant material concentrations on callus growth
In the current step, there were significant differences in the fresh and dry callus growth of l-proline treatment. Firstly, in the proline experiment, the highest and lowest callus’ fresh weights (0.26 gm) were observed in MS media supplemented with 50 mg L−1 proline and control (0.21 gm). On the other hand, the highest and lowest dry weights were found in 50 mg L−1 proline (0.068 gm) and 200 mg L−1 proline (0.04 gm) (Fig. 8a).
Although there were significant differences between callus’ fresh weights in the casein hydrolysate treatments, there was no significant differences found in callus dry weights in casein hydrolysate treatments. In the casein hydrolysate treatment, the highest and lowest rates of callus’ fresh growth rates were observed in 100 mg L−1 casein hydrolysate(0.26 gm) and control (0.201) (Fig. 8a). While the callus’ dry weights in casein hydrolysate were not significant, the highest and lowest rates were found in100 mg L−1 casein hydrolysate (0.07 gr) and control (0.05) (Fig. 8a).
Figure 8b shows that callus’ fresh weights were significantly (p ≤ 0.01) different, but callus’ dry weights were not significantly different in the potassium metasilicate treatments. In parallel with the above results, the highest and lowest callus fresh weights were seen in 30 mg L−1(0.24 gm) and control (0.05 gm) (Fig. 8b). The highest and lowest callus dry weights were also found in30 mg L−1 (0.05 gm) and control (0.04 gm).
Nutritional additions such as proline and casein hydrolysate have been reported to improve callusing reaction (Lin and Zhang 2005). Accumulation of proline occurs in plants, marine invertebrates, eubacteria and protozoa after adverse conditions (Pawar et al. 2015). Accumulation of this anti-stress has been described after drought, low temperature, high temperature, salt heavy metal, UV irradiation, anaerobiosis, atmospheric pollution and pathogen infection (Hare and Cress 1997; Rachmawati and Anzai 2006; Narciso and Hattori 2010; Pawar et al. 2015). Generally speaking, proline accumulation in plants leads to enzymatic regulator functions, osmo-protection, carbon reverse and nutritional nitrogen storage under stress (Holme et al. 1997). Proline is added to the media as an organic nitrogen source. The promotive effects of l-proline on inducing and proliferating embryogenesis callus were observed in different plants such as Oryza sativa, Dactylis glomerata and Zea maize (Siripornadulsil et al. 2002; Pawar et al. 2015). Reportedly, the proline’s presence in the medium appears to produce a required stress condition where there is a decrease in water potential, increasing the nutritional elements accumulation in cells, callus and enhance embryogenesis. Although the biochemical and physiological roles of proline in tissue culture are still unclear, the interaction of proline with nitrogen sources of media such as nitrate and ammonium content has been reported. Hence, proline accumulation is effective for the proliferation, initiation and maintenance of embryogenic callus (Chowdhry et al. 1993; Verbruggen and Hermans 2008). Nonetheless, high accumulation of proline causes toxicity affects, which leads to negative impact on callus growth rate (Hare and Cress 1997).
Casein hydrolysate is a rich source of phosphate, calcium, vitamins, several microelements and a mixture of up to 18 amino acids. This nutrient may overcome the shortage or lack of glutamine while there is inadequate phosphorus for adequate biosynthesis. It has been reported that the addition of casein hydrolysate is more effective than the addition of main amino acids alone (Verbruggen and Hermans 2008; Pawar et al. 2015) due to the fact that proline and casein hydrolysate provide a more accessible to nitrogen source. A low concentration of proline and casein hydrolysate is non-toxic which helps maintain the cell for a longer period (Siripornadulsil et al. 2002; Verbruggen and Hermans 2008).
Numerous investigations have reported that biosilica treatment can improve the growth rate and production of different plants, especially while plants are subjected to adverse conditions (Moghaddam et al. 2000; Ma 2004; Vogel 2005; Abro et al. 2009; Sivanesan and Park 2014). The availability of silicon in crop production area and hydroponic system is limited. Accumulation of silicon in soilless substrate or nutrient solutions increases growth traits, quality and yield of diverse plants (Ma 2004). In another report, the highest callus induction frequency in rice was achieved in MS media containing CaSiO3 (He et al. 2013). Generally, the biosilica effect on physiological and morphological potential of in vitro crop cultures depends on genotype, species and silicon concentrations (Sivanesan and Park 2014). In the current experiment, the calli in the MS media supplemented with 30 mg L−1 absorbed silicon from the media (Fig. 9). Silicon may increase the structural stability of callus during its growth and proliferation as has been observed by George and Sherrington (1984). Interestingly, from our observation, the accumulation of silicon in the MS media promoted calli production obtained from root explants and stem nodal, whereas the promotion of somatic embryogenesis was dependent of the explants. For example, in Phragmites australis sodium silicate (Na2SiO3) induced somatic embryos in calli from root explants, but could not stimulate embryogenesis of stem nodal calli (He et al. 2013). The callus in our experiment was induced from root explants, and the high frequency of calli growth on MS media supplemented with 30 mg L−1 of silicon may be due to this explants source, root.
Plant regeneration symphony
Effect of various cytokinin concentrations on plant regeneration
To identify the ability of cytokinin, MS media supplemented with 2 mg L−1 of 2,4-D and four different types of cytokinin were implemented on the callus regeneration. The regeneration results showed significant differences between all the cytokinin concentrations (Fig. 10). MS media supplemented with KIN and BAP were more efficient in enhancing regeneration in comparison with Zeatin or TDZ combinations where no callus regeneration were observed. Based on the results, 3 mg L−1 of KIN (7%) was found to be the most efficient for the regeneration, and all the KIN combinations showed better efficiency as compared to BAP combinations. Therefore, KIN was chosen to be used for the subsequent experiments. No callus regenerations were observed in control (no cytokinin) and TDZ. In a related experiment, Azizi et al. (2015) successfully studied the regeneration of different Indica rice varieties and found that KIN had the best efficiency compared with other cytokinin concentrations.
In the current experiment, the colour of some parts of transferred calli (Fig. 11a) changed to green spots in regeneration media (Fig. 11b). The lustrous green spot calli covered with sickle-shaped trichomes (Fig. 11c) grow to shoots (Fig. 11d) and regenerate (Fig. 11e), whereas the pale green calli with whitish hairs did not incuse shoots in the regeneration media. On the other hand, non-somatic embryogenic calli did not induce green spots and after one month; it changed to a brown mass and died (Fig. 11f).
Effects of various auxin concentrations on plant regeneration
There were significant differences in the plant regenerations from various auxin concentrations. A comparison of plant regeneration percentage showed that the MS medium supplemented with 2 mg L−1 of NAA at 9.3% was statistically significant and the best auxin concentration as compared to others (Fig. 12). The second best auxin combination was the MS medium supplemented with ABA (all concentrations), NAA with 1 mg L−1 and IAA with 3 mg L−1 where the average of regeneration percentage was superior compared to other treatments. Control treatment (MS media with cytokinin and without additional auxins) regenerated less explants. Based on the results of the current steps, the highest regenerated percentages were achieved in MS media supplemented with 3 mg L−1 of KIN and 2 mg L−1 of NAA (Fig. 12).
In general, plant tissue culturists have applied two key methods to exhibit the hormone regulation effects on somatic embryogenesis. Firstly, culture mediums are supplemented with PGRs (assessing diverse substances, applications period and concentrations) to induce the desired developmental pattern. It is usually done in trial-and-error experiments to propose suitable conditions, combination and concentrations of PGRs (Park et al. 2005; Jiménez 2005; Stefanello et al. 2005; Máthé et al. 2012). Secondly, with the improvement of adequate techniques to investigate small molecules such as PGRs, the role of compounds in controlling somatic embryogenesis was recognized. To understand the mentioned mechanisms, the relation of endogenous concentrations and callus morphogenesis plays significant roles (Máthé et al. 2012). Therefore, the interaction of high auxin/cytokinin ratio prompts root induction, while a low ratio induces shoot induction (Arunyanart and Chaitrayagun 2005).
Effects of various adjuvant material concentrations on plant regeneration
In the current study, the effects of three different adjuvant tissue culture materials were evaluated. Our results show that among all l-proline concentrations, 100 mg L−1 had the highest percentage of regeneration. Nonetheless, the regenerations were not significant between other proline concentrations and control. On the other hand, the casein hydrolysate analysis demonstrated no significant differences between various concentrations of casamino acids and the control (Fig. 13a). The silicon experiment results showed that the most efficient treatment was 30 mg L−1, followed by 50 mg L−1, whereas other concentrations of silicon were not significantly different (Fig. 13b).
Another important parameter for a highly effective micropropagation is the capability to produce a uniform, extensive and rapid shoot proliferation (Su et al. 2011). The development and multiplication of shoots in a medium depend upon various aspects (Chen and Ziv 2003), among which is supplementing of exogenous proline, casamino and silicon that contribute to the growth in vitro conditions (Haque et al. 2003). While in vitro cells are usually able to synthesize their essential amino acids, the addition of casein hydrolysate and proline may increase the cell development. The use of amino acid such as proline and casein hydrolysate has been reported to have positive consequences on callus frequency and regeneration in rice (Siripornadulsil et al. 2002; Verbruggen and Hermans 2008; Sugimoto et al. 2010; Pawar et al. 2015).
As mentioned earlier, one of the most important effects of silicon on plants is to increase the plants photosynthetic activity and nutrient uptake (Sahebi et al. 2016). Although silicon is not being a part of any plant tissue culture media, the positive role of Si to improve physiological and morphologic potentials such as increasing callus growth and regeneration has been shown in different in vitro cultures (Ma 2004; He et al. 2013; Sivanesan and Park 2014). Biosilica, for example, is preferentially placed in leaf hull and sheath, leaf blades, vascular and epidermal stem tissues to improve proliferations (De Paiva Neto and Otoni 2003) and by using silicon as a fertilizer, some plants achieve thickness in their tissues (Moghaddam et al. 2000). In rice, it has been found that silicon is deposited in epidermal cell walls with a silicon-cuticle double-layer form (Ma 2004). In rice leaves, silicon is deposited in a “dumbbell”-type structure by lignification process which plant leaf size rises as it matures. Silicon was found to be deposited also in the lumen of cells through needle-like silica structures moulding the inner cell walls (Ning et al. 2014). In our experiment, we found the silicon accumulation in the treated plants (30 mg L−1 of silicon treatment; Fig. 14a, b). The differences between the regenerations may occur due to the decrease in hyperhydric shoot induction and increase in mechanical strength. Silicon has been reported to decrease the hydrogen peroxide content and oxidative reductive enzyme activity such as ascorbate oxidase, APX and GPX. The energy-dispersive X-ray spectroscopy (EDX) and scanning electron microscopy (SEM) results confirmed the existence of silicon in the regenerated root explants (Fig. 14). The biosilica accumulation in the root’s cell as well as cell walls’ inner space showed the positive effects of silicon on the regeneration and development of the plants. Silicon is usually transferred from the rice roots to shoots and is unloaded into stem and leaf. Still, additional research is necessary to understand the molecular and biochemical network of biosilica on somatic embryogenesis and organogenesis.
Electron microscopy image showing the root. a control and b 30 mg L−1 of silicon (white spots). Electron microscopy image of root samples (control) in three spectrum sa1/sa2/sa3 and silicon treatment sb1/sb2 and sb3. The comparison of quantitative measurements of three random selected samples of roots under control and silicon treatments
Regenerated plantlets’ protection art
Due to the restriction in root induction and length after one month of regeneration, various concentrations of auxins were needed to induce more roots and increase root length. In general, our results indicated that the percentages of increase in root length were significant (p ≤ 0.01) at 0.4 mg L−1 of IBA (52%). The lowest percentage belongs to the control (34%) (Fig. 15). Rooting stage that needs to be induced possesses diverse PGRs requirements (Kido et al. 2015). Among the PGRs, auxin has shown an essential function in regulating root growth and is involved in the adventitious rooting process (Zhang et al. 2013). Furthermore, the rooting system re-establishment from tissues without pre-existing meristems often depends on the use of exogenous auxins (De Klerk et al. 1995). Previous researches have shown that auxin treatments either inhibit or stimulate root production system depending on the concentration utilized (Pan and Tian 1999). Reportedly, root growth is a part of a quantitative genetic character controlled by PGR signals, mainly by auxins. Synthetic auxins such as IAA, NAA and IBA have been described to be better in root development compared to diverse explants (Pan and Tian 1999). In our experiment, 0.4 mg L−1 of IBA was more effective auxins as compared to the other auxin such as IAA and ABA in developing the root systems. This observation has also been reported elsewhere, for example IBA showed a better auxin for adventitious root growth and development in W. somnifera (Baraldi et al. 1995), O. prostrata (Martin et al. 2008), O. stamineus (Leng and Lai-Keng 2004), P. corylifolia (Baskaran and Jayabalan 2009) and E. angustifolia (Baskaran and Jayabalan 2009).
Conclusion
Generally, the in vitro system is a reliable tool in biotechnology to study cell signalling, molecular biology, physiology, morphogenesis, and plant growth and development. To this point, a number of protocols have been optimized for rice tissue culture. However, several restrictive factors have been observed in the micropropagation of Indica rice. The MR219 cultivar that is hybrid rice possesses high quality (taste and shape) and yield in Malaysia. Although different protocols have been introduced for callogenesis and plant regeneration Indica rice, the current experiment focused on the different sides of MR219 tissue culture such as root explant, addition of silicon and evaluation of in vitro factors in different culturing steps. The finding of this investigation revealed that to achieve high-efficiency protocol based on plants’ requirements, researchers should focus on specific factors separately. Although 2,4,5-T is an Agent Orange like 2,4-D, the reaction of these auxins was significantly different in response to callogenesis. In this investigation, we introduced root as an appropriate explant for callogenesis of MR219. We have found also l-proline and casein hydrolysate are showing reliable effects on tissue culture procedures, and as far as usage of silicon, this is the first report to show the suitability of silicon as adjuvant for MR219 micropropagation. In conclusion, for the callogenesis of MR219 the best recipe is MS media added with 2 mg L−1 of 2,4-D with root as the explant. For the proliferation phase, the highest efficiency was observed at week eight in the MS media supplemented with 2 mg L−1 of 2,4-D, 2 mg L−1 of kinetin, 50 mg L−1 of l-proline, 100 mg L−1 of casein hydrolysate and 30 mg L−1 of potassium metasilicate. Finally, MS media supplemented with 3 mg L−1 of KIN, 30 mg L−1 of potassium metasilicate and 2 mg L−1 of NAA were selected as the best media condition for regeneration section. To promote the roots of regenerated explants, 0.4 mg L−1 of IBA has shown potential as an appropriate activator.
References
Abiri R, Valdiani A, Maziah M, Shaharuddin NA, Sahebi M, Yusof ZN, Atabaki N, Talei D (2015) A critical review of the concept of transgenic plants: insights into pharmaceutical biotechnology and molecular farming. Curr Issues Mol Biol 18:21–42
Abro SA, Qureshi R, Soomro FM, Mirbahar AA, Jakhar G (2009) Effects of silicon levels on growth and yield of wheat in silty loam soil. Pak J Bot 41:1385–1390
Aggarwal D, Kumar A, Sharma J, Reddy MS (2012) Factors affecting micropropagation and acclimatization of an elite clone of Eucalyptus tereticornis Sm. In Vitro Cell Dev Biol Plant 48:521–529. doi:10.1007/s11627-012-9446-z
Ahmad N, Fatima N, Ahmad I, Anis M (2015) Effect of PGRs in adventitious root culture in vitro: present scenario and future prospects. Rend Lincei 26:307–321. doi:10.1007/s12210-015-0445-y
Arunyanart S, Chaitrayagun M (2005) Induction of somatic embryogenesis in lotus (Nelumbo nucifera Geartn.). Sci Hortic 105:411–420. doi:10.1016/j.scienta.2005.01.034
Atta R, Laurens L, Boucheron Dubuisson E, Guivarc’h A, Carnero E, Giraudat Pautot V, Rech P, Chriqui D (2009) Pluripotency of Arabidopsis xylem pericycle underlies shoot regeneration from root and hypocotyl explants grown in vitro. Plant J 57:626–644. doi:10.1111/j.1365-313X.2008.03715.x
Azizi P, Rafii MY, Mahmood M, Hanafi MM, Abdullah SNA, Abiri R, Sahebi M (2015) Highly efficient protocol for callogenesis, somagenesis and regeneration of Indica rice plants. C R Biol 338:463–470. doi:10.1016/j.crvi.2015.04.004
Baraldi P, Bertazza G, Bregoli A, Fasolo F, Rotondi A, Predieri S, Serafini-Fracassini D, Slovin J, Cohen J (1995) Auxins and polyamines in relation to differential in vitro root induction on microcuttings of two pear cultivars. J Plant Growth Regul 14:49–59. doi:10.1007/BF00212646
Baskaran P, Jayabalan N (2009) Psoralen production in hairy roots and adventitious roots cultures of Psoralea coryfolia. Biotechnol Lett 31:1073–1077. doi:10.1007/s10529-009-9957-9
Benlioglu B, Tuna D, Birsin M, Ozgen A (2015) Effect of growth regulators on tissue culture parameters in rice (Oryza sativa L.). Ekin J Crop Breed and Gen 2:43–46
Bhojwani SS, Dantu PK (2013) Plant tissue culture: an introductory text. Springer, Berlin. doi:10.1007/978-81-322-1026-9
Birch RG (1997) Plant transformation: problems and strategies for practical application. Annu Rev Plant Biol 48:297–326. doi:10.1146/annurev.arplant.48.1.297
Blackman FF, Matthaei GL (1901) On the reaction of leaves to traumatic stimulation. Ann Bot 3:533–546
Buah J, Tachie-Menson J, Addae G, Asare P (2011) Sugarcane juice as an alternative carbon source for in vitro culture of plantains and bananas. Am J Food Technol 6:685–694. doi:10.3923/ajft.2011.685.694
Che Radziah C, Siti Nurkhalida A, Zamri Z, Ismanizan I (2012) Effect of illumination, casein hydrolysate and proline on callus induction of Oryza sativa L. var. MR219. Malays Appl Biol 41:37–41
Chen J, Ziv M (2003) Carbohydrate, metabolic, and osmotic changes in scaled-up liquid cultures of Narcissus leaves. In Vitro Cell Dev Biol Plant 39:645–650. doi:10.1079/IVP2003451
Chowdhry CN, Tyagi A, Maheshwari N, Maheshwari S (1993) Effect of l-proline and l-tryptophan on somatic embryogenesis and plantlet regeneration of rice (Oryza sativa L. cv. Pusa 169). Plant Cell Tissue Organ Cult 32:357–361. doi:10.1007/BF00042300
Christianson M, Warnick D (1985) Temporal requirement for phytohormone balance in the control of organogenesis in vitro. Dev Biol 112:494–497. doi:10.1016/0012-1606(85)90423-3
Dahot MU (2007) Morpho-physiological aspects of micro-propagating banana under different hormonal conditions. Asian J Plant Sci 6:496–501
De Klerk GJ, Keppel M, Ter Brugge J, Meekes H (1995) Timing of the phases in adventitous root formation in apple microcuttings. J Exp Bot 46:965–972. doi:10.1093/jxb/46.8.965
De Paiva Neto VB, Otoni WC (2003) Carbon sources and their osmotic potential in plant tissue culture: does it matter? Sci Hortic 97:193–202. doi:10.1016/S0304-4238(02)00231-5
Din ARJM, Ahmad FI, Wagiran A, Samad AA, Rahmat Z, Sarmidi MR (2016) Improvement of efficient in vitro regeneration potential of mature callus induced from Malaysian upland rice seed (Oryza sativa cv. Panderas). Saudi J Biol Sci 23:S69–S77. doi:10.1016/j.sjbs.2015.10.022
Egan JF, Barlow KM, Mortensen DA (2014) A meta-analysis on the effects of 2,4-D and dicamba drift on soybean and cotton. Weed Sci 62:193–206. doi:10.1614/WS-D-13-00025.1
Elhiti M, Stasolla C (2016) Somatic embryogenesis: the molecular network regulating embryo formation, somatic embryogenesis in ornamentals and its applications, Springer, pp. 217–229. doi: 10.1007/978-81-322-2683-3-14
George EF, Sherrington PD (1984) Plant propagation by tissue culture. Exegetics Ltd, Eversley
Haque MS, Wada T, Hattori K (2003) Effects of sucrose, mannitol and KHsub2/subPOsub4/sub on proliferation of root tip derived shoots and subsequent bulblet formation in garlic. Asian J Plant Sci 2:903–908
Hare P, Cress W (1997) Metabolic implications of stress-induced proline accumulation in plants. Plant Growth Regul 21:79–102. doi:10.1023/A:1005703923347
He C, Wang L, Liu J, Liu X, Li X, Ma J, Lin Y, Xu F (2013) Evidence for ‘silicon’ within the cell walls of suspension cultured rice cells. New Phytol 200:700–709. doi:10.1111/nph.12401
Holme IB, Krogstrup P, Hansen J (1997) Embryogenic callus formation, growth and regeneration in callus and suspension cultures of Miscanthus x ogiformis Honda Giganteus’ as affected by proline. Plant Cell Tiss Org Cult 50:203–210. doi:10.1023/A:1005981300847
Hoque ME, Mansfield JW (2004) Effect of genotype and explant age on callus induction and subsequent plant regeneration from root-derived callus of Indica rice genotypes. Plant Cell Tiss Org Cult 78:217–223. doi:10.1023/B:TICU.0000025640.75168.2d
Ikeuchi M, Sugimoto K, Iwase A (2013) Plant callus: mechanisms of induction and repression. Plant Cell 25:3159–3173. doi:10.1105/tpc.113.116053
Islam MM, Ahmed M, Mahaldar D (2005) In vitro callus induction and plant regeneration in seed explants of rice (Oryza Sativa L.). Res J Agric Biol Sci 1:72–75
Islam MM, Roly ZY, Lee Y, Khalekuzzaman M (2014) In vitro propagation and genetic transformation system using immature embryo in elite rice (Oryza sativa L.) cultivars. Plant Breed Biotechnol 2:88–96. doi:10.9787/PBB.2014.2.1.088
Iwai H, Masaoka N, Ishii T, Satoh S (2002) A pectin glucuronyltransferase gene is essential for intercellular attachment in the plant meristem. Proc Natl Acad Sci 99:16319–16324. doi:10.1073/pnas.252530499
Ji XH, Wang YT, Zhang R, Wu SJ, An MM, Li M, Wang CZ, Chen XL, Zhang YM, Chen XS (2015) Effect of auxin, cytokinin and nitrogen on anthocyanin biosynthesis in callus cultures of red-fleshed apple (Malus sieversii f. niedzwetzkyana). Plant Cell. Tissue and Organ Cult (PCTOC) 120:325–337. doi:10.1007/s11240-014-0609-y
Jia Y, Zhang QX, Pan HT, Wang SQ, Liu QL, Sun LX (2014) Callus induction and haploid plant regeneration from baby primrose (Primula forbesii Franch.) anther culture. Sci Hortic 176:273–281. doi:10.1016/j.scienta.2014.07.018
Jiménez VM (2005) Involvement of plant hormones and plant growth regulators on in vitro somatic embryogenesis. Plant Growth Regul 47:91–110. doi:10.1007/s10725-005-3478-x
Kandasamy MK, Gilliland LU, McKinney EC, Meagher RB (2001) One plant actin isovariant, ACT7, is induced by auxin and required for normal callus formation. Plant Cell 13:1541–1554. doi:10.1105/TPC.010026
Khaleda L, Al-Forkan M (2006) Genotypic variability in callus induction and plant regeneration through somatic embryogenesis of five deepwater rice (Oryza sativa L.) cultivars of Bangladesh. Afr J Biotechnol 17:5–16
Kido N, Yokoyama R, Yamamoto T, Furukawa J, Iwai H, Satoh S, Nishitani K (2015) The matrix polysaccharide (1; 3, 1; 4)-β-D-glucan is involved in silicon-dependent strengthening of rice cell wall. Plant Cell Physiol 56:268–276. doi:10.1093/pcp/pcu162
Konaté S, Koné M, Kouakou H, Kouadio J, Zouzou M (2013) Callus induction and proliferation from cotyledon explants in Bambara groundnut. Afr Crop Sci J 21:255–263
Leng LW, Lai-Keng C (2004) Plant regeneration from stem nodal segments of Orthosiphon stamineus Benth., a medicinal plant with diuretic activity. In Vitro Cell Dev Biol Plant 40:115–118. doi:10.1079/IVP2003500
Li Y, Gao J, Sz Fei (2009) High frequency in vitro embryogenic callus induction and plant regeneration from indiangrass mature caryposis. Sci Hortic 119:306–309. doi:10.1016/j.scienta.2008.07.035
Lin YJ, Zhang Q (2005) Optimising the tissue culture conditions for high efficiency transformation of indica rice. Plant Cell Rep 23:540–547. doi:10.1007/s00299-004-0843-6
Ma JF (2004) Role of silicon in enhancing the resistance of plants to biotic and abiotic stresses. Soil Sci Plant Nutr 50:11–18. doi:10.1080/00380768.2004.10408447
Martin KP, Zhang CL, Hembrom ME, Slater A, Madassery J (2008) Adventitious root induction in Ophiorrhiza prostrata: a tool for the production of camptothecin (an anticancer drug) and rapid propagation. Plant Biotechnol Rep 2:163–169. doi:10.1007/s11816-008-0057-4
Máthé C, Mosolygó Á, Surányi G, Beke A, Demeter Z, Tóth VR, Beyer D, Mészáros I, Márta M (2012) Genotype and explant-type dependent morphogenesis and silicon response of common reed (Phragmites australis) tissue cultures. Aquat Bot 97:57–63. doi:10.1016/j.aquabot.2011.11.005
Moghaddam BE, Mesbah M, Yavari N (2000) The effect of in planta TIBA and proline treatment on somatic embryogenesis of sugar beet (Beta vulgaris L.). Euphytica 112:151–156. doi:10.1023/A:1003879914856
Murashige T, Skoog F (1962) A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol Plant 15:473–497. doi:10.1111/j.1399-3054.1962.tb08052.x
Narciso JO, Hattori K (2010) Genotypic differences in morphology and ultrastructures of callus derived from selected rice varieties. Phillipine Sci Lett 3:59–65
Neely D (1979) Tree wounds and wound closure. J Arboricult (USA) 5:135–140
Ngomuo M, Ndakidemi P (2013) The effects of auxins and cytokinin on growth and development of (Musa sp.) var. “Yangambi” explants in tissue culture. American. J Plant Sci 4:2174. doi:10.4236/ajps.2013.411269
Ning D, Song A, Fan F, Li Z, Liang Y (2014) Effects of slag-based silicon fertilizer on rice growth and brown-spot resistance. PLoS ONE 9:e102681. doi:10.1371/journal.pone.0102681
Nwe NH, Mahmood M, Ho CL, Qamaruz Zaman F, Md Zain A (2011) Regeneration capacity of cell suspension culture in Malaysian rice genotypes under salinity stress. Asian J Biotechnol 3:357–367. doi:10.3923/ajbkr.2011.357.367
Pan R, Tian X (1999) Comparative effect of IBA, BSAA and 5, 6-Cl2-IAA-Me on the rooting of hypocotyl in mung bean. Plant Growth Regul 27:91–98. doi:10.1023/A:1006154426941
Panjaitan SB, Abdullah SNA, Abdul Aziz M, Meon S, Omar O (2009) Somatic embryogenesis from scutellar embryo of Oryza sativa L. var. MR219. Pertanika J Trop Agric Sci 32:185–194
Park SY, Ahn JK, Lee WY, Murthy HN, Paek KY (2005) Mass production of Eleutherococcus koreanum plantlets via somatic embryogenesis from root cultures and accumulation of eleutherosides in regenerants. Plant Sci 168:1221–1225. doi:10.1016/j.plantsci.2004.12.023
Pawar B, Prashant K, Bahurupe J, Jadhav A, Anil K, Pawar S (2015) Proline and Glutamine Improve in vitro Callus Induction and Subsequent Shooting in Rice. Rice Sci 22:283–289. doi:10.1016/j.rsci.2015.11.001
Rachmawati D, Anzai H (2006) Studies on callus induction, plant regeneration and transformation of Javanica rice cultivars. Plant Biotechnol 23:521–524. doi:10.5511/plantbiotechnology.23.521
Rahman A, Bannigan A, Sulaman W, Pechter P, Blancaflor EB, Baskin TI (2007) Auxin, actin and growth of the Arabidopsis thaliana primary root. Plant J 50:514–528. doi:10.1111/j.1365-313X.2007.03068.x
Rashid U, Ali S, Ali GM, Ayub N, Masood MS (2009) Establishment of an efficient callus induction and plant regeneration system in Pakistani wheat (Triticum aestivum) cultivars. Electron J Biotechnol 12:4–5. doi:10.4067/S0717-34582009000300004
Rodríguez-Serrano M, Pazmiño D, Sparkes I, Rochetti A, Hawes C, Romero-Puertas M, Sandalio L (2014) 2, 4-Dichlorophenoxyacetic acid promotes S-nitrosylation and oxidation of actin affecting cytoskeleton and peroxisomal dynamics. J Exp Bot:eru237. doi: 10.1093/jxb/eru237
Roy P, Hasan M, Rasul M, Hossain M (2015) Seed culture of aromatic rice varieties under salt stress. Am J Biol Life Sci 3:260
Saharan V, Yadav RC, Yadav NR, Chapagain BP (2004) High frequency plant regeneration from desiccated calli of indica rice (Oryza sativa L.). Afr J Biotechnol 3:256–259
Sahebi M, Hanafi MM, Siti Nor Akmar A, Rafii MY, Azizi P, Tengoua FF, Nurul Mayzaitul Azwa J, Shabanimofrad M (2015) Importance of silicon and mechanisms of biosilica formation in plants. BioMed Res Int 2015:1–16. doi:10.1155/2015/396010
Sahebi M, Hanafi MM, Azizi P (2016) Application of silicon in plant tissue culture. In Vitro Cell Dev Biol Plant 2016:1–7. doi:10.1007/s11627-016-9757-6
Shahsavari E (2010) Evaluation and optimizations of media on the tissue culture system of upland rice. Int J Agric Biol Eng 12:537–540
Shukla R, Dube A, Koshy E (2014) Production of high quality embryogenic callus of rice. Int Quarterly J Life Sci 9:1077–1080
Siripornadulsil S, Traina S, Verma DPS, Sayre RT (2002) Molecular mechanisms of proline-mediated tolerance to toxic heavy metals in transgenic microalgae. Plant Cell 14:2837–2847. doi:10.1105/tpc.004853
Sivanesan I, Park SW (2014) The role of silicon in plant tissue culture. Front Plant Sci 5:571. doi:10.3389/fpls.2014.00571
Stefanello S, Dal Vesco LL, Ducroquet JPH, Nodari RO, Guerra MP (2005) Somatic embryogenesis from floral tissues of feijoa (Feijoa sellowiana Berg). Sci Hortic 105:117–126. doi:10.1016/j.scienta.2004.11.006
Su YH, Liu YB, Zhang XS (2011) Auxin–cytokinin interaction regulates meristem development. Mol Plant 4:616–625. doi:10.1093/mp/ssr007
Sugimoto K, Jiao Y, Meyerowitz EM (2010) Arabidopsis regeneration from multiple tissues occurs via a root development pathway. Dev Cell 18:463–471. doi:10.1016/j.devcel.2010.02.004
Sugiyama M (2015) Historical review of research on plant cell dedifferentiation. J Plant Res 128:349–359. doi:10.1007/s10265-015-0706-y
Szabados L, Savoure A (2010) Proline: a multifunctional amino acid. Trends Plant Sci 15:89–97. doi:10.1016/j.tplants.2009.11.009
Talei D, Valdiani A, Maziah M, Mohsenkhah M (2013) Germination response of MR 219 rice variety to different exposure times and periods of 2450 MHz microwave frequency. Sci World J. doi:10.1155/2013/408026
Thokozani BL, Zulu D, Sileshi C, Teklehaimanot Z, Gondwe DS, Sarasan V, Stevenson P (2013) Seed germination and in vitro regeneration of the African medicinal and pesticidal plant, Bobgunnia madagascariensis. Afr J Biotechnol 10:5959–5966
Valvekens D, Van Montagu M, Van Lijsebettens M (1988) Agrobacterium tumefaciens-mediated transformation of Arabidopsis thaliana root explants by using kanamycin selection. Proc Natl Acad Sci 85:5536–5540. doi:10.1073/pnas.85.15.5536
Vennapusa AR, Vemanna RS, Rajashekar RBH, Babitha K, Kiranmai K, Nareshkumar A, Sudhakar C (2015) An efficient callus induction and regeneration protocol for a drought tolerant rice indica genotype AC39020. J plant Sci 3:248–254
Verbruggen N, Hermans C (2008) Proline accumulation in plants: a review. Amino Acids 35:753–759. doi:10.1007/s00726-008-0061-6
Visarada K, Sarma N (2002) Qualitative assessment of tissue culture parameters useful in transformation of indica rice. Curr Sci 82:343–346
Vogel G (2005) How does a single somatic cell become a whole plant? Science 309:86. doi:10.1126/science.309.5731.86
Walles B, Buvat R (1989) Ontogeny, cell differentiation, and structure of vascular plants. Nord J Bot 9:498–499. doi:10.1111/j.1756-1051.1990.tb00540.x
Wani SH, Sanghera GS, Gosal SS (2011) An efficient and reproducible method for regeneration of whole plants from mature seeds of a high yielding Indica rice (Oryza sativa L.) variety PAU 201. New Biotech 28:418–422. doi:10.1016/j.nbt.2011.02.006
Wu H-X, Ma Y-Z, Xiao J-P, Zhang Z-H, Shi Z-H (2013) Photosynthesis and root characteristics of rice (Oryza sativa L.) in floating culture. Photosynthetica 51:231–237. doi:10.1007/s11099-013-0015-4
Yaseen M, Ahmad T, Sablok G, Standardi A, Hafiz IA (2013) Review: role of carbon sources for in vitro plant growth and development. Mol Biol Rep 40:2837–2849. doi:10.1007/s11033-012-2299-z
Yinxia Z, Te-chato S (2012) Callus induction and plantlet regeneration from mature embryos of indica rice (Oryza Sativa L.) cultivar Kra Dang Ngah. J AgricTech 8:2423–2433
Zhang J, Feng Q, Jin C, Qiu D, Zhang L, Xie K, Yuan D, Han B, Zhang Q, Wang S (2005) Features of the expressed sequences revealed by a large scale analysis of ESTs from a normalized cDNA library of the elite indica rice cultivar Minghui 63. Plant J 42:772–780. doi:10.1111/j.1365-313X.2005.02408.x
Zhang G, Cui Y, Ding X, Dai Q (2013) Stimulation of phenolic metabolism by silicon contributes to rice resistance to sheath blight. J Plant Nutr Soil Sci 176:118–124. doi:10.1002/jpln.201200008
Zuraida A, Rahiniza K, Nurul Hafiza M, Roowi S, Zamri Z, Subramaniam S (2010) Factors affecting delivery and transient expression of gusA gene in Malaysian indica rice MR 219 callus via biolistic gun system. Afr J Biotechnol 9:8810. doi:10.5897/AJB10.1467
Zuraida A, Naziah B, Zamri Z, Sreeramanan S (2011) Efficient plant regeneration of Malaysian indica rice MR 219 and 232 via somatic embryogenesis system. Acta Physiol Plant 33:1913–1921. doi:10.1007/s11738-011-0739-3
Acknowledgements
The authors would like to appreciate the Long-term Research Grants Scheme (LRGS), Food Security Rice Research Program of the Ministry of Higher Education, Malaysia, for creating an opportunity to conduct the present research article.
Author information
Authors and Affiliations
Corresponding author
Additional information
Editorial responsibility: Xu Han.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Abiri, R., Maziah, M., Shaharuddin, N.A. et al. Enhancing somatic embryogenesis of Malaysian rice cultivar MR219 using adjuvant materials in a high-efficiency protocol. Int. J. Environ. Sci. Technol. 14, 1091–1108 (2017). https://doi.org/10.1007/s13762-016-1221-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13762-016-1221-y