Abstract
Introduction
Mechanical ventilation with control of partial arterial CO2 pressures (PaCO2) is used to treat or stabilize intracranial pressure (ICP) in patients with traumatic brain injury (TBI). Pressure-regulated volume control (PRVC) is a ventilator mode where inspiratory pressures are automatically adjusted to deliver the patient a pre-set stable tidal volume (TV). This may result in a more stable PaCO2 and thus a more stable ICP compared with conventional pressure control (PC) ventilation. The aim of this study was to compare PC and PRVC ventilation in TBI patients with respect to ICP and PaCO2.
Methods
This is a randomized crossover trial including eleven patients with a moderate or severe TBI who were mechanically ventilated and had ICP monitoring. Each patient was administered alternating 2-h periods of PC and PRVC ventilation. The outcome variables were ICP and PaCO2.
Results
Fifty-two (26 PC, 26 PRVC) study periods were included. Mean ICP was 10.8 mmHg with PC and 10.3 mmHg with PRVC ventilation (p = 0.38). Mean PaCO2 was 36.5 mmHg (4.87 kPa) with PC and 36.1 mmHg (4.81 kPa) with PRVC (p = 0.38). There were less fluctuations in ICP (p = 0.02) and PaCO2 (p = 0.05) with PRVC ventilation.
Conclusions
Mean ICP and PaCO2 were similar for PC and PRVC ventilation in TBI patients, but PRVC ventilation resulted in less fluctuation in both ICP and PaCO2. We cannot exclude that the two ventilatory modes would have impact on ICP in patients with higher ICP values; however, the similar PaCO2 observations argue against this.
Similar content being viewed by others
Introduction
Traumatic brain injury (TBI) is one of the main causes of death and disability with an incidence in Europe of 235 per 100,000 per year and a mortality rate of 15 per 100,000 per year [1]. Intracranial hypertension increases mortality rate and affects functional outcome [2, 3]. The main treatment goal is to avoid secondary brain injury by ensuring adequate circulation and oxygenation to the brain by avoiding elevation of the intracranial pressure (ICP) and securing an adequate cerebral perfusion pressure (CPP). There are however controversies regarding how to deliver such care [4, 5].
Elevated partial arterial pressure of CO2 (PaCO2) causes cerebral vasodilation which increases ICP, while too low PaCO2 causes vasoconstriction and decreases blood flow to the brain [6–10]. In order to secure a stable PaCO2 and ICP, most patients with severe TBI and some patients with moderate TBI are initially sedated and mechanically ventilated. While the TBI guidelines include desired levels of oxygen saturation and PaCO2 targets, there is no consensus on which ventilation mode to prefer in TBI patients [11, 12].
This study compares two modes of mechanical ventilation in TBI patients: pressure control (PC) ventilation and pressure-regulated volume control (PRVC) ventilation. Both modes use a decelerating inspiratory flow that is thought to be close to the normal physiology of the lungs and may give a lower peak inspiratory pressure than standard volume control ventilation [13–15]. In our ICU, we usually ventilate TBI patients with PC ventilation. PC ventilation maintains a stable airway pressure but may give fluctuations in tidal volumes (TV) depending on variable pulmonary atelectasis, secretions, or lung compliance. In PRVC ventilation, the inspiratory pressure above PEEP is automatically adjusted so that the ventilator delivers a constant, pre-set TV. A stable TV given by PRVC ventilation can be hypothesized to give a more stable PaCO2 resulting in a more stable ICP. Thus, the primary aim of the study was to compare PC and PRVC ventilation in patients with TBI with respect to possible effects on ICP. The secondary aim was to study whether different ventilation modes led to differences in PaCO2.
Materials and Methods
Trial Design
This phase II study is a crossover randomized trial comparing two ventilation modes, PC and PRVC ventilation, in mechanically ventilated patients with a moderate or severe TBI.
Participants and Setting
The study was done at the neurosurgical intensive care unit (ICU) and the general ICU at St. Olav University Hospital, Trondheim, Norway. The hospital is the only neurosurgical center in a geographical catchment region with 680 000 inhabitants. Patients who were ≥16 years old with a severity of TBI indicating continuous measurement of ICP, continuous infusions of sedatives, and mechanical ventilation were considered for inclusion. Patients were excluded if they were pregnant, had an ICP ≥ 25 mmHg > 5 min, an ongoing cerebral antiedema therapy corresponding to Step III at St Olav University Hospital TBI therapy guidelines (Table 1), an open external drainage of cerebrospinal fluid (CSF); or if they had a clinical pulmonary condition limiting changes in respiratory therapy.
Interventions
The patients entered the study after initial emergency surgery and stabilization in the ICU. All patients were sedated to motor activity assessment scale (MAAS) score 0–1 [16] with midazolam or propofol and received analgesia with morphine, fentanyl, or remifentanil. The general ICP directed therapy was given according to the hospitals therapy guideline for TBI patients (Table 1). All patients were ventilated using a Maquet SERVO-i ventilator system V6.0 (Maquet Critical Care AB, Solna, Sweden).
After inclusion, the patients were randomized by a web interface to alternating 2-h periods with PC or PRVC ventilation. After each study period, the patients were crossed-over to the alternative ventilation mode in the next study period after an interval needed for interventions and adjustment of ventilator settings. Each patient was subject to a maximum of 6 2-h study periods, i.e., 3 PC and 3 PRVC periods.
Before each study period, the ventilator settings were adjusted to achieve PaCO2 within 34–41 mmHg (4.5–5.5 kPa) (normocapnia). The relevant adjustments were positive end expiratory pressure (PEEP), respiratory rate (RR), and pressure support above PEEP (in PC) or TV (in PRVC). The inspiration:expiration (I:E) ratio was set to 1:2 in all patients. The inspired oxygen fraction (FiO2) was adjusted to achieve oxygen saturation measured by pulse oximetry (SpO2) above 95 %. Changes in ventilator settings were avoided during each study period, except for the FiO2 that could be adjusted to maintain SpO2 ≥ 95 %.
All interventions that could cause changes in ICP such as tracheal suction and change of wound dressings had to be performed before or between the 2-h study periods. Fluid therapy, the use of vasoactive agents, the use of sedatives, and antipyretics were given as required according to the TBI treatment protocol.
A study period was terminated if the ICP was above 20 mmHg for more than 10 min or ICP ≥ 25 mmHg for more than 5 min and rescue therapy (e.g., opening CSF drainage, osmotic therapy, or respiratory intervention) was initiated. The cause of terminating the study period was registered.
ICP Monitoring
The ICP was measured continuously by an intraparenchymal (n = 9) or subdural (n = 2) pressure sensor (Spiegelberg (GmbH & Co.) KG, Hamburg, Germany) [17, 18]. For calculating the CPP, the zero level of the arterial blood pressure was at the level of the heart.
Registrations
Patient demographics (age, gender, simplified acute physiology scale 3 (SAPS 3) [19], concomitant diseases, injury history, GCS score before intubation, intracranial CT findings, injury severity score (ISS) [20], sequential organ failure assessment (SOFA) score [21] ), surgical interventions, all medications (vasoactive drugs, sedatives, other), and chest X-ray findings within 24 h were registered at inclusion.
The following variables were registered at the beginning and during each study period: ICP, CPP, MAAS, ventilator settings, observed TV, RR, peak pressures, SpO2, partial arterial pressure of O2 (PaO2), PaCO2, end-tidal CO2 (Capnostat etCO2 sensor, Maquet, Solna, Sweden), intra-arterial blood pressure, heart rate, the use of vasoactive drugs (type/dose), serum sodium concentration, blood glucose, and hemoglobin. Observations were registered every 10 min except for blood gases and clinical chemistry, which were obtained every 30 min (Siemens RAPIDLab 1200 Systems or Radiometer ABL 800 Flex).
Pulmonary complications [pneumonia and acute respiratory distress syndrome (ARDS)] during the ICU stay, ICU length of stay, and in-hospital mortality were also registered [22].
Outcome Variables
The pre-specified primary outcome variable was ICP during the PC and PRVC ventilation periods. The secondary outcome variable was PaCO2. We also analyzed the fluctuations (standard deviations) of ICP and PaCO2 during the study periods with PC and PRVC ventilation.
Randomization Procedure and Blinding
The randomization procedure was computerized and performed by “Unit for Applied Clinical Research” at the Norwegian University of Science and Technology. Each patient was randomized to either of two sequences: PC, PRVC, PC, PRVC, PC, PRVC, or PRVC, PC, PRVC, PC, PRVC, PC. The researcher was blinded to the sequence of interventions before the patients were included and received the randomization by a web interface after inclusion. The enrollment and the assignment of patients were done by the investigators (K.S.M. and P.K.). The interventions (ventilator settings) were not possible to blind to the investigators during the study periods, but it was blinded to the statistician assessing the outcome data (E.S).
Statistical Methods and Sample Size Calculation
Descriptive statistics are given as mean, median, range, confidence interval and standard deviation or absolute numbers, and percentage as appropriate. The primary outcome variable was ICP during the 2-h ventilation periods. If a study period was terminated before 2 h, the data obtained until termination were used in the analyses, and for the rest of the study period, values were imputed using “the last value carried forward”.
Two neurosurgeons (O.S. and A.V.) considered the minimum clinical difference of interest regarding ICP between the two ventilation modes to be 2 mmHg. Pilot data obtained retrospectively from clinically stable TBI patients in the ICU were analyzed in order to assess expected within- and between-patient variability, suggesting a within-patient standard deviation of 2 mmHg. With these assumptions, 80 % power, and a significance level of 5 %, a total of 32 observations (2-h ventilation periods) were required. The outcome was modeled in a linear mixed effects model with ventilation mode as a fixed effect and study period nested within patient as random effects [23]. We used the statistical software SPSS ver.22 (IBM SPSS statistics, Armonk, New York, USA) and STATA SE ver.13.1 (StataCorp LP, College Station, Texas, USA) for the mixed model analysis.
Ethical Considerations
The study was done according to the principles of the Helsinki Declaration. The Regional Committee for Medical Research Ethics, Health region IV, Norway, approved the study. Written informed consent prior to inclusion in the study was given by the patients’ next of kin since the patients were unconscious. For patients who regained capacity to give an informed consent, a deferred consent was obtained.
Results
Patients
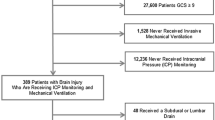
The patients were recruited during a total period of 14 months (Sept 26th 2013 to June 13th 2014 and August 10th 2014 to January 23rd 2015). During this period, 30 TBI patients were treated with mechanical ventilation. Sixteen patients did not meet the inclusion criteria (Fig. 1). Three patients were not included due to early withdrawal of sedatives, leaving 11 patients included in this study.
The median age was 45.5 years (range 16–74), and 9 of the 11 patients were male. The cause of the accident was either traffic (n = 7) or fall injuries (n = 4). The median GCS score before intubation was 5 (3–13). Three of the patients had unilateral pupil dilation on arrival at the hospital, and the most common cerebral CT findings were traumatic subarachnoid hemorrhage (n = 8), subdural hematoma (n = 3), epidural hematoma (n = 3), and multiple contusions (n = 8). Three patients needed surgery for their intracranial injury while 3 patients underwent extracranial surgery. Median SAPS 3 score for patients who were ≥18 years was 54 (30–75), while the median ISS score was 29 (20–45). Median initial SOFA score was 10 (7–15). No patients had a known pulmonary or cardiac disease, but three patients showed abnormal chest X-ray findings due to the injury.
Baseline Data
The median time from injury to inclusion in the study was 24 (16–51) h. Seven of the patients had been stabilized at a local hospital before transferal. Each patient completed from 1 to 6 study periods resulting in a total number of 52 study periods, 26 with PC and 26 with PRVC. Baseline observations obtained at the start of each study period are shown in Table 2. The median time interval between two study periods was 70 min, and the median time from adjusting the ventilator settings to start of the observation period was 35 min.
Outcome Variables
For the primary outcome ICP, we observed no statistical difference between the two study groups (PC 10.8 mmHg (mean), PRVC 10.3 mmHg (mean), p = 0.38) (Table 3, Fig. 2). In one study period with PC ventilation, the patient received rescue therapy after 85 min. For PaCO2, we found no significant difference between the two ventilation modes (PC 36.5 mmHg (4.87 kPa) (mean), PRVC 36.1 mmHg (4.81 kPa) (mean), p = 0.38). Episodes of hypoventilation (PaCO2 > 41 mmHg (5.5 kPa)) occurred in one study period with PRVC and in 3 study periods with PC ventilation.
There were less fluctuations in the ICP within each ventilation period when using PRVC ventilation compared to PC ventilation (residual SD 1.47 and 1.72 mmHg, respectively, p = 0.019; significant autocorrelation with ρ = 0.72). The fluctuation in PaCO2 was also slightly less during the PRVC than during the PC ventilation periods (residual SD 2.0 mmHg (0.27 kPa) and 2.5 mmHg (0.33 kPa), respectively, p = 0.05; significant autocorrelation with ρ = 0.83).
Other respiratory values such as PaO2, FiO2, TV, and peak inspiratory pressures during the ventilation periods are described in Table 4. The mean CPP was 70 mmHg in the PC periods and 69 mmHg during the PRVC periods. The mean difference between PaCO2 and etCO2 in all patients was 2.2 ± 3.0 mmHg (0.3 ± 0.4 kPa). All except one patient received noradrenaline to maintain an adequate CPP. During the study sessions all patients had normoglycemia. The mean hemoglobin was identical in both groups, 10.4 g/dl.
Ten out of 11 patients received treatment for pneumonia during their ICU stay, and two patients had both pneumonia and ARDS (one with mild and one with severe ARDS according to the Berlin definition [22]). The median ICU length of stay for the included patients was 10 days (range 5–36). Hospital mortality was 18 %; one patient died during the ICU stay due to intracranial herniation 8 days after the injury, while one patient died at a local hospital after 42 days due to sequels from the TBI.
Discussion
The main finding in this randomized phase II study was that there was no difference in ICP or PaCO2 when comparing PC to PRVC ventilation in patients with a moderate or severe TBI. However, we observed less fluctuation in ICP and PaCO2 in study periods with PRVC ventilation.
There are studies that have compared the effect of PC, PRVC, and VC ventilation on cardiopulmonary parameters [15, 24, 25]. However, we could not find any previous studies comparing PC, PRVC, and VC ventilation modes in respect to their influence on ICP in neurocritical care. This lack of evidence regarding a central component of intensive care for a large group of patients reflects that studies comparing medical technical devices or settings are scarce. There is a large discrepancy between the scientific and regulatory rigor needed to introduce new drugs compared to the level of evidence needed to implement new medical technical devices. That such studies are needed are repeatedly demonstrated for instance by the negative studies for oscillation therapy in ARDS patients [26].
While ICP and PaCO2 were similar in the ventilatory modes, we observed that applying a ventilation mode with constant TV (PRVC) resulted in less fluctuation in ICP and PaCO2. Still, the difference in absolute numbers is small demonstrating that both ICP and PaCO2 were relatively stable in both the PC and the PRVC group. Thus, short-term changes in pulmonary compliance are not frequent in sedated TBI patients. We cannot exclude that study periods longer than 2 h might have shown larger differences between the two ventilation modes. Study period duration was selected so that other interventions (e.g., changes in position or tracheal suction) that would confound the observations could be postponed to after the study period. Also Guldager et al. who compared VC and PRVC in patients with acute respiratory failure without intracranial pathology used 2-h study periods [14].
Respiratory treatment is a central part of ICU treatment in TBI patients and control of PaCO2 is usually needed in order to avoid intracranial hypertension. In mechanically ventilated patients, the current recommendation in the TBI guidelines for patients without intracranial hypertension (ICP < 20 mmHg) is to maintain normocapnia (i.e., PaCO2 34–41 mmHg (4.5–5.5 kPa)), and a study by Lee et al. [27] confirmed that CO2 reactivity remains relatively intact in the acute phase after a TBI. However, PaCO2 levels within normal values are not always achieved in TBI patients [28]. TBI patients are at high risk of developing respiratory complications such as pneumonia and ARDS [29, 30]. This indicates a need for lung protective ventilation with a higher PEEP, low TV, and acceptance of hypercapnia [31–33]. The discrepancy between a lung protective strategy and cerebral protective ventilation complicates what ventilator mode to prefer in TBI patients. In a multicenter study on mechanically ventilated neurologic patients by Pelosi et al. [34], the most common primary ventilation mode was volume-cycled assist-control ventilation, whereas the use of PC and PRVC were less frequent. Most of the patients had TV 6–12 ml/kg and were ventilated with a PEEP ≤ 5 cm H2O, but the different ventilation modes were not compared. Mascia et al. found that high TV in TBI patients is associated with the development of acute lung injury (ALI) [35]. The use of a ventilator mode that protects against high TV, such as PRVC could potentially lower the risk of developing ALI/ARDS. In our study, we used two variants of PC ventilation modes to normo-ventilate the patients according to our TBI protocol while still keeping the mean TV within accepted limits recommended for lung protective ventilation.
We recognize that this study has some limitations. Firstly, this is a single-center study with a limited number of patients. However, 52 study periods with a total of 674 ICP measurements were studied and compared. Also, by using the patient as his/her own control, findings are less influenced by inter-individual variability due to differences between the patients’ pre-injury characteristics or their acute illnesses. Secondly, the crossover design precludes any comparisons of long-term outcomes related to the two ventilation modes. This study is therefore designed to discover as a proof-of-concept if an alternative ventilator strategy influences TBI-related observations. Such studies are needed and should be performed before considering using resources on a large-scale phase III interventional study and before doing studies in patients with critically high ICP. Thirdly, due to the pressure–volume relationship in the brain (the Monro-Kelly hypothesis), effects of interventions on ICP are small in patients without significantly elevated ICP. In order to do a crossover trial, the patients’ clinical conditions had to be relatively stable, and moreover, before introducing a new treatment to patients in critical neurological state, studies have to be done in patients less vulnerable. We cannot exclude that the two ventilator modes may have impact on ICP in patients with high ICP; however, the similar PaCO2 observations argue against this. Fourthly, the value of clinical difference in ICP of 2 mmHg is debatable. Since we could not identify any similar studies, we decided to define this low ICP difference in order to eventually err on the side of safety in regard to the number of observations. Finally, in this study, we measured ICP and CPP. Other methods for neurocritical monitoring such as brain tissue oxygen pressure or central venous oxygen saturation may have given additional information and could be applied in further studies.
Conclusion
In conclusion, we did not observe any difference in ICP and PaCO2 when comparing PC and PRVC ventilation in patients with TBI. PRVC ventilation resulted in less fluctuation in both ICP and PaCO2, but the magnitude of this difference is minor and probably not clinically important.
Abbreviations
- TBI:
-
Traumatic brain injury
- ICP:
-
Intracranial pressure
- CPP:
-
Cerebral perfusion pressure
- PC:
-
Pressure control
- PRVC:
-
Pressure-regulated volume control
- TV:
-
Tidal volume
- PaCO2 :
-
Partial arterial pressure of CO2
- PaO2 :
-
Partial arterial pressure of O2
- ICU:
-
Intensive care unit
- MAAS:
-
Motor activity assessment scale
- SAPS 3:
-
Simplified acute physiology scale 3
- SOFA:
-
Sequential organ failure assessment score
- GCS:
-
Glasgow coma scale
- CSF:
-
Cerebrospinal fluid
- ARDS:
-
Acute respiratory distress syndrome
- ISS:
-
Injury severity score
References
Tagliaferri F, Compagnone C, Korsic M, Servadei F, Kraus J. A systematic review of brain injury epidemiology in Europe. Acta Neurochir. 2006;148:255–68.
Vik A, Nag T, Fredriksli OA, et al. Relationship of “dose” of intracranial hypertension to outcome in severe traumatic brain injury. J Neurosurg. 2008;109:678–84.
Stocchetti N, Zanaboni C, Colombo A, et al. Refractory intracranial hypertension and “second-tier” therapies in traumatic brain injury. Intensive Care Med. 2008;34:461–7.
Grande PO, Asgeirsson B, Nordstrom CH. Volume-targeted therapy of increased intracranial pressure: the Lund concept unifies surgical and non-surgical treatments. Acta Anaesthesiol Scand. 2002;46:929–41.
Chesnut RM, Temkin N, Carney N, et al. A trial of intracranial-pressure monitoring in traumatic brain injury. N Engl J Med. 2012;367:2471–81.
Mascia L, Grasso S, Fiore T, Bruno F, Berardino M, Ducati A. Cerebro-pulmonary interactions during the application of low levels of positive end-expiratory pressure. Intensive Care Med. 2005;31:373–9.
Stocchetti N, Maas AI, Chieregato A, van der Plas AA. Hyperventilation in head injury: a review. Chest. 2005;127:1812–27.
Asgari S, Bergsneider M, Hamilton R, Vespa P, Hu X. Consistent changes in intracranial pressure waveform morphology induced by acute hypercapnic cerebral vasodilatation. Neurocrit Care. 2011;15:55–62.
van Hulst RA, Hasan D, Lachmann B. Intracranial pressure, brain PCO2, PO2, and pH during hypo- and hyperventilation at constant mean airway pressure in pigs. Intensive Care Med. 2002;28:68–73.
Brian JE Jr. Carbon dioxide and the cerebral circulation. Anesthesiology. 1998;88:1365–86.
Maas AI, Dearden M, Teasdale GM, et al. EBIC-guidelines for management of severe head injury in adults. European Brain Injury Consortium. Acta Neurochir. 1997;139:286–94.
Bratton SL, Chestnut RM, Ghajar J, et al. Guidelines for the management of severe traumatic brain injury. J Neurotrauma. 2007;24(Suppl 1):S14–95.
Kocis KC, Dekeon MK, Rosen HK, et al. Pressure-regulated volume control vs volume control ventilation in infants after surgery for congenital heart disease. Pediatr Cardiol. 2001;22:233–7.
Guldager H, Nielsen SL, Carl P, Soerensen MB. A comparison of volume control and pressure-regulated volume control ventilation in acute respiratory failure. Crit Care. 1997;1:75–7.
Alvarez A, Subirana M, Benito S. Decelerating flow ventilation effects in acute respiratory failure. J Crit Care. 1998;13:21–5.
Devlin JW, Boleski G, Mlynarek M, et al. Motor Activity Assessment Scale: a valid and reliable sedation scale for use with mechanically ventilated patients in an adult surgical intensive care unit. Crit Care Med. 1999;27:1271–5.
Lang JM, Beck J, Zimmermann M, Seifert V, Raabe A. Clinical evaluation of intraparenchymal Spiegelberg pressure sensor. Neurosurgery. 2003;52:1455–9 discussion 9.
Vender J, Waller J, Dhandapani K, McDonnell D. An evaluation and comparison of intraventricular, intraparenchymal, and fluid-coupled techniques for intracranial pressure monitoring in patients with severe traumatic brain injury. J Clin Monit Comput. 2011;25:231–6.
Moreno RP, Metnitz PG, Almeida E, et al. SAPS 3–From evaluation of the patient to evaluation of the intensive care unit. Part 2: development of a prognostic model for hospital mortality at ICU admission. Intensive Care Med. 2005;31:1345–55.
Baker SP, O’Neill B, Haddon W Jr, Long WB. The injury severity score: a method for describing patients with multiple injuries and evaluating emergency care. J Trauma. 1974;14:187–96.
Janssens U, Graf C, Graf J, Radke PW, Königs B, Koch KC, Lepper W, vom Dahl J, Hanrath P. Evaluation of the SOFAscore: a single-center experience of a medical intensive care unit in 303 consecutive patients with predominantly cardio-vascular disorders. Intensive Care Med. 2000;26:1037–45.
Ranieri VM, Rubenfeld GD, Thompson BT, et al. Acute respiratory distress syndrome: the Berlin Definition. JAMA. 2012;307(23):2526–33.
Rabe-Hesketh S, Skrondal A. Multilevel and longitudinal modeling using Stata, vol. 1. 3rd ed. Texas: Stata Press; 2012. p. 168–71.
Kallet RH, Campbell AR, Dicker RA, Katz JA, Mackersie RC. Work of breathing during lung-protective ventilation in patients with acute lung injury and acute respiratory distress syndrome: a comparison between volume and pressure-regulated breathing modes. Respir Care. 2005;50:1623–31.
Pierce JD, Gilliland E, Smith-Blair N, Clancy RL. Effects of volume control, pressure control, and pressure-regulated volume control on cardiopulmonary parameters in a normal rat lung. Mil Med. 1998;163:625–30.
Ferguson ND, Cook DJ, Guyatt GH, et al. High-frequency oscillation in early acute respiratory distress syndrome. N Engl J Med. 2013;368:795–805.
Lee JH, Kelly DF, Oertel M, et al. Carbon dioxide reactivity, pressure autoregulation, and metabolic suppression reactivity after head injury: a transcranial Doppler study. J Neurosurg. 2001;95:222–32.
Schirmer-Mikalsen K, Moen KG, Skandsen T, Vik A, Klepstad P. Intensive care and traumatic brain injury after the introduction of a treatment protocol: a prospective study. Acta Anaesthesiol Scand. 2013;57:46–55.
Schirmer-Mikalsen K, Vik A, Gisvold SE, Skandsen T, Hynne H, Klepstad P. Severe head injury: control of physiological variables, organ failure and complications in the intensive care unit. Acta Anaesthesiol Scand. 2007;51:1194–201.
Pelosi P, Severgnini P, Chiaranda M. An integrated approach to prevent and treat respiratory failure in brain-injured patients. Curr Opin Crit Care. 2005;11:37–42.
Young N, Rhodes JK, Mascia L, Andrews PJ. Ventilatory strategies for patients with acute brain injury. Curr Opin Crit Care. 2010;16:45–52.
Mascia L. Acute lung injury in patients with severe brain injury: a double hit model. Neurocrit Care. 2009;11:417–26.
Lowe GJ, Ferguson ND. Lung-protective ventilation in neurosurgical patients. Curr Opin Crit Care. 2006;12:3–7.
Pelosi P, Ferguson ND, Frutos-Vivar F, et al. Management and outcome of mechanically ventilated neurologic patients. Crit Care Med. 2011;39:1482–92.
Mascia L, Zavala E, Bosma K, et al. High tidal volume is associated with the development of acute lung injury after severe brain injury: an international observational study. Crit Care Med. 2007;35:1815–20.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest and Source of funding
Authors Kari Schirmer-Mikalsen and Kent Gøran Moen have received a Research grant from the Liaison Committee between the Central Norway Regional Health Authority (RHA) and the Norwegian University of Science and Technology (NTNU). For the remaining authors, none were declared. The participants received no compensation for participating in the study. Contributions from health care personnel at the ICU were agreed without any additional financial compensation.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Additional information
The study was performed at St. Olav University Hospital, Trondheim, Norway.
The study is registered in clinicaltrials.gov (NCT01955785, 2013/1346).
The full trial protocol can be accessed by contacting the corresponding author (KSM).
Rights and permissions
About this article
Cite this article
Schirmer-Mikalsen, K., Vik, A., Skogvoll, E. et al. Intracranial Pressure During Pressure Control and Pressure-Regulated Volume Control Ventilation in Patients with Traumatic Brain Injury: A Randomized Crossover trial. Neurocrit Care 24, 332–341 (2016). https://doi.org/10.1007/s12028-015-0208-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12028-015-0208-8