Abstract
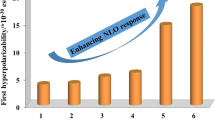
A new class of chiral phthalimides functionalized with aryl piperazines was designed anticipating their strong candidature for crystal engineering and technological applications. Five new phthalimides were synthesized, characterized and subjected to single crystal X-ray diffraction study that directed their non-centrosymmetric structures. Four phthalimides crystallized in P21 space group with monoclinic crystal system, however, one was found to possess P212121 space group with orthorhombic system. The supramolecular architectures of phthalimide crystals were analysed by an approach based on consideration of energy of intermolecular interaction. The molecular hyperpolarizability (β) calculation for all the listed phthalimides indicated their promising candidature for NLO materials. Further, the crystalline form of all phthalimides was evaluated for their second harmonic generation (SHG) response. A significant response of 16.4 mV was measured for phthalimide possessing t-butyl substituent at the para position of 4-benzylpiperazine. This high SHG response may be attributed to the molecular chirality and helical supramolecular frameworks stabilized by C-H ⋯O hydrogen bonds in the solid state. The current study attests chiral phthalimides possessing arylpiperazines as effective nominees to the area of crystal engineering and nonlinear optics.

New chiral phthalimides possessing terminal arylpiperazines have been studied for their solid-state properties. The second order NLO response of all the listed phthalimides was substantiated with the aid of computational study and crystal engineering approach.










Similar content being viewed by others
References
(a) Badan J, Hierle R, Perigaud A and Zyss J 1993 In American Chemical Society Symposium Series 233 (American Chemical Society: Washington, DC); (b) Prasad P N and Williams D J 1991 In Introduction to non-linear optical effects in organic molecules and polymers (Wiley: New York); (c) Brédas J L and Meyers F 1995 Nature 375 362; (d) Yu H, Wu H, Pan S, Yang Z, Hou X, Su X, Jing Q, Poeppelmeier K R and Rondinelli J M 2014 J. Am. Chem. Soc. 136 1264; (e) Stokes G Y and Conboy J C 2014 J. Am. Chem. Soc. 136 1409; (f) Eaton D F 1991 Science 253 28; (g) Lambert C, Nöll G, Schmälzlin E, Meerholz K and Bräuchle C 1998 Chem. Eur. J. 4 2129; (h) Wojciechowski A, Ozga K, Reshak AH, Miedzinski R, Kityk I V, Berdowski J and Tylczyński Z 2010 Mater. Lett. 64 1957; (i) Ozga K, Reshak A H, Berdowski J, Tylczyński Z, Wojciechowski A, Ślęzak A and Kityk I V 2011 Mater. Lett. 65 1734
(a) Zyss J and Chemla D S 1987 In Nonlinear Optical properties of Organic Molecules and Crystals, Vol. 1 (Academic Press: New York); (b) Bosshard C, Sutter K, Prgtre P, Hulliger J, Florsheimer M, Kaatz P and Gunter P 1995 In Organic Nonlinear Optical Materials (Gordon and Breach Science Publishers: Amsterdam); (c) Adur J, Carvalho H F, Cesar C L and Casco V H 2014 Cancer Inform. 13 67; (d) Diederichs C, Tignon J, Dasbach G, Ciuti C, Lemaitre A, Bloch J, Roussignol P and Delalande C 2006 Nature 440 904; (e) Samoc M, Gauthier N, Cifuentes M P, Paul F, Lapinte C and Humphrey M G 2006 Angew. Chem. Int. Ed. 45 7376; (f) Davydyuk G E, Khyzhun O Y, Reshak A H, Kamarudin H, Myronchuk G L, Danylchuk S P, Fedorchuk A O, Piskach L V, Mozolyuk M Y and Parasyuk O V 2013 Phys. Chem. Chem. Phys. 15 6965
(a) Gunter P 2000 In Nonlinear Optical Effects and Materials Springer Series in Optical Sciences Vol. 72 (Springer: Berlin); (b) Champagne B, Plaquet A, Pozzo J L, Rodriguez V and Castet F 2012 J. Am. Chem. Soc. 134 8101; (c) Deussen H-J, Boutton C, Thorup N, Geisler T, Hendrickx E, Bechgaard K, Persoons A Â and Bjùrnholm T 1998 Chem. Eur. J. 4 240; (d) Coluccini C, Sharma A K, Caricato M, Sironi A, Cariati E, Righetto S, Tordin E, Botta C, Forni A and Pasini D 2013 Phys. Chem. Chem. Phys. 15 1666
Malfant I, Cordente N, Lacroix P G and Lepetit C 1998 Chem. Mater. 10 4079
(a) Zhao S, Gong P, Bai L, Xu X, Zhang S, Sun Z, Lin Z, Hong M, Chen C and Luo J 2014 Nat. Commun. 5 4019; (b) Compain J D, Mialane P, Dolbecq A, Marrot J, Proust A, Nakatani K, Yu P and Secheresse F 2009 Inorg. Chem. 48 6222; (c) Lacroix PG 1995 Chem. Mater. 7 1293; (d) Hsiue G-H, Lee R-H and Jeng R-J 1997 Chem. Mater. 9 883; (e) Lebeau B, Brasselet S, Zyss J and Sanchez C 1997 Chem. Mater. 9 1012; (f) Lacroix P G 2001 Chem. Mater. 13 3495; (g) Sun H, Tian X, Wang J, Zhang J, Yuan Y and Sun Z R 2011 J. Phys. Chem. A 115 14495
(a) Kanis D R, Ratner M A and Marks T J 1994 Chem. Rev. 94 195; (b) Torre G, Vázquez P, López F A and Torres T 2004 Chem. Rev. 104 3723; (c) Ostroverkhova O and Moerner W E 2004 Chem. Rev. 104 3267
Patil P S, Dharmaprakash S M, Ramakrishna K, Fun H -K, Sai R, Kumar S and Rao D N 2007 J. Cryst. Growth 303 520
(a) Russell V A, Etter M C and Ward M D 1994 Chem. Mater. 6 1206; (b) Radhakrishnan T P 2008 Acc. Chem. Res. 41 367
Zhao B, Lu W -Q, Zhou Z -H and Wub Y 2000 J. Mater. Chem. 10 1513
Oudar J and Hieple R 1977 J. Appl. Phys. 48 2699
(a) Wang Y and Eaton D F 1985 Chem. Phys. Lett. 120 441; (b) Barron L D 1982 In Molecular Light Scattering and Optical Activity (Cambridge Univ. Press: Cambridge)
(a) Moerner W E and Silence S M 1994 Chem. Rev. 94 127; (b) Burland D M, Miller R D and Walsh C A 1994 Chem. Rev. 94 31; (c) Marks T J and Ratner M A 1995 Angew. Chem. Int. Ed. Engl. 34 155
(a) Bossi D E and Ade R W 1992 Laser Focus World 28 135; (b) Higgins T V 1992 Laser Focus World 28 125
Prakash M J and Radhakrishnan T P 2005 Cryst. Growth. Des. 5 1831
(a) Singh A K, Kishan R, Vijayan N, Balachandran V, Singh T, Tiwari H K, Singh B K and Rathi B 2013 RSC Adv. 3 14750; (b) Singh A K, Kishan R, Bahadur V, Vijayan N, Balachandran V, Tiwari H K, Singh B K and Rathi B 2014 J. Phy. Org. Chem. 27 490
Schwarzer A and Weber E 2008 Cryst. Growth Des. 8 2862
(a) Pal D, Suhnel J and Weiss M S 2002 Angew. Chem. Int. Ed. 41 4663; (b) Larsen P L, Parolin T J, Powell D R, Hedrich M P and Borovik A S 2003 Angew. Chem. Int. Ed. 42 85
Barooah N, Sarma R J and Baruah J B 2003 Cryst. Growth Des. 3 639
Mallakpour S and Rafiee Z 2008 Polym. Adv. Technol. 19 1474
Shishkin O V, Dyakonenko V V and Maleev A V 2012 Cryst. Eng. Comm. 14 1795
Yufit D S, Zubatyuk R, Shishkin O V and Howard J A K 2012 Cryst. Eng. Comm. 14 8222
Macrae C F, Bruno I J, Chisholm J A, Edgington P R, McCabe P, Pidcock E, Rodriguez-Monge L, Taylor R and van S. J 2008 de and Wood P A J. Appl. Cryst. 41 466
Hohenberg P and Kohn W 1964 Phys. Rev. 136B 864
Parr R G and Yang W 1989 In Density Functional Theory of Atoms and Molecules (Oxford University Press: New York)
Becke A D 1988 Phys. Rev. A 38 3098
Becke A D 1993 J. Chem. Phys. 98 5648
Lee C, Yang W and Parr R G 1988 Phys. Rev. B 37 785
Grimme S, Antony J, Ehrlich S and Krieg H 2010 J. Chem. Phys. 132 154104
Boys S F and Bernardi F 1970 Mol. Phys. 19 553
Neese F 2010 ORCA 28 0 (Universitaet Bonn: Germany)
Frisch M J, Trucks G W, Schlegel H B et al. 2009 GAUSSIAN 09: Revision A. 02 (Gaussian. Inc.:Wallingford, CT)
Kudin K N and Scuseria G E 2000 J. Chem. Phys. 113 7779
Kleinman D A 1962 Phys. Rev. 126 1977
Taylor R and Kennard O 1982 J. Am. Chem. Soc. 104 5063
(a) Desiraju G R 1991 Acc. Chem. Res. 24 290; (b) Desiraju G R 1996 Acc. Chem. Res. 29 441; (c) Steiner T 1996 Crystallogr. Rev. 6 1; (d) Steiner T 1997 Chem. Commun. 727
Zefirov Yu V 1997 Kristallografiya 42 936 (in Russian)
Dyakonenko V V, Maleev A V, Zbruyev A I, Chebanov V A, Desenko S M and Shishkin O V 2010 Cryst. Eng. Comm. 12 1816
Kurtz S K and Perry T T 1968 J. Appl. Phys. 39 3798
Acknowledgements
BR is grateful to the Department of Science and Technology, Ministry of Science and Technology, India for financial support (SR/FT/CS-108/2010). Scientific collaboration was possible due to India-Ukraine Bilateral Scientific Cooperation program supported by Department of Science and Technology, Ministry of Science & Technology, India and The Ukrainian State Agency for Science, Innovation, and Informatization (0114U003690). We are thankful to Dr. Svetlana V. Shishkina for her valuable corrections. AKS and VB are thankful to CSIR, India for providing Senior Research Fellowship.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary Information
All additional information relating to characterization of the compounds such as NMR spectras (Figures S1–S15), TGA-DTA plots (Figures S16–S20), UV-Vis spectra (Figure S21), supramolecular architecture of crystals presented as packing of molecules and hedgehogs of intermolecular interactions (Figures S23–S27), Crystallographic data (Table S1), Geometrical parameters of intermolecular interactions in crystals (Table S2) and Numbering of dimers, symmetry operation of second molecule of dimer and energy of intermolecular interactions in dimers formed by the basic molecule in the crystals (Tables S3–S7) are given in the supporting information available at www.ias.ac.in/chemsci.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
SINGH, A.K., RATHI, B., MEDVIEDIEV, V.V. et al. Functionalized organic frameworks explored as second order NLO agents. J Chem Sci 128, 297–309 (2016). https://doi.org/10.1007/s12039-015-1012-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12039-015-1012-x