Abstract
Polyacrylamide (PAM) hydrogel filled with different types and contents of hydrophilic silica was synthesized by in situ polymerization. Rheological behaviors of the hydrogels were measured with an oscillation rheometer. The results indicated that the addition of silica efficiently improved the mechanical properties of the hydrogel, both for the uncrosslinked system and crosslinked system; simultaneously, the storage modulus (G′), loss tangent (tanδ) and viscosity (η) of the hydrogel were obtained. These results suggest that a nonbonded complexation existed between the silica and the molecular chains of PAM that endowed the hydrogel with special behaviors. Results from Fourier Transform infrared spectroscopy (FTIR) showed that the action of the nonbonded complexation was derived from hydrogen bonds between hydroxyl groups of silica and amide groups of PAM. The formed secondary networks endowed the hydrogels with high mechanical properties.
Similar content being viewed by others
Introduction
Chemical flood is one of the most important enhanced oil recovery (EOR) techniques for oil production and superabsorbent polymers, such as polyacrylamide is widely used in EOR. Super absorbent polymers (SAPs) are three-dimensional crosslinked networks of hydrophilic functional groups of polyelectrolytes; they can absorb a large amount of water that is much heavier than their masses because of their ionic nature and interconnected structural features (Buchholz 1996; Wan et al. 2008; Chen et al. 2010). Due to this unique characteristic, SAPs have been extensively used in many fields, such as personal care products, agriculture and horticulture, bioengineering, pharmaceutical, drug-delivery systems, food industry, enhanced oil recovery and other advanced technologies (Hua and Wang 2009; Lokhande and Varadarajan 1992; Raju et al. 2003, 2004; Guo et al. 2005; Liang et al. 2007; Sakiyama et al. 1993; Shiga et al. 1992; Yoshida et al. 1989; Hassan and Peppas 2000; Zohuriaan-Mehr et al. 2010; Pourjavadi and Kurdtabar 2010). In many application cases, the SAPs hydrogels are often broken down if they undergo large shear deformation; thus it is important to study and improve their elasticity to resist the shear destroy (Guo et al. 2005; Gosavi et al. 1999; Lee and Huang 2007; Yılmaz et al. 2007).
To improve the elasticity of the hydrogel, SAPs filled with different inorganic fillers have been studied. Santiago et al. (2007) prepared SAP/bentonite composites and studied the effect of the bentonite. Zhang et al. (2006) studied the swelling behaviors of poly(acrylic acid)/organo-attapulgite composite hydrogels in aqueous electrolyte solution. Wang et al. studied the swelling behaviors, and slow-release properties of a poly(acrylic acid-co-acrylamide)/sodium humate superabsorbent composite (Zhang et al. 2006). From the above research, it was found that filling an inorganic filler in a hydrogel is an effective way to improve the mechanical properties of hydrogel, especially their elasticity and anti-shear property.
Silica is a common filler which has a huge specific surface area. Abundant groups on the surface of silica make them easily form nonbonded complexations with polymer matrices. Therefore, silica has been widely used in the modification of rubber and plastic. However, only a few works have reported the use of silica in SAPs (Chinthamanipeta et al. 2008; Achilias et al. 2008; Meissner and Matějka 2008).
In the study, different types and contents of silica were novelly introduced into the polymerization system to improve the elasticity of the hydrogel. Both crosslinked and uncrosslinked polyacrylamide hydrogels containing silica were synthesized by in situ solution polymerization. FTIR was conducted to determine the structure of association between the silica and the polymer chains. Oscillation rheometer was performed to measure the rheological behaviors of the hydrogel SAPs. Effect comparisons among the different polyacrylamide hydrogels were made and the mechanism of silica improving polyacrylamide hydrogel with high mechanical properties was proposed.
Experiment
Materials
Acrylamide (AM, Chengdu Kelong Chemical Reagent Factory, China), and polymerizations were initiated by potassium persulfate (KPS, Tianjin Bodi Chemical Industry Ltd, China). Polyethylene glycol diacrylate-600 (PEGDA-600) as the crosslink agent was purchased from Aldrich and sodium chloride was purchased from Kermel Chemical Regent Ltd (China). Silica (H-T40, O-T40, H2000, QS20) was supplied by China BlueStar Chengrand Research and Design Institute of Chemical Industry. All these reagents were analytical grade and used as received. Water used in this study was deionized water unless otherwise mentioned.
Synthesis of silica/acrylamide polymer
The hydrogel was synthesized by free radical in situ polymerization in aqueous solution. The procedure was as follows: AM was dissolved in deionized water and added to a three-necked round-bottom flask equipped with a mechanical stirrer and a nitrogen gas inlet/outlet. Then silica dispersed in the water was also poured into the flask and the reaction solution was stirred under 300 rad min−1 to make sure the silica was completely dispersed. The flask was then put into a water bath at 60 °C and the polymerization mixture was flushed with nitrogen gas for 30 min, and a mechanical stirrer to exclude oxygen from the system. An aqueous solution of initiator was then added into the reactor. The reaction was carried out at 60 °C for 5 h with purging nitrogen. At the end of the reaction, the gel was granulated and dried in a vacuum oven at 60 °C for 10 h. Then a white powdered sample was obtained by pulverizing.
Different hydrogels were prepared by changing the silica types (H-T40, O-T40, H2000 and QS20) and content (0, 1%, 2 %, 3 %, 4 % wt). The difference of the four kinds of silica was illustrated in Table 1 and they will be discussed in detail later.
Measurement of properties
FTIR tests
Crosslinked polyacrylamide (CPAM) samples were extracted by Soxhlet extraction using distilled water at 60 °C for 72 h, and then the extracted sample was dried in a vacuum oven at 60 °C for 10 h. The dried CPAM powder was characterized by Nicolet 560 Fourier transform infrared spectra (FTIR).
Rheological measurements
1 g polymer powder was added to 29 g NaCl saline solution with the mineralization of 50,000 mg l−1 by gentle magnetic stir for 30 min to obtain the hydrogel.
The viscoelastic properties of the hydrogel were investigated by oscillatory measurements on a Bohlin VOR Rheometer (Bohlin Gemini 200, Malvern Instruments Ltd, UK). Samples were squeezed between stainless steel parallel plates (diameter 40 mm, gap 1 mm) and subjected to oscillating rotational deformations at frequencies from 0.01 to 10 Hz at a constant stress (0.1 Pa). Steady-state shear viscosity measurements were performed using the same instrument at shear rates ranging from 0.01 to 10 s−1.
Results and discussion
Rheological properties of linear polyacrylamide (LPAM) hydrogel and crosslinked polyacrylamide (CPAM) hydrogel filled with different contents of hydrophilic H-T40 silica
The elasticity and viscosity are important properties to water-soluble polymer. The change of the polymeric structure will cause the difference of the properties. The rheological behaviors of the hydrogel were measured to study the differences.
Figure 1 showed the rheological curves of the LPAM hydrogel (uncrosslinked), which was filled with different contents of hydrophilic H-T40 silica. It is clear that the G′ increases when the silica content rises from 0 to 3 % and decreases when the silica content rises to 4 %. The tanδ curves of LPAM are shown in Fig. 1b. When the silica content is 3 %, the tanδ reaches the smallest. It has been proved that tanδ can reflect the crosslink degree of the CPAM hydrogel (Rubinstein and Colby 2003; Wu and Wu 2002). The smaller the tanδ is the bigger crosslink degree of the network is. So, from Fig. 1b it can be deduced that silica can act as the crosslink points in the secondary networks generated from the hydrogen bond between polyacrylamide and silica, and the secondary networks improved the elasticity of the hydrogel. However, when the silica content increased further, the excessive silica might play a role as the polymerization inhibitor, which caused the crosslink reaction incomplete and the network incomplete. The inhibited reaction had also been found during the polymerization. When the content of silica was 4 % wt, after reaction there was some dilute solution in the three-necked flask, which did not react and the phenomenon was not found when the content was 0–3 %.
The steady-state viscosity (η) curves of silica-filled LPAM hydrogel are showed in Fig. 2. It can be seen that with the increase of silica from 0 to 3 %, the η continues to increase; however, with the further increase of the silica content, the η goes down. The reason is consistent with that of the increase in G′. From the results above, it can be concluded that the silica could improve the G′ and viscosity of the hydrogel indeed, which could also be an evidence for that the increase of silica would be effective to the improvement of elasticity of the hydrogel.
Figure 3 showed rheological curves of CPAM hydrogel crosslinked with PEGDA-400 filled with different contents of silica. The change trend of G′ and tanδ is similar as that of hydrogel without crosslink one, which means that the addition of silica can improve the crosslink network no matter there is chemical crosslinking points or not.
Rheological properties of LPAM hydrogel and CPAM hydrogel filled with different kinds of silica
The difference of specific area and methanol value of the four silica is shown in Table 1. The content of −OH of the hydrophobic silica can be represented by the methanol value, which is the possessive volume of 1 g hydrophobic silica dispersed in water. H-T40 and QS20 are hydrophilic, and the other two are hydrophobic.
Figure 4 showed rheological curves of LPAM hydrogel filled with different types of silica. It is obvious that the more hydrophilic the silica is, the larger the G′ is, i.e., G′ of hydrogel filled with H-T40 or QS20 is larger than that with H2000 or O-T40. The reason can be attributed that the hydrophilic silica has more −OH and they could combine with the polymer matrix better than the hydrophobic silica with less −OH does, so the crosslink networks formed more completely, resulting in a larger G′. It also can be found from Fig. 4a that G′ of hydrogel with H-T40 and O-T40 is larger than that with QS20 and H2000, respectively, which indicates the specific area of silica plays another important role in forming secondary network. That is, the silica with larger specific area has more −OH and stronger capability to combine with the polyacrylamide matrix, so the network becomes more complete and the G′ larger.
The effects of different types of silica on the CPAM hydrogel are similar as those of LPAM hydrogel, which is shown in Fig. 5. It could be concluded that the hydrophilic silica with larger specific area can effectively form secondary crosslink network and improve the mechanical properties of the hydrogel for both chemical crosslinked and uncrosslinked system of polyacrylamide.
The zero-shear viscosity and flow index of the CPAM hydrogel filled with different contents of H-T40
To study the effect of incorporation silica on the behaviors of the hydrogel, the viscosity–shear rate curves are measured. The rheological behaviors of the hydrogel could be express by \(\tau = f(\dot{\gamma })\). And the viscosity–shear rate curve could be fit by Ostwald–de Waele power-law equation (Han 1976).
Here η is viscosity, k a constant and n the flow index. When the shear rate of the curve \(\eta = f(\dot{\gamma })\) is extrapolated to zero, η 0 was obtained. η 0 was the zero-shear viscosity value, which is not affected by the shear rate but by the molecular structure and conformation. So the η 0 can be an evidence of the molecular weight and the molecular interaction. While it is Newton flow, the value of n is 1. The more the departure of the value of n from 1, the more the trend towards the non-Newton flow. So the value of n can be an evidence of the degree of nonbonded complexation between silica and polyacrylamide.
According to the test method above (Fig. 6), the η 0 and n of the CPAM hydrogel filled with H-T40 silica of different water absorbency ratio was shown in Figs. 7 and 8. Obviously, with the increasing content of H-T40 from 0 to 3 %, η 0 increases and n decreases, indicating the storage modulus of the hydrogel increase. When the content of H-T40 arrives to 4 %, the value of n increases, the reason is similar to that of G′. It is also found in Figs. 7 and 8 that with the increase of water absorbency, η 0 decreases and n increases. It is a result of the competition complex between polyacrylamide–silica and polyacrylamide–water. Obviously the secondary network between polyacrylamide and silica is stronger than the interaction between polyacrylamide and water.
FTIR evidence and model of nonbonded complex
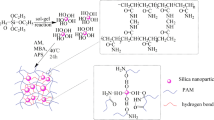
From the results of rheological measurements of LPAM and CPAM hydrogel filled with silica, the nonbonded secondary complexation between silica and molecular chains of polyacrylamide is put forward and proved indirectly. To directly prove existence of the nonbonded complexation, the FTIR was used to test the CPAM hydrogel filled with silica. As can be seen in Fig. 9, for the sample without silica, the stretching vibration of −NH2 appears at 3439.84 cm−1. The stretching vibration of −NH2 can be found at 3392.60 cm−1 for the samples filled with silica which indicates a phenomenon of red shift. Simultaneously, in the same FTIR spectra the symmetric and asymmetric vibration of Si–O groups appear at about 1050 and 1180 cm−1, respectively. It is evident that more the content of silica, the bigger the red shift, which implies that some associations must be generated, i.e., one kind of nonbonded complex network, between the −NH2 groups on the polyacrylamide molecular chain and the −OH on the surface of silica. Based on the conclusion above, a sketch for explaining association between polyacrylamide chain and silica particles was illustrated in Fig. 10. With the increase of the silica content, more silica combines with the polyacrylamide matrix which makes the polyacrylamide network become more complete, resulting in G′ increase. However, when the silica content goes up further, the excessive silica might play a role as the polymerization inhibitor, which makes the crosslink reaction incomplete and the network imperfect, so the G′ decreases. From the FTIR results, we have known that the silica has strong association with the polyacrylamide matrix.
Conclusions
Hydrogel of polyacrylamide filled with different types and contents of silica was successfully prepared by aqueous polymerization. The results of rheological measurements indicated that with the increase of silica content, the secondary network forms more completely. But the excessive silica might play a role as the polymerization inhibitor, which caused the crosslink reaction incomplete and the network incomplete. And the hydrophilic silica with larger specific area can effectively form secondary crosslink network and improve the mechanical properties of the hydrogel for both chemical crosslinked and uncrosslinked system of polyacrylamide. The above conclusions were proved by FTIR tests.
References
Achilias D, Bikiaris D, Karavelidis V (2008) Effect of silica nanoparticles on solid state polymerization of poly(ethylene terephthalate). Europ Polym J 44:3096–3107
Buchholz F (1996) Superabsorbent polymers: an idea whose time has come. J Chem Educ 73:512–515
Chen Y, Liu Y, Tan H (2010) Hydroxyethyl chitosan-g-poly(acrylic acid-co-sodium acrylate) superabsorbent polymers. J Appl Polym Sci 117:2233–2240
Chinthamanipeta P, Kobukata S, Nakata H (2008) Synthesis of poly(methyl methacrylate)–silica nanocomposites using methacrylate-functionalized silica nanoparticles and RAFT polymerization. Polymer 49:5636–5642
Gosavi U, Deopurkar R, Ghole V (1999) Microbial degradation of superabsorbent hspan gel by an indigenously isolated bacterial culture. Macromol 32:4264–4271
Guo M, Liu M, Zhan F (2005) Preparation and properties of a slow-release membrane-encapsulated urea fertilizer with superabsorbent and moisture preservation. Ind Eng Chem Res 44:4206–4211
Han CD (1976) Rheology in polymer processing [M]. Academic Press, New York, pp 89–109
Hassan C, Peppas N (2000) Structure and applications of poly(vinyl alcohol) hydrogels produced by conventional crosslinking or by freezing/thawing methods. Adv Polym Sci 153:37–65
Hua S, Wang A (2009) Synthesis, characterization and swelling behaviors of sodium alginate-g-poly(acrylic acid)/sodium humate superabsorbent. Carbohyd Polym 75:79–84
Lee W, Huang Y (2007) Swelling and antibacterial properties for the superabsorbent hydrogels containing silver nanoparticles. J Appl Polym Sci 106:1992–1999
Liang R, Liu M, Wu L (2007) Controlled release NPK compound fertilizer with the function of water retention. React Funct Polym 67:769–779
Lokhande H, Varadarajan P (1992) A new guargum-based superabsorbent polymer synthesised using gamma radiation as a soil additive. Bioresour Technol 42:119–122
Meissner B, Matějka L (2008) Constitutive equation describing the biaxial stress–strain behavior of poly(dimethylsiloxane) networks reinforced with silica generated in situ. Europ Polym J 44:1940–1948
Pourjavadi A, Kurdtabar M (2010) Effect of different bases and neutralization steps on porosity and properties of collagen-based hydrogels. Polym Int 59:36–42
Raju K, Raju M, Mohan Y (2003) Synthesis of superabsorbent copolymers as water manageable materials. Polym Int 52:768–772
Raju K, Raju M, Mohan Y (2004) Synthesis and swelling behavior of superabsorbent polymeric materials. Int J Polym Mater 53:419–429
Rubinstein M, Colby R (2003) Polymer Physics. Oxford University Press, USA
Sakiyama T, Chu C, Fujii T (1993) Preparation of a polyelectrolyte complex gel from chitosan and κ-carrageenan and its pH-sensitive swelling. J Appl Polym Sci 50:2021–2025
Santiago F, Mucientes A, Osorio M (2007) Preparation of composites and nanocomposites based on bentonite and poly(sodium acrylate). Effect of amount of bentonite on the swelling behaviour. Euro Polym J 43:1–9
Shiga T, Hirose Y, Okada A (1992) Bending of poly(vinyl alcohol)–poly(sodium acrylate) composite hydrogel in electric fields. J Appl Polym Sci 44:249–253
Wan T, Yao J, Ma X (2008) Preparation of poly (AA-AM) water superabsorbent by inverse microemulsion polymerization. J Appl Polym Sci 110:3859–3864
Wu Q, Wu J (2002) Polymer rheology. Higher Education Press, Bejing
Yılmaz S, Kul D, Erdöl M (2007) Synthesis of a novel crosslinked superabsorbent copolymer with diazacyclooctadecane crown ether and its sorption capability. Europ Polym J 43:1923–1932
Yoshida M, Asano M, Kumakura M (1989) A new temperature-sensitive hydrogel with α-amino acid group as side chain of polymer. Euro Polym J 25:1197–1202
Zhang J, Chen H, Li P (2006a) Preparation of poly(acrylic acid)/organo-attapulgite composite hydrogels and swelling behaviors in aqueous electrolyte solution. Macromol Mater Eng 291:1529–1538
Zhang J, Liu R, Li A (2006b) Preparation, swelling behaviors, and slow-release properties of a poly(acrylic acid-co-acrylamide)/sodium humate superabsorbent composite. Ind Eng Chem Re 45:48–53
Zohuriaan-Mehr M, Omidian H, Doroudiani S (2010) Advances in non-hygienic applications of superabsorbent hydrogel materials. J Mater Sci 45:5711–5735
Acknowledgments
This research is supported by National Science & Technology Major Projects of China (2011ZX05011-004) and China Postdoctoral Science Foundation (2014M560573).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Jiang, Z., Cao, X., Li, Z. et al. Rheological behaviors and secondary networks of polyacrylamide hydrogel filled with silica. J Petrol Explor Prod Technol 6, 93–99 (2016). https://doi.org/10.1007/s13202-015-0163-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13202-015-0163-0