Abstract
Zirconia (ZrO2)-modified graphitic carbon nitride (g-C3N4) nanocomposite was used for effective photodegradation of 4-nitrophenol (4-NP) in water. The ZrO2 nanoparticles, g-C3N4 nanosheets, and ZrO2/g-C3N4 nanocomposite were well characterized by including N2 adsorption, X-ray diffraction, Fourier transform infrared spectroscopy, field emission scanning electron microscopy, UV–Vis diffuse reflectance spectroscopy, photoelectrochemical measurements, and photoluminescence spectroscopy methods. ZrO2/g-C3N4 nanocomposites were formed at room temperature using sonication and used for effective for photodegradation of 4-NP under irradiation with visible light. The nanocomposite samples resulted in a significant increase in photocatalytic activity compared with single-component samples of g-C3N4. In particular, the ZrO2/g-C3N4 nanocomposite exhibited the significant increase in the photocatalytic activity. The ZrO2/g-C3N4 nanocomposite showed an excellent catalytic activity toward the reduction of 4-NP in aqueous medium. Further, ZrO2/g-C3N4 nanocomposite can be reused several times for photocatalytic degradation as well as for 4-NP adsorption.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Extensive global industrial activities have resulted in severe negative impacts on the environment through air and water pollution and generation of large amounts of waste materials. Industrial waste materials consist of both organic and inorganic substances such as pesticide residues, chlorinated and volatile compounds, nitro aromatics, detergents, chemical dyes, and fertilizers. Among the various toxic wastes, dyes and nitro aromatic compounds caused the greatest concern due to their high chemical and biological stability. The US Environmental Protection Agency has declared 4-NP a hazardous pollutant (Atlow et al. 1984; Klibanov et al. 1983). 4-NP can disturb functioning of the human central network system and cause damage to kidneys and the liver (Pohanish 2008; Zarei et al. 2016). Thus, removal of 4-NP from polluted water is essential. Traditional water remediation treatments are not effective for the removal of 4-NP due to its stability and solubility in water. Therefore, recently, the scientific community has given focused on inventing efficient catalysts for the degradation of 4-NP (Chang et al. 2012; Pradhan et al. 2002).
Semiconductor photocatalysis is the emerging advanced oxidation method for abatement of organic pollutants and possesses several advantages over traditional oxidation methods. One of the merits of semiconductor photocatalysis is the ability of adsorbing a wide range of organic and inorganic species on the surface of the photocatalyst which chemically alters by light-induced redox reaction. Various types of photocatalysts for use in air or water purification applications have been designed (Pawar et al. 2015). Combinations of metal/metal oxide nanoparticles (NPs) such as Au, Ag, Pt, TiO2, ZnO, SnO2, WO3, Nb2O5, BiVO4, and Fe2O3 with conducting polymers such as polyaniline, polypyrrole, and polythiophene have been utilized for the degradation of organic pollutants (Ge et al. 2013; Jiang et al. 2014; Reimer et al. 2014; Wang and Astruc 2014; Wang et al. 2014; Xu et al. 2014; Zhang et al. 2015b). The small dimensions induce a significant change in the reduction potential of metal NPs compared to bulk materials. This unique characteristic improves the electron transfer process in various catalysis reactions. However aggregation, agglomeration, and high surface energy of NPs reduce the feasibility of NP applications as photocatalysts. In order to reduce aggregation, NPs can be stabilized on the surface of a solid support (Campelo et al. 2009). Carbonaceous nanomaterials are interesting candidates due to their exotic physical and chemical characteristics (Geim 2009) such as high surface area, high electrical-thermal conductivity, high charge carrier mobility, and extraordinary mechanical strength, and graphene allotropes are a promising material for support of different NPs. The graphene support which contains metal or semiconductor NPs has been confirmed to be effective nanocomposite material for the photovoltaic cell (Ong et al. 2015a; Yeh et al. 2015), catalysis, and biosensor applications (Guo and Dong 2011). Recently, another two-dimensional carbonaceous material, g-C3N4, has attracted significant attention (Gao et al. 2014; Maeda et al. 2014; Tan et al. 2015; Tian et al. 2014; Wang et al. 2012; Wu et al. 2014; Zheng et al. 2012). Unique properties of g-C3N4 including good visible-light absorption, moderate band gap (2.7 eV), high thermal and chemical stability, and photocatalytic properties introduce a new material for application in photocatalysis (Pawar et al. 2016a, 2017). The g-C3N4 and g-C3N4-based structures have been used in various applications such as photo catalytic degradation of microbial, organic, and inorganic pollutants (Lan et al. 2015; Li et al. 2015; Papailias et al. 2015; Pawar et al. 2015, 2016b), hydrogen evolution from water splitting (Liang et al. 2015; Liu et al. 2015b; Xu et al. 2015; Yang et al. 2015b), sensors applications (Pany and Parida 2015; Wang et al. 2015; Zhang et al. 2015a), adsorption (Anbia and Haqshenas 2015; Chen et al. 2015; Hu et al. 2015), reduction of oxygen (Lu et al. 2015; Zhao et al. 2015), lithium-ion batteries (Liu et al. 2015a; Luo et al. 2015), biofuel cells (Yi et al. 2015), and photochemo combination methods (Yang et al. 2015a). Due to repeating triazine units, the g-C3N4 consists of a large number of binding sites which can stabilize the NPs, making this g-C3N4 a promising support for NPs (Huang et al. 2015; Ong et al. 2015b). Thus, g-C3N4 nanosheet can be used as a feasible candidate for stabilizing metal NPs for catalytic applications (Wang et al. 2009). This material is used for the degradation of organic pollutants and photocatalytic reduction of CO2 under visible light (Mao et al. 2013; Yan et al. 2009). However, it suffers from a high charge carrier recombination rate and low quantum efficiency which lead to poor photocatalytic performance (Wang et al. 2012). Sensitization and combination with semiconductor NPs (namely Au, Ag, Pt, and Pd) and carbon-based materials (CNTs and graphene) are the most viable routes for improving the g-C3N4-based photocatalysts (Sridharan et al. 2014). Among the metal oxide NPs, ZrO2 is a material with favored properties including high stability, high toughness, high chemical strength, desirable corrosion, chemical and microbial resistance (Polisetti et al. 2011; Singh and Nakate 2014). Moreover, ZrO2 NPs have plenty of oxygen vacancies on surfaces with wideband-gap P-type semiconductors. The high ion exchange ability and redox movement make it useful in many catalytic processes as a catalyst (Fidelus et al. 2012).
Here, we report a facile ultrasonication method for the fabrication of hybrid nanocomposites using ZrO2 NPs, and g-C3N4 nanosheets and their application for photocatalytic degradation of 4-NP in water. Mono-dispersed ZrO2 NPs were dispersed on carbon nitride sheets by ultrasound. The ZrO2/g-C3N4 composite exhibited superior catalytic activity toward the reduction of 4-NP. Possible mechanism for degradation of 4-NP upon ZrO2/g-C3N4 catalyst under light irradiation was proposed. The ZrO2/g-C3N4 catalyst is very efficient for the removal of 4-NP from aqueous environment by its dual actions with adsorption and photocatalytic degradation of 4-NP.
Materials and methods
Materials
All reagents were of analytical grade. Hydrochloric acid and ammonia were purchased from Merck. ZrOCl2·8H2O, KOH, and nitric acid were purchased from Sigma-Aldrich. Melamine (99%) was prepared from Sigma-Aldrich and used without further purification. P25 titanium dioxide (TiO2) powder was obtained from Degussa (rutile/anatase: 85:15, 99.9%).
Synthesis of ZrO2 NPs
In a typical synthesis, 0.1 M of ZrOCl2·8H2O was dissolved in 100 mL of distilled water. Then, 0.3 M of KOH was added to the above solution. The final solution was transferred into a stainless steel Teflon-lined sterilized capacity of 100 mL and kept in an oven at 180 °C for 16 h. The resulting precipitates were washed with distilled water and ethanol. The resultant was dried for 3 h in vacuum at 80 °C.
Synthesis of g-C3N4 nanosheets
In a typical synthesis, 30 g melamine was placed in a crucible and then heated at 600 °C for 2 h, resulting in yellow powder. Liquid exfoliating method was applied to produce the g-C3N4 nanosheets from bulk material. In brief, 70 mg of the bulk g-C3N4 powder was dispersed in 100 mL water and the mixture was ultrasounded for 6 h. The final suspension was then centrifuged at 10,000 rpm and dried for 3 h in vacuum at 80 °C (Tian et al. 2013).
Fabrication of ZrO2/g-C3N4 hybrids
The ZrO2 NPs was incorporated into g-C3N4 sheets using ultrasonication at room temperature. Then, 50 mg of g-C3N4 powder was added to 20 mL of the ZrO2 colloidal dispersion and ultrasonicated for 2 h at 30 °C. The ZrO2/g-C3N4 dispersion was then filtered and washed several times using ethanol. The resulting ZrO2/g-C3N4 powder was collected and dried overnight at 60 °C.
Characterization methods
The X-ray diffraction (XRD) pattern of the nanomaterials was obtained by using a powder X-ray diffractometer model Shimadzu XRD 6000 using CuKa with a diffraction angle between 20° and 80°. The crystallite size was determined using Scherrer’s formula. Field emission Scanning electron microscopy (FESEM) studies were carried out on TSCAN. UV–Vis absorption spectrum for the samples was recorded using a Varian spectrophotometer in the range of 300–700 nm. The Fourier transform infrared spectroscopy (FTIR) spectrum of the ZrO2 NPs was taken using an FTIR model THERMO NICOLET Spectrometer. The photoluminescence (PL) spectrum of the ZrO2 particles was recorded by the PerkinElmer lambda spectrophotometer with a Xenon lamp as the excitation light source.
Photocatalytic degradation
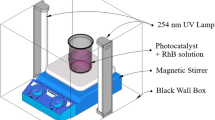
The photocatalytic activities of ZrO2/g-C3N4 hybrids were evaluated at room temperature. Thirty–sixty milligrams of sample was dispersed into the 100 mL of 30 mg/L 4-NP solution with magnetic stirring in each experiment. Prior to irradiation, the suspensions were magnetically stirred in the dark for 60 min to reach an adsorption/desorption equilibrium between the catalyst and 4-NP solution. Then, the solution was exposed to simulated solar light under magnetic stirring. At given reaction time of approximately 2 h, 4 mL of the solution was pipetted every 10 min and centrifuged to separate the catalyst. Concentration of 4-NP solution was measured by a UV–visible spectrophotometer at λ = 500 nm to evaluate the degradation rate. The examination experiment of reactive species is similar to the photodegradation experiment. The catalytic degradation efficiency of 4-NP was calculated using Eq. (1):
where C0 is initial concentration of 4-NP solution (mg L−1) and Ce is the equilibrium concentration of 4-NP solution (mg L−1).
Photoelectrochemical measurements
Photoelectrochemical (PEC) performance was analyzed using a three-electrode in quartz cell. Graphite served as the counter electrode, and Ag/AgCl served as the reference. The PEC measurements were taken using a Xe arc lamp (300 W, model 66984; Oriel, USA) with the electrolyte solution of Na2SO4 (0.5 M). For preparation of working electrodes, 10 mg of g-C3N4 powder was added to 10 mL of Liquion solution, and the resulted mixture was ultrasonicated for 1 h and stirred overnight. This suspension was spin-coated on a fluorine-doped tin oxide (FTO) glass substrate at ambient conditions. At final stage, in order to increase the uniform distribution of the catalyst on the substrate, the prepared FTO films spin-coated with the catalysts were heated in oven at 60 °C for 2 h (Pawar et al. 2016a).
Results and discussion
Characterizations of ZrO2/g-C3N4 composites
Figure 1a–c shows the XRD patterns of the g-C3N4, ZrO2, and ZrO2/g-C3N4 nanocomposites. The diffraction peaks of ZrO2 NPs can be assigned to the monoclinic phase. ZrO2 NPs are spherical with an average particle size of 15 nm, which leads to its high surface area of 73.9 m2 g−1. However, pure g-C3N4 has a low surface area of 51.8 m2 g−1, which can be attributed to its special morphology. The BET surface area analysis applied using the N2 adsorption method illustrates that the incorporation of ZrO2 NPs increases the surface area of g-C3N4 nanosheets. The BET surface area of 10 wt% ZrO2/g-C3N4 and 30 wt% ZrO2/g-C3N4 samples was 59.12 and 65.3 m2 g−1, respectively. The BET surface areas result shows that the addition of ZrO2 increases the surface area of g-C3N4. The strong and sharp ZrO2 diffraction peaks demonstrate that the samples have high crystallinity. The XRD patterns show no extra diffraction peaks indicating the high level of purity of the samples. Diffraction peaks of g-C3N4 at 12.8° and 27.6 ° confirmed that g-C3N4 was formed. The XRD patterns of ZrO2/g-C3N4 hybrid samples contain the peaks of both ZrO2 NPs and g-C3N4 nanosheets and show that after synthesis of ZrO2/g-C3N4 composite, the crystal structure of ZrO2 and g-C3N4 did not change. Figure 1d–f illustrates field emission scanning emission microscopy (FESEM) images of the ZrO2, g-C3N4, and ZrO2/g-C3N4 hybrid samples which show the planar structure of g-C3N4 nanosheets as well as the spherical structure of ZrO2 NPs.
XRD patterns of a g-C3N4, b ZrO2 and c ZrO2/g-C3N4 composites with different ZrO2 concentrations. FESEM images of d g-C3N4, e ZrO2, and f 20.9 wt% ZrO2/g-C3N4 composite (e and f). (green color: g-C3N4, violet color: ZrO2, blue color: 10 wt% ZrO2/g-C3N4, red color: 30 wt% ZrO2/g-C3N4, block color: 40 wt% ZrO2/g-C3N4)
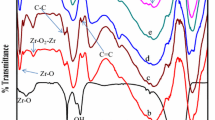
Figure 2a shows the FTIR spectra for ZrO2, g-C3N4, and ZrO2/g-C3N4 nanocomposites. For ZrO2, the broad bands at the range 500–550 cm−1 and 800 cm−1 are related to the Zr–O vibration absorption (Zhang et al. 2013). Furthermore, the broad region with a maximum of about 3350 cm−1 can be related to the hydroxyl groups of hydrated oxide. For g-C3N4, the sharp peaks in the range of 1200–1700 cm−1 can be attributed to the stretching vibration of the breathing mode of triazine units (Sayama and Arakawa 1993). In addition, a broad absorption band with a maximum of around 3200–3700 cm−1 can be attributed to the N–H groups (Sayama and Arakawa 1993). There is a similarity between the FTIR spectra of ZrO2/g-C3N4 and pure g-C3N4. However, the change in the bands of 800 and 3300 cm−1 with the variation of ZrO2 concentration still demonstrates the presence of ZrO2 NPs. The FTIR result shows good correlation with XRD results and shows the hybrids structure of ZrO2/g-C3N4 nanocomposite (Sayama and Arakawa 1993).
Figure 2b shows the influence of the ZrO2/g-C3N4 hetero-junction on the separation efficiency of electron–hole pairs which was investigated by PL spectra. Sharp emission peak at around 380 nm in unmodified g-C3N4 related to the band gap transition emission which is equal to the band gap energy of unmodified g-C3N4. However, fluorescence quenching can be observed in the similar position in the PL spectrum of ZrO2/g-C3N4. Further, charge transfer between g-C3N4 and ZrO2 component prevents the electron–hole recombination.
Compared with that of g-C3N4, the emission intensities of ZrO2/g-C3N4 are much lower, which indicate that a good separation of electrons and holes is achieved by introducing ZrO2 NPs to the g-C3N4 nanosheets.
The ability to absorb the light by photocatalysts is important to generate the charge carriers. Therefore, we measured the optical characteristics in g-C3N4, ZrO2, and ZrO2/g-C3N4 nanocomposites using the UV–Vis diffuse reflectance spectroscopy (DRS) technique. As shown in Fig. 3a, ZrO2 NPs absorbed the light with a wavelength shorter than 170 nm have band gap energy of about 4.8 eV. In pure g-C3N4, the absorbance edge is located at 415 nm, revealing the standard absorption threshold of g-C3N4 structure (Wang et al. 2009; Yan et al. 2009). Because of interactions between ZrO2 and g-C3N4, the ZrO2/g-C3N4 nanocomposite (10, 30 wt% ZrO2/g-C3N4) shows better photo absorption performance than pure g-C3N4. Different chemical bonds formed between the ZrO2 and g-C3N4 might result in the enhanced optical property which is similar to that in the N-doped ZrO2 (Zhao et al. 2014) and TiO2-doped graphene photocatalyst (Leary and Westwood 2011; Woan et al. 2009). According to DRS results, ZrO2/g-C3N4 nanocomposite shows an efficient visible-light photocatalytic activity. When ZrO2 was incorporated into g-C3N4 nanosheets, the corresponding band edge absorption was shifted to longer wavelength in comparison with the pure g-C3N4. The nanocomposite structures of 30 wt% ZrO2/g-C3N4 exhibited maximum absorption in the visible region of spectrum. Moreover, we estimated band gap energy of pure g-C3N4 and ZrO2/g-C3N4 nanocomposite from optical absorption, and the corresponding plots are shown in Fig. 3b. The direct band gap of pure ZrO2 is calculated to be 5.20 eV, which is seen to be not useful in visible region of solar spectrum. However, after making nanocomposite with g-C3N4, it shifted toward visible region. The obtained band gap of pure g-C3N4 is found to be 2.75 eV, which can be used in visible irradiation. Additionally, nanocomposite structure exhibited band edge absorption around 2.6 eV, indicating its applicability in visible light. Figure 3a shows the UV–Vis spectra of all the ZrO2 samples have extended a red shift and significant absorption between 100 and 450 nm. This red shift translates into a decrease in the band gaps of the nanocomposites which leads to more absorption in the visible region and hence better photocatalytic activity. In general, the red shift and absorption intensity both increased with higher ZrO2 concentration. In addition, the optical spectra of the ZrO2/g-C3N4 display clear red shifts with respect to that of pure g-C3N4 which is consistent with the band gaps calculated from the DRS results. The enhanced visible-light absorption of ZrO2/g-C3N4 materials makes them ideal candidates for photocatalysts. Hence, the band gap value of the nanocomposite decreases by increasing the amount of g-C3N4. This result indicated the strong interaction between ZrO2 and g-C3N4 nanosheets in the nanocomposite.
Photocatalytic degradation of 4-NP
Figure 4 shows the degradation of 4-NP under visible-light irradiation for determination of photocatalytic activity of ZrO2, g-C3N4, and ZrO2/g-C3N4 nanocomposites. For comparison, the degradation of 4-NP by TiO2 powder was analyzed as a standard material. The blank test shows that 4-NP is stable under visible-light irradiation, which indicates that the contribution of 4-NP photolysis can be neglected. Also, ZrO2 NP has a high surface area and demonstrated the stable adsorption for 4-NP. Elevated 4-NP concentration at the beginning of the light-on was attributed to desorption of some 4-NPs due to the increased temperature on the ZrO2 surface. In irradiation time longer than 30 min, the concentration of 4-NP gradually decreases.
a Photodegradation of 4-NP on ZrO2/g-C3N4 nanocomposites under light irradiation and b the corresponding reactive constant (green color triangle: g-C3N4, violet color circle: ZrO2, red color diamond: 10 wt% ZrO2/g-C3N4, blue color down triangle: 30 wt% ZrO2/g-C3N4, brown color pentagon: TiO2, block color square: blank)
Compared to ZrO2, pure g-C3N4 shows weaker adsorption of 4-NP but higher photoactivity under visible-light irradiation. The rate of 4-NP degradation is 0.0116 min−1 which is higher than that of ZrO2 (0.0022 min−1). The incorporation of ZrO2 NPs on g-C3N4 nanosheets can improve the electron–hole separation efficiency and also in absorption of light. Thus, the catalytic activity for 4-NP degradation is enhanced. By loading of ZrO2 NPs with concentration from 10 to 30 wt%, the photocatalytic activity of ZrO2/g-C3N4 increases. According to the results, the 30 wt% ZrO2/g-C3N4 sample demonstrates the highest degradation rate of 0.0167 min−1 which is higher than that of pure g-C3N4 nanosheets. Also, the prepared ZrO2/g-C3N4 exhibited higher catalytic activity and degradation rate than TiO2 powder.
Figure 5 shows the photocatalytic activity of 30 wt% ZrO2/g-C3N4 with different scavengers (2-propanol (IPA), KBrO3, benzoquinone (BQ), KI, and NaF). From Fig. 5, it can be observed that IPA which is ·OH scavenger (He et al. 2013; Li et al. 2009) showed little impact on the reaction rate (k) of 4-NP degradation. Although, the addition of BQ, which is ·O−2 scavenger (Cui et al. 2013; Yang et al. 2013), decreased the k from 0.0317 to 0.0063 min−1. The k also had a clear drop to 0.0078 min−1 in the existence of KI (h+ and ·OH scavenger) (He et al. 2013; Li et al. 2009). This result demonstrates the role of h+ and ·O−2 in the photocatalytic degradation of 4-NP on ZrO2/g-C3N4 nanocomposites.
Photoelectrochemical measurement
Transient photocurrent response was measured to investigate the separation efficiency of photogenerated electrons and holes pairs of the ZrO2 NPs, g-C3N4 nanosheets, ZrO2/g-C3N4 nanocomposite. As shown in Fig. 6a, ZrO2, g-C3N4, and ZrO2/g-C3N4 samples demonstrated good reproducibility and stability under several on–off cycles of light illumination. However, the ZrO2/g-C3N4 exhibited stronger and higher photocurrent than ZrO2 and g-C3N4. The reason for this response in hybrid sample related to the existence of numerous charge carriers which enhances the photocatalytic activity. Furthermore, it confirms the strong interaction of ZrO2 NPs and g-C3N4 nanosheets in hybrid sample.
Photocatalytic mechanism
Incorporation of ZrO2 NPs on g-C3N4 nanosheets generates an efficient photocatalyst for degradation of 4-NP under visible-light irradiation. The addition of ZrO2 NPs can enhance the surface area and light absorption capacity of g-C3N4 nanosheets. In general, two possible reaction mechanisms should be considered for the 4-NP degradation: (a) photolysis and (b) photocatalytic process. In photolysis, energy could be transferred from 4-NP to O2 to produce an oxygen atom (O) which can oxidize 4-NP as follows:
Figure 4a shows no 4-NP degradation in the blank experiment under visible light, which confirms that the 4-NP is stable and the photolysis has a negligible contribution in the degradation of 4-NP. In the photocatalytic degradation by NPs, it can be described that the promotion of an electron from the valence band (VB) to the conduction band (CB) by light irradiation generates the active site (hole) for the oxidation of the 4-NP. According to the band gap structure of ZrO2/g-C3N4 and the influence and role of scavengers, possible pathway of the photocatalytic reaction with ZrO2/g-C3N4 was proposed (Eqs. 5–9). The schematic diagram of 4-NP degradation on ZrO2/g-C3N4 is illustrated in Fig. 6:
Separation efficiency of electron–hole pairs plays an important role in photocatalytic degradation of 4-NP. Electrons of ZrO2 NPs and g-C3N4 were excited from VB to CB which generated the holes in the VB for both semiconductors under the light irradiation. The CB of g-C3N4 nanosheet is more negative than that of O2 molecules (Song and Zhang 2008) which led to excitation of the electrons and diffusion to the g-C3N4 surface followed by reaction with oxygen molecules and generation of ·O−2 radicals. The highly reactive ·O−2 radicals have high oxidation capability and can degrade the 4-NP molecules on photocatalyst surface. Further, the CB of g-C3N4 is more negative than that of ZrO2 and electrons on g-C3N4 can directly move to the CB of ZrO2 NPs. Also, the VB of ZrO2 is more positive than the VB of g-C3N4 and the holes on the VB of ZrO2 can directly move to the VB of g-C3N4. This process can effectively enhance the separation of electron–hole pairs and greatly decrease the charge recombination, resulting in the high photoactivity of ZrO2/g-C3N4 nanocomposites. The VB of ZrO2/g-C3N4 nanocomposites was evaluated according to the concepts of electronegativity. The VB of a semiconductor can be calculated by following equation (Zhang et al. 2009):
where EVB is the VB potential, Ec is the energy of free electrons (about 4.5 eV), and X is the electronegativity of the semiconductor. The value of X for ZrO2 is 5.92 eV (Jiang et al. 2010), and the EVB for ZrO2 was calculated to be 4.11 eV. The conduction band edge potential, ECB, is calculated by:
The band gap of the ZrO2 NPs is about 5.20 eV. The values of EVB and ECB for g-C3N4 were reported as 1.53 and − 1.01 eV, respectively (Yan et al. 2010).
Efficiency of the catalyst
To study the efficiency and stability of ZrO2/g-C3N4 nanocomposite, the photocatalytic degradation was repeated by a five-run cycling test under the same condition (Fig. 7). For each run, the photocatalyst was recycled, cleaned, and dried. In each cycle, the suspension was magnetically stirred for 2 h in the presence of light irradiation. The photodegradation of 4-NP was completed in each cycle. Hence, it is plausible to say that there is no significant loss of catalytic activity even after five cycles.
Conclusions
Nanotechnology-based photocatalysis plays an important role in the future of environmental decontamination, water remediation, and renewable energy generation from water splitting. In this work, ZrO2/g-C3N4 composites were successfully synthesized via simple ultrasound method. The prepared ZrO2/g-C3N4 catalysts were used for photocatalytic degradation of 4-NP. ZrO2/g-C3N4 exhibited higher photocatalytic activity than pure g-C3N4 for the degradation of 4-NP under light irradiation, and the 30 wt% ZrO2/g-C3N4 sample showed the highest activity. The XRD, nitrogen sorption, and FESEM results revealed that the ZrO2/g-C3N4 catalysts have high specific surface area. The ZrO2/g-C3N4 nanocomposite is not only a good adsorbent but also a very efficient heterogeneous photocatalyst for the degradation of 4-NP. Moreover, this ZrO2/g-C3N4 nanocomposite can be reused several times for photocatalytic degradation as well as for 4-NP adsorption. Thus, this ZrO2/g-C3N4 composite is a suitable catalyst for water cleaning. It has been shown the ZrO2 NPs and g-C3N4 nanosheets have unique properties such as antibacterial and antimicrobial behavior, environmental friendliness, and non-toxicity (Bing et al. 2015; Gowri et al. 2014). In fact, these characteristics besides the photocatalytic behaviors of g-C3N4 and ZrO2 NPs present a great potential for application of ZrO2/g-C3N4 composite for water remediation purposes.
References
Anbia M, Haqshenas M (2015) Adsorption studies of Pb(II) and Cu(II) ions on mesoporous carbon nitride functionalized with melamine-based dendrimer amine. Int J Environ Sci Technol 12:2649–2664
Atlow SC, Bonadonna-Aparo L, Klibanov AM (1984) Dephenolization of industrial wastewaters catalyzed by polyphenol oxidase. Biotechnol Bioeng 26:599–603
Bing W, Chen Z, Sun H, Shi P, Gao N, Ren J, Qu X (2015) Visible-light-driven enhanced antibacterial and biofilm elimination activity of graphitic carbon nitride by embedded Ag nanoparticles. Nano Res 8:1648–1658
Campelo JM, Luna D, Luque R, Marinas JM, Romero AA (2009) Sustainable preparation of supported metal nanoparticles and their applications in catalysis. Chemsuschem 2:18–45
Chang G, Luo Y, Lu W, Qin X, Asiri AM, Al-Youbi AO, Sun X (2012) Ag nanoparticles decorated polyaniline nanofibers: synthesis, characterization, and applications toward catalytic reduction of 4-nitrophenol and electrochemical detection of H2O2 and glucose. Catal Sci Technol 2:800–806
Chen Q, Zhao Y, Huang X, Chen N, Qu L (2015) Three-dimensional graphitic carbon nitride functionalized graphene-based high-performance supercapacitors. J Mater Chem A 3:6761–6766
Cui H et al (2013) Facile synthesis of graphene oxide-enwrapped Ag3PO4 composites with highly efficient visible light photocatalytic performance. Mater Lett 93:28–31
Fidelus JD, Karbowski A, Mariazzi S, Werner-Malento E, Brusa RS, Zhou W, Karwasz GP (2012) Combined positron-annihilation and structural studies of hydrothermally grown zirconia. Nanomater Energy 1:97–105
Gao H, Yan S, Wang J, Zou Z (2014) Ion coordination significantly enhances the photocatalytic activity of graphitic-phase carbon nitride. Dalton Trans 43:8178–8183
Ge L, Han C, Xiao X, Guo L (2013) Synthesis and characterization of composite visible light active photocatalysts MoS2–gC3N4 with enhanced hydrogen evolution activity. Int J Hydrog Energy 38:6960–6969
Geim AK (2009) Graphene: status and prospects. Science 324:1530–1534
Gowri S, Gandhi RR, Sundrarajan M (2014) Structural, optical, antibacterial and antifungal properties of zirconia nanoparticles by biobased protocol. J Mater Sci Technol 30:782–790
Guo S, Dong S (2011) Graphene nanosheet: synthesis, molecular engineering, thin film, hybrids, and energy and analytical applications. Chem Soc Rev 40:2644–2672
He Y, Cai J, Li T, Wu Y, Lin H, Zhao L, Luo M (2013) Efficient degradation of RhB over GdVO4/g-C3N4 composites under visible-light irradiation. Chem Eng J 215:721–730
Hu R, Wang X, Dai S, Shao D, Hayat T, Alsaedi A (2015) Application of graphitic carbon nitride for the removal of Pb(II) and aniline from aqueous solutions. Chem Eng J 260:469–477
Huang Z-F et al (2015) Carbon nitride with simultaneous porous network and O-doping for efficient solar-energy-driven hydrogen evolution. Nano Energy 12:646–656
Jiang H, Gomez-Abal RI, Rinke P, Scheffler M (2010) Electronic band structure of zirconia and hafnia polymorphs from the G W perspective. Phys Rev B 81:085119
Jiang F, Yan T, Chen H, Sun A, Xu C, Wang X (2014) A g-C3N4–CdS composite catalyst with high visible-light-driven catalytic activity and photostability for methylene blue degradation. Appl Surf Sci 295:164–172
Klibanov AM, Tu T-M, Scott KP (1983) Peroxidase-catalyzed removal of phenols from coal-conversion waste waters. Science 221:259–261
Lan M, Fan G, Yang L, Li F (2015) Enhanced visible-light-induced photocatalytic performance of a novel ternary semiconductor coupling system based on hybrid Zn–In mixed metal oxide/g-C3N4 composites. RSC Adv 5:5725–5734
Leary R, Westwood A (2011) Carbonaceous nanomaterials for the enhancement of TiO2 photocatalysis. Carbon 49:741–772
Li G, Wong K, Zhang X, Hu C, Jimmy CY, Chan R, Wong P (2009) Degradation of acid orange 7 using magnetic AgBr under visible light: the roles of oxidizing species. Chemosphere 76:1185–1191
Li G et al (2015) Enhanced visible-light-driven photocatalytic inactivation of Escherichia coli using g-C3N4/TiO2 hybrid photocatalyst synthesized using a hydrothermal-calcination approach. Water Res 86:17–24
Liang Q, Li Z, Yu X, Huang ZH, Kang F, Yang QH (2015) Macroscopic 3D porous graphitic carbon nitride monolith for enhanced photocatalytic hydrogen evolution. Adv Mater 27:4634–4639
Liu G, Wang Y, Shen C, Ju Z, Yuan D (2015a) A facile synthesis of microporous organic polymers for efficient gas storage and separation. J Mater Chem A 3(6):3051–3058
Liu J et al (2015b) Metal-free efficient photocatalyst for stable visible water splitting via a two-electron pathway. Science 347:970–974
Lu Y-C, Chen J, Wang A-J, Bao N, Feng J-J, Wang W, Shao L (2015) Facile synthesis of oxygen and sulfur co-doped graphitic carbon nitride fluorescent quantum dots and their application for mercury (II) detection and bioimaging. J Mater Chem C 3:73–78
Luo WB, Chou SL, Wang JZ, Zhai YC, Liu HK (2015) A metal-free, free-standing, macroporous graphene@ g-C3N4 composite air electrode for high-energy lithium oxygen batteries. Small 11:2817–2824
Maeda K, Kuriki R, Zhang M, Wang X, Ishitani O (2014) The effect of the pore-wall structure of carbon nitride on photocatalytic CO2 reduction under visible light. J Mater Chem A 2:15146–15151
Mao J, Peng T, Zhang X, Li K, Ye L, Zan L (2013) Effect of graphitic carbon nitride microstructures on the activity and selectivity of photocatalytic CO2 reduction under visible light. Catal Sci Technol 3:1253–1260
Ong W-J, Tan L-L, Chai S-P, Yong S-T (2015a) Graphene oxide as a structure-directing agent for the two-dimensional interface engineering of sandwich-like graphene–gC3N4 hybrid nanostructures with enhanced visible-light photoreduction of CO2 to methane. Chem Commun 51:858–861
Ong W-J, Tan L-L, Chai S-P, Yong S-T (2015b) Heterojunction engineering of graphitic carbon nitride (gC3N4) via Pt loading with improved daylight-induced photocatalytic reduction of carbon dioxide to methane. Dalton Trans 44:1249–1257
Pany S, Parida K (2015) A facile in situ approach to fabricate N, S-TiO2/g-C3N4 nanocomposite with excellent activity for visible light induced water splitting for hydrogen evolution. Phys Chem Chem Phys 17:8070–8077
Papailias I, Giannakopoulou T, Todorova N, Demotikali D, Vaimakis T, Trapalis C (2015) Effect of processing temperature on structure and photocatalytic properties of g-C3N4. Appl Surf Sci 358:278–286
Pawar RC, Kang S, Ahn SH, Lee CS (2015) Gold nanoparticle modified graphitic carbon nitride/multi-walled carbon nanotube (g-C3N4/CNTs/Au) hybrid photocatalysts for effective water splitting and degradation. RSC Adv 5:24281–24292
Pawar RC, Kang S, Park JH, Kim J-H, Ahn S, Lee CS (2016a) Room-temperature synthesis of nanoporous 1D microrods of graphitic carbon nitride (g-C3N4) with highly enhanced photocatalytic activity and stability. Sci Rep 6:31147
Pawar RC, Son Y, Kim J, Ahn SH, Lee CS (2016b) Integration of ZnO with g-C3N4 structures in core–shell approach via sintering process for rapid detoxification of water under visible irradiation. Curr Appl Phys 16:101–108
Pawar RC, Kang S, Park JH, Kim J-H, Ahn S, Lee CS (2017) Evaluation of a multi-dimensional hybrid photocatalyst for enrichment of H 2 evolution and elimination of dye/non-dye pollutants. Catal Sci Technol 7:2579–2590
Pohanish RP (2008) Sittig’s handbook of toxic and hazardous chemicals and carcinogens. William Andrew, Norwich
Polisetti S, Deshpande PA, Madras G (2011) Photocatalytic activity of combustion synthesized ZrO2 and ZrO2–TiO2 mixed oxides. Ind Eng Chem Res 50:12915–12924
Pradhan N, Pal A, Pal T (2002) Silver nanoparticle catalyzed reduction of aromatic nitro compounds. Colloids Surf A 196:247–257
Reimer T et al (2014) Single step integration of ZnO nano-and microneedles in Si trenches by novel flame transport approach: whispering gallery modes and photocatalytic properties. ACS Appl Mater Interfaces 6:7806–7815
Sayama K, Arakawa H (1993) Photocatalytic decomposition of water and photocatalytic reduction of carbon dioxide over zirconia catalyst. J Phys Chem 97:531–533
Singh A, Nakate UT (2014) Microwave synthesis, characterization, and photoluminescence properties of nanocrystalline zirconia. Sci World J Article ID 349457:1–7
Song C, Zhang J (2008) Electrocatalytic oxygen reduction reaction. In: PEM fuel cell electrocatalysts and catalyst layers. Springer, pp 89–134
Sridharan K, Kuriakose T, Philip R, Park TJ (2014) Transition metal (Fe, Co and Ni) oxide nanoparticles grafted graphitic carbon nitrides as efficient optical limiters and recyclable photocatalysts. Appl Surf Sci 308:139–147
Tan L-L, Ong W-J, Chai S-P, Goh BT, Mohamed AR (2015) Visible-light-active oxygen-rich TiO2 decorated 2D graphene oxide with enhanced photocatalytic activity toward carbon dioxide reduction. Appl Catal B Environ 179:160–170
Tian J, Liu Q, Asiri AM, Al-Youbi AO, Sun X (2013) Ultrathin graphitic carbon nitride nanosheet: a highly efficient fluorosensor for rapid, ultrasensitive detection of Cu2+. Anal Chem 85:5595–5599
Tian J, Ning R, Liu Q, Asiri AM, Al-Youbi AO, Sun X (2014) Three-dimensional porous supramolecular architecture from ultrathin g-C3N4 nanosheets and reduced graphene oxide: solution self-assembly construction and application as a highly efficient metal-free electrocatalyst for oxygen reduction reaction. ACS Appl Mater Interfaces 6:1011–1017
Wang D, Astruc D (2014) Fast-growing field of magnetically recyclable nanocatalysts. Chem Rev 114:6949–6985
Wang X et al (2009) A metal-free polymeric photocatalyst for hydrogen production from water under visible light. Nat Mater 8:76–80
Wang Y, Wang X, Antonietti M (2012) Polymeric graphitic carbon nitride as a heterogeneous organocatalyst: from photochemistry to multipurpose catalysis to sustainable chemistry. Angew Chem Int Ed 51:68–89
Wang H, Lu J, Wang F, Wei W, Chang Y, Dong S (2014) Preparation, characterization and photocatalytic performance of g-C3N4/Bi2WO6 composites for methyl orange degradation. Ceram Int 40:9077–9086
Wang L, Zhao F, Han Q, Hu C, Lv L, Chen N, Qu L (2015) Spontaneous formation of Cu2O–g-C3N4 core–shell nanowires for photocurrent and humidity responses. Nanoscale 7:9694–9702
Woan K, Pyrgiotakis G, Sigmund W (2009) Photocatalytic carbon-nanotube–TiO2 composites. Adv Mater 21:2233–2239
Wu Z, Gao H, Yan S, Zou Z (2014) Synthesis of carbon black/carbon nitride intercalation compound composite for efficient hydrogen production. Dalton Trans 43:12013–12017
Xu H, Ouyang S, Liu L, Reunchan P, Umezawa N, Ye J (2014) Recent advances in TiO2-based photocatalysis. J Mater Chem A 2:12642–12661
Xu L et al (2015) Insights into enhanced visible-light photocatalytic hydrogen evolution of g-C3N4 and highly reduced graphene oxide composite: the role of oxygen. Chem Mater 27:1612–1621
Yan S, Li Z, Zou Z (2009) Photodegradation performance of g-C3N4 fabricated by directly heating melamine. Langmuir 25:10397–10401
Yan S, Lv S, Li Z, Zou Z (2010) Organic–inorganic composite photocatalyst of g-C3N4 and TaON with improved visible light photocatalytic activities. Dalton Trans 39:1488–1491
Yang X, Cui H, Li Y, Qin J, Zhang R, Tang H (2013) Fabrication of Ag3PO4-graphene composites with highly efficient and stable visible light photocatalytic performance. ACS Catal 3:363–369
Yang J, Zhang H, Chen B, Tang H, Li C, Zhang Z (2015a) Fabrication of the gC3N4/Cu nanocomposite and its potential for lubrication applications. RSC Adv 5:64254–64260
Yang Q, Wang W, Zhao Y, Zhu J, Zhu Y, Wang L (2015b) Metal-free mesoporous carbon nitride catalyze the Friedel–Crafts reaction by activation of benzene. RSC Adv 5:54978–54984
Yeh T-F, Chen S-J, Teng H (2015) Synergistic effect of oxygen and nitrogen functionalities for graphene-based quantum dots used in photocatalytic H2 production from water decomposition. Nano Energy 12:476–485
Yi J, Liao K, Zhang C, Zhang T, Li F, Zhou H (2015) Facile in situ preparation of graphitic-C3N4@ carbon paper as an efficient metal-free cathode for nonaqueous Li–O2 battery. ACS Appl Mater Interfaces 7:10823–10827
Zarei M, Seif A, Azizi K, Zarei M, Bahrami J (2016) Effect of phenolic radicals on the geometry and electronic structure of DNA base pairs: computational study. Int J Mod Phys C 27:1650119
Zhang X, Zhang L, Xie T, Wang D (2009) Low-temperature synthesis and high visible-light-induced photocatalytic activity of BiOI/TiO2 heterostructures. J Phys Chem C 113:7371–7378
Zhang Q, Zhang Y, Li H, Gao C, Zhao Y (2013) Heterogeneous CaO-ZrO2 acid–base bifunctional catalysts for vapor-phase selective dehydration of 1, 4-butanediol to 3-buten-1-ol. Appl Catal A Gen 466:233–239
Zhang H et al (2015a) Self-assembly of graphitic carbon nitride nanosheets–carbon nanotube composite for electrochemical simultaneous determination of catechol and hydroquinone. Electrochim Acta 176:28–35
Zhang P, Wang T, Gong J (2015b) Mechanistic understanding of the plasmonic enhancement for solar water splitting. Adv Mater 27:5328–5342
Zhao Y, Zhang Y, Li J, Du X (2014) Solvothermal synthesis of visible-light-active N-modified ZrO2 nanoparticles. Mater Lett 130:139–142
Zhao L et al (2015) A chromium nitride/carbon nitride containing graphitic carbon nanocapsule hybrid as a Pt-free electrocatalyst for oxygen reduction. Chem Commun 51:12399–12402
Zheng Y, Liu J, Liang J, Jaroniec M, Qiao SZ (2012) Graphitic carbon nitride materials: controllable synthesis and applications in fuel cells and photocatalysis. Energy and Environ Sci 5:6717–6731
Acknowledgements
The authors acknowledge University of Kurdistan for supporting this project.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Zarei, M., Bahrami, J. & Zarei, M. Zirconia nanoparticle-modified graphitic carbon nitride nanosheets for effective photocatalytic degradation of 4-nitrophenol in water. Appl Water Sci 9, 175 (2019). https://doi.org/10.1007/s13201-019-1076-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13201-019-1076-8