Abstract
Objective
In patients with severe brain injury and acute lung injury the use of positive end-expiratory pressure (PEEP) is limited by conflicting results on its effect on intracranial pressure. We hypothesised that the occurrence of alveolar hyperinflation during the application of PEEP would lead to an increase in PaCO2 responsible for a rise in intracranial pressure.
Design
Prospective interventional study.
Setting
Intensive Care Unit of University Hospitals.
Patients and participants
Twelve severely brain-injured patients with acute lung injury and intracranial pressure higher than applied PEEP.
Interventions
5 and 10 cmH2O of PEEP was randomly applied.
Measurements and results
In all patients intracranial pressure, flow velocity by transcranial Doppler of middle cerebral artery, and jugular oxygen saturation were recorded. Static volume-pressure curves of the respiratory system were obtained, recruited volume and elastance calculated to classify patients as recruiters and non-recruiters. In recruiters (= 6 patients), elastance decreased (P<0.01) and PaO2 increased (P<0.005), while in non-recruiters (= 6 patients) elastance and PaCO2 significantly increased (P<0.001). Intracranial pressure, Doppler flow velocity, and jugular saturation remained constant in recruiters but significantly increased (P<0.0001) in non-recruiters. A significant correlation was found between changes in intracranial pressure and elastance (r2 = 0.8 P<0.0001) and between changes in PaCO2 and intracranial pressure (P<0.001, r2 = 0.4) and elastance (P<0.001, r2 = 0.4), respectively.
Conclusions
When PEEP induced alveolar hyperinflation leading to a significant increase in PaCO2, intracranial pressure significantly increased, whereas when PEEP caused alveolar recruitment intracranial pressure did not change.
Similar content being viewed by others
Introduction
Development of acute lung injury (ALI) occurs in 20–25% of patients with isolated severe brain injury and is associated with a threefold increased risk of dying or remaining in a vegetative state [1, 2, 3, 4]. Ventilatory support for acute lung injury involves the application of positive end-expiratory pressure (PEEP) to recruit collapsed alveoli, improve arterial oxygenation and reduce elastance of the respiratory system [5]. Although improvement in oxygenation is a key factor for optimizing O2 delivery to the brain [6], clinical studies provide contradictory information on the use of PEEP in patients with acute lung injury complicating severe brain injury [7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18].
Application of PEEP may affect cerebral circulation by hemodynamic mechanisms mainly reducing cerebral venous drainage. Indeed, effects of PEEP on intracranial pressure (ICP) have been best explained by the Starling resistor model [14, 15, 19]. Recently, McGuire et al. [18] confirmed in patients that PEEP increased ICP when the baseline ICP value was lower than PEEP, but had less effect on cerebral perfusion when ICP was above the highest applied PEEP. Besides this, application of PEEP may affect cerebral circulation by CO2-mediated mechanisms depending on recruitment/hyperinflation of alveolar units: if alveolar hyperinflation is the predominant event with PEEP, an increase in pulmonary elastance and dead space leading to a rise in arterial PCO2 and ICP should occur [20]. If alveolar recruitment is the paramount effect of PEEP, one would expect lung elastance to decrease and arterial PO2 to substantially increase due to the reopening of previously collapsed and perfused regions [20]. Consequently, even if hemodynamic transmission is minimized because ICP values are higher than applied PEEP, ICP may still be affected by changes in PaCO2.
The current study tested the hypothesis that the occurrence of alveolar hyperinflation during the application of PEEP will lead to an increase in PaCO2 responsible for an increase in ICP. To test this hypothesis, only patients with baseline ICP values higher than applied PEEP were included. Some of the results of this study have been previously reported in the form of an abstract [21].
Methods
The institutional ethical committee approved the research protocol and informed consent was obtained. Twelve patients admitted to the Intensive Care Unit of the Policlinico, Di Venere (University of Bari) and S. Giovanni Battista (University of Turin) were recruited according to the following inclusion criteria: 1) diagnosis of severe brain injury defined as a Glasgow Coma Score <9 on admission after resuscitation [22]; 2) diagnosis of ALI made according to the American-European Consensus Conference criteria: acute onset, arterial O2 partial pressure/inspired O2 fraction (PaO2/FiO2 mmHg) <300 for acute lung injury (<200 for acute respiratory distress syndrome) regardless of PEEP level, bilateral and diffuse opacities on frontal chest X-ray film, absence of left ventricular failure or history of lung disease [23]; 3) patients with ICP values >10 mmHg and <15 mmHg were included. An ICP level >10 mmHg (equal to 13.6 cmH2O) was chosen to prevent the application of 10 cmH2O of PEEP being transmitted through the cerebral veins according to the Starling resistor model; ICP values >15 mmHg were excluded to avoid any potential rise in PaCO2 from increasing ICP over the threshold for active treatment [24] (i.e., 20 mmHg). Exclusion criteria were: 1) hemodynamic instability; and 2) absence of temporal acoustic window for TCD examination. According to the protocol, the study was to be discontinued and PEEP returned to initial values if a sustained (>30 s) increase in ICP (>20 mmHg) occurred. This, however, was not the case in any patient during the study.
Clinical management
All patients were intubated, mechanically ventilated (900C; Siemens-Elema, Berlin, Germany) by volume control modality and nursed in supine position with an approximately 30° head tilt. At the time of the study, patients were sedated (0.05–0.15 mg·kg·h midazolam and 0.5 μg·kg·h fentanyl) and paralyzed (atracurium 0.5 mg/kg). A physician not involved in the study was always present to provide for patient care.
Tidal volume was targeted to maintain a PaCO2 equal to 30–35 mmHg according to the guidelines for brain injury management [24]. Before study entry, tidal volume, respiratory rate, and PEEP were 647±197 ml, 14±3 breaths per min, and 2±2 cmH2O, respectively.
Study protocol
Cerebral and systemic hemodynamics
Arterial and right atrial pressures were measured using standard pressure transducers, intracranial pressure was monitored continuously using an intraparenchimal probe with a tip transducer (Camino Laboratories, San Diego, Calif., USA); cerebral perfusion pressure was calculated as the difference between mean arterial pressure and mean ICP with the zero reference to the external auditory meatus [25]. Cerebral venous hemoglobin oxygen saturation (SjO2) was monitored on the dominant side of cerebral venous drainage by standard central venous catheter with the tip catheter positioned in the jugular bulb, above the lower border of cervical vertebra C1 [25] as confirmed by X-ray study; intermittent samples were obtained for blood gas analysis. The dominant side was determined by ICP response to neck compression [25]. Transcranial Doppler (TCD) insonation of the middle cerebral artery was performed with a 2 MHz range-gated pulsed Doppler ultrasound (EME TC2-64 B Uberlingen, Germany) on the same side of ICP monitoring; mean middle cerebral artery flow velocity was calculated as previously described [25]. The depth of insonation giving the highest mean flow velocity was chosen for recording; TCD measurements were averaged over at least 15 cardiac cycles during periods of hemodynamic stability.
Respiratory mechanics
Respiratory flow was measured with a heated pneumotacograph (Fleish No. 2; Fleish, Lausanne, Switzerland) connected to a differential pressure transducer (Special Instruments, Diff-Cap ±1 cmH2O; Nordlingen, Germany); volume was determined by electronic integration of the flow signal. Airway pressure was measured proximal to the endotracheal tube with a pressure transducer (Special Instruments Digima-Clic ±100 cmH2O). Equipment dead space (not including the endotracheal tube) was 70 ml. The increase in functional residual capacity with PEEP (ΔFRC) was measured as the difference between end-expiratory lung volume on PEEP and on zero end-expiratory pressure (ZEEP) [5]. The static inspiratory volume-pressure curves of the respiratory system on ZEEP and PEEP were obtained by performing single end-inspiratory occlusions at different inflating volumes, achieved by randomly changing the respiratory frequency of the ventilator [5]. Each occlusion was maintained until the airway pressure signal reached a plateau that corresponds to the static alveolar pressure [5]. The volume-pressure curves were hence constructed by plotting the different inflation volumes against the corresponding values of alveolar pressure. The volume of collapsed alveoli recruited with PEEP (recruited volume) was calculated from the volume-pressure curves on ZEEP and PEEP as the difference in lung volume between PEEP and ZEEP for the same degree of static alveolar pressure (20 cmH2O) [5]. According to previous studies [5], patients were defined a priori as recruiters if recruited volume with PEEP was ≥ 110 ml; otherwise they were considered non-recruiters. Stiffness of the respiratory system was evaluated by measuring the static elastance of the respiratory system (reciprocal of compliance) as: end-inspiratory occlusion airway pressure minus end-expiratory occlusion airway pressure divided by baseline inflation volume [5]. Ventilator settings were kept constant during the study period. PEEP at 0 cmH2O, 5 cmH2O, and 10 cmH2O was randomly applied and maintained for 30–35 min; respiratory mechanics and hemodynamics measurements were obtained during the last 5–10 min of each level of PEEP.
All physiological signals were collected on a personal computer through a 12-bit resolution analog-to-digital converter board (National Instrument DAQCard 700; Austin, Tex.,, USA) at a sample rate of 150 Hz (ICU Lab, KleisTEK Engineering; Bari, Italy).
Statistical analysis
Data are presented as mean±standard deviation (SD). Differences between experimental conditions were evaluated using a two-way analysis of variance for repeated measurements (ANOVA); if significant (P<0.05), values obtained at different levels of PEEP were compared with the ZEEP condition using the paired t-test with Bonferroni/Dunn correction. Differences between the two groups for each experimental condition were evaluated using the unpaired t-test. Regression analysis was performed with the least-square method (StatView, Abacus).
Results
Clinical data on study entry are shown in Table 1. All patients had severe acute lung and brain injury. The application of 5 cmH2O and 10 cmH2O of PEEP caused an upward volume displacement along the volume-pressure curve obtained during ZEEP in six patients indicating recruitment of previously collapsed alveoli (recruiters), while in the remaining six patients there was no recruitment because with PEEP the experimental points moved along the volume-pressure curve on ZEEP (non-recruiters). In recruiters, application of 5 cmH2O and 10 cmH2O of PEEP recruited 220±94 ml and 318±39 ml, decreased elastance of 10±4% and 20±15% (P<0.01), and increased PaO2 of 28±10% and 47±20% (P<0.005), respectively (Table 2). In non-recruiters, application of 5 cmH2O and 10 cmH2O of PEEP recruited 34±9 ml and 46±20 ml, increased elastance of 16±12% and 28±4% (P<0.0001), and PaCO2 of 9±6% and 16±6% (P<0.0001), respectively (Table 2).
Baseline ICP values were 12±2 mmHg and 13±2 mmHg (corresponding to 16.3±2.7 cmH2O and 17.7±2.7 cmH2O, Table 3) in the two groups, respectively, being higher than 10 cmH2O (i.e the highest level of applied PEEP). The effects of PEEP on hemodynamics in recruiters and non-recruiters are shown in Table 3. In both groups mean arterial pressure and cerebral perfusion pressure remained constant while right atrial pressure (RAP) significantly increased with PEEP from 4.7±1.7 mmHg to 7.3±1.9 mmHg in recruiters and from 4±1.6 mmHg to 6.8±1.6 mmHg in non-recruiters (P<0.0001). In non-recruiters ICP, mean Doppler flow velocity and SjO2 significantly (P<0.001) increased while remained stable in recruiters after application of PEEP (Table 3).
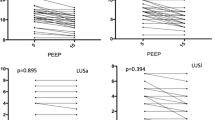
A significant correlation was found between changes in ICP and changes in elastance (r2 = 0.8 P <0.0001; Fig. 1) following the application of PEEP. Besides, significant correlations were found between changes in PaCO2 and ICP (r2 = 0.4 P <0.001) and static elastance of the respiratory system respectively (r2 = 0.4 P <0.001; Fig. 2).
Relationship between percentage changes in PaCO2 and static elastance of the respiratory system (P <0.001, r2 = 0.4, left part) and between percentage changes in PaCO2 and intracranial pressure (P <0.001, R2 = 0.4, right part) following the application of 5 cmH2O and 10 cmH2O of positive end-expiratory pressure. (closed circles recruiters, open circles non-recruiters.)
Discussion
The main finding of the present study was that, in brain-injured patients with “relatively normal” ICP applying low levels of PEEP, different cerebral hemodynamic consequences occurred according to the effects of PEEP on respiratory mechanics and gas exchange. When the application of PEEP induced alveolar hyperinflation and rise in PaCO2, ICP, Doppler flow velocity, and SjO2 significantly increased, whereas when PEEP caused alveolar recruitment there were no effects on ICP and cerebral perfusion.
Before discussing our results, a few methodological issues will be addressed. In the present study patients with spontaneous and traumatic severe brain injury were included because in 20–25% of both these populations acute lung injury occurs and is associated with high mortality and morbidity [1, 2, 3, 4]. Indeed, PaO2/FiO2 <300 represents a potential secondary insult that should be carefully avoided to optimize intensive care management of severe brain injury [6]. A narrow range of ICP values was chosen for both methodological and ethical reasons: patients with baseline ICP values higher than applied PEEP (equal to 10 cmH2O) were included to avoid the possibility that changes in ICP could be related to the hemodynamic transmission through cerebral veins according to the Starling resistor model [14, 15, 19]. ICP values higher than 15 mmHg were excluded to avoid that any potential rise in PaCO2 could increase ICP over the threshold for active treatment (i.e., 20 mmHg) [24].
Physiological consequences of the application of PEEP depend on its effects on pulmonary elastance, systemic hemodynamics, and gas exchange. These effects are strongly conditioned by whether or not PEEP recruits previously collapsed alveoli. If hyperinflation of normal alveoli with PEEP is the predominant effect, increase in pulmonary elastance will result in a rise in PaCO2 due to the increase in dead space [20, 26], while PaO2 will slightly change according to the variations in cardiac output [27]. If PEEP induces alveolar recruitment, the reduction in pulmonary elastance will lead to reduction in shunt with improvement in oxygenation [21] and reduction in dead space [20].
Cerebral circulation of patients with acute brain and lung injury is influenced by these complex cardiopulmonary interactions. The application of PEEP may indeed affect cerebral circulation by hemodynamic and CO2-mediated mechanisms. The hemodynamic mechanism may alter cerebral circulation both on the arterial side, reducing arterial pressure and on the venous side, reducing cerebral venous drainage. The gas exchange mechanism may alter cerebral circulation through a CO2-induced vasodilation responsible for an increase in cerebral blood volume.
Gas exchange mechanism
The increase in PaCO2 directly causes vasodilation of cerebral arteries increasing cerebral blood volume; this may cause a rise in ICP, if intracranial compliance is reduced [27]. Most of the previous studies in brain-injured patients did not report the effects of PEEP on gas exchange [7, 8, 10, 14, 15, 17, 18, 29]. In our study, in non-recruiters, overdistension of alveolar areas contributed to the increase in dead space and then in PaCO2 causing cerebral vasodilation (increase in ICP, Doppler flow velocity, and SjO2). In recruiters, reduction in shunt with improvement in oxygenation was the predominant effect, while decrease in PaCO2 due to reduction in dead space was less pronounced; consequently ICP and cerebral perfusion did not change significantly. When elastance, ICP, and PaCO2 were treated as continuous variables, a significant relationship was found.
Hemodynamic mechanism
According to the concept of the vasodilatory cascade, the decrease in arterial pressure caused by PEEP may diminish cerebral blood flow in patients whose cerebral autoregulation is impaired, while may cause a compensatory vasodilation if autoregulation is preserved [30]. In the latter case, vasodilation will lead to an increase in cerebral blood volume and ICP, given a reduced intracranial compliance [30]. However, most of the studies, including the present one, showed that the application of PEEP did not induce a significant reduction in arterial and cerebral perfusion pressure, probably because euvolemia was guaranteed [8]. Doppler flow velocity of the middle cerebral artery and SjO2 were monitored as independent measures of perfusion and its adequacy for metabolic requirements [25, 31]. In non-recruiters the significant increase in Doppler flow velocity and SjO2 paralleled the increase in ICP, confirming the CO2-mediated cerebral vasodilation [28, 32]. Application of PEEP may also affect cerebral circulation through an impairment in local venous return and an increase in RAP due to the passive trasmission of pleural pressure to right atrium [14, 15, 18, 19]. In our study, the application of PEEP significantly increased RAP in both groups, although RAP values were always well below ICP values. Thus, the increase in ICP seen only in the non-recruiters should not be due to a selective transmission of pleural pressure to cerebral veins but only to the increase in PaCO2.
The Starling resistor model describes flow dynamics in collapsible tubes [33]. In cerebral circulation [19] the upstream pressure is represented by arterial pressure and the downstream pressure by ICP which surrounds collapsible cerebral veins. The application of PEEP increases intrathoracic pressure leading to an increase in RAP, responsible for a rise in sagittal sinus pressure. The increase in sagittal sinus pressure decreases cerebral venous outflow and increases ICP. Luce et al. demonstrated in an animal model that consequences of the application of 20 cmH2O of PEEP on ICP were more evident if the initial ICP was lower than 20 cmH2O, but less when ICP was higher than applied PEEP [19]. McGuire et al. confirmed in patients that application of PEEP up to 15 cmH2O was not transmitted if baseline ICP values were higher than PEEP (baseline ICP was equal to 18.8 mmHg i.e., 24.5 cmH2O) [18]. In our study we tested the hypothesis that the increased PaCO2 in non-recruiters should be responsible for the rise in ICP independently of small changes in RAP. To test this hypothesis we applied PEEP up to 10 cmH2O only in patients with ICP values higher than 10 mmHg (i.e., 13.6 cmH2O). To further minimize the interference with the venous outflow due to the increase in RAP, our patients were treated with 30° head-up tilt [34, 35, 36]. Indeed, during head elevation, the following protective mechanisms may attenuate pressure transmission after application of PEEP: collapsible jugular veins, the vertebral venous plexus and eventually the presence of valves on the jugular veins. If we assume that during head elevation the entire increase in RAP consequent to the application of PEEP is transmitted through the jugular venous channel, its collapse at the thoracic inlet could be the main resistance to pressure transmission upward. The jugular veins will then act as a Starling resistor where the upstream pressure is sagittal sinus pressure and the downstream pressure is represented by RAP. Toung et al. showed that an increase in RAP equal to 20 mmHg is required to overcome the collapse in jugular veins occurring at the thoracic inlet and to induce an upward pressure transmission [29]. In our study PEEP induced an average increase in RAP of 3 mmHg remaining well below ICP values. therefore we may assume that the collapsible jugular veins offset the increase in RAP. However, during head elevation cerebral venous blood also drains through the vertebral venous system [37] because this venous drainage channel is not directly connected to superior vena cava and therefore it is not subjected to immediate intrathoracic pressure variations responsible for jugular veins collapse. The presence of valves on jugular veins at the thoracic inlet protects the brain from sudden rise in venous pressure, such as during coughing, but this mechanism should only be momentary because the outflow from the brain must continue [29]. Therefore it is unlikely that this mechanism can play a major role in attenuating pressure transmission after application of PEEP.
Feldman et al. showed that 10 cmH2O of PEEP reduced intracranial compliance but did not increase ICP [9], whereas no changes in PaCO2 were reported. In our study in non-recruiters ICP significantly increased, suggesting that the CO2-mediated vasodilation increased cerebral blood volume and exhausted the intracranial compensatory reserve.
We have proven that the occurrence of alveolar hyperinflation with PEEP leads to an increase in PaCO2 responsible for a rise in ICP in brain-injured patients with “relatively normal” ICP. This effect could be even more enhanced in patients with reduced intracranial compliance while the hemodynamic mechanism altering cerebral circulation on the venous side is somehow attenuated in patients with intracranial hypertension.
In conclusion our data show that, in patients with ICP values higher than applied PEEP, effects of PEEP on cerebral hemodynamics depend on recruitment/hyperinflation of alveolar units and PaCO2 variations may have major impact on brain perfusion.
References
Contant CF, Valadka AB, Gopinath SP, Hannay HJ, Robertson CS (2001) Adult respiratory distress syndrome: a complication of induced hypertension after severe head injury. J Neurosurg 95:560–568
Gruber A, Reinprecht AU, Illievich M, Fitzgerald R, Dietrich W, Czech T, Richling B (1999) Extracerebral organ dysfunction and neurologic outcome after aneurysmal subarachnoid hemorrhage. Crit Care Med 27:505–514
Bratton SL, Davis RL (1997) Acute lung injury in isolated traumatic brain injury. Crit Care Med 40:707–712
Holland MC, Mackersie RC, Morabito D, Campbell AR, Kivett VA, Patel R, Erickson VR, Pittet JF (2003) The development of acute lung injury is associated with worse neurologic outcome in patients with severe traumatic brain injury. J Trauma 55:106–111
Ranieri VM, Eissa NT, Corbeil C, Chasse M, Braidy J, Matar N, Milic-Emili J (1991) Effects of positive end-expiratory pressure on alveolar recruitment and gas exchange in patients with the adult respiratory distress syndrome. Am Rev Respir Dis 544–551
Miller JD, Sweet RC, Narayan R, Becker DP (1978) Early insults to the injured brain. JAMA 240:439–442
Cooper KR, Boswell PA, Choi SC (1985) Safe use of PEEP in patients with severe head injury. J Neurosurg 63:552–555
Doblar DD, Santiago TV, Kahn AU, Edelman NH (1981) The effect of positive end-expiratory pressure ventilation (PEEP) on cerebral blood flow and cerebrospinal fluid pressure in goats. Anesthesiology 55:244–250
Feldman Z, Robertson CS, Contant CF, Gopinath SP, Grossman RG (1997) Positive end-expiratory pressure reduces intracranial compliance in the rabbit. J Neurosurg Anesthesiol 9:175–179
Burchiel KJ, Steege TD, Wyler AR (1981) Intracranial pressure changes in brain-injured patients requiring positive end-expiratory pressure ventilation. Neurosurgery 8:443–449
Shapiro HM, Marshall LF (1978) Intracranial pressure responses to PEEP in head-injured patients. J Trauma 18:254–256
Frost EA (1977) Effects of positive end-expiratory pressure on intracranial pressure and compliance in brain-injured patients. J Neurosurg 47:195–200
Aidinis SJ, Lafferty J, Shapiro HM (1976) Intracranial responses to PEEP. Anesthesiology 45:275–286
Huseby JS, Luce JM, Cary JM, Pavlin EG, Butler J (1981) Effects of positive end-expiratory pressure on intracranial pressure in dogs with intracranial hypertension. J Neurosurg 55:704–705
Huseby JS, Pavlin EG, Butler J (1978) Effect of positive end-expiratory pressure on intracranial pressure in dogs. J Appl Physiol 44:25–27
Apuzzo M, Weiss M, Petersons V, Small B, Kurze T, Heiden J (1977) Effect of positive end-expiratory pressure on intracranial pressure in man. J Neurosurg 46:227–232
Georgiadis D, Schwarz S, Baumgartner RW, Veltkamp R, Schwab S (2001) Influence of positive end-expiratory pressure on intracranial pressure and cerebral perfusion pressure in patients with acute stroke. Stroke 32:2088–2092
McGuire G, Crossley D, Richards J, Wong D (1997) Effects of varying levels of positive end-expiratory pressure on intracranial pressure and cerebral perfusion pressure. Crit Care Med 25:1059–1062
Luce JM, Huseby JS, Kirk W, Butler J (1982) A Starling resistor regulates cerebral venous outflow in dogs. J Appl Physiol 53:1496–1503
Blanch L, Fernandez R, Benito S, Mancebo J, Net A (1987) Effect of PEEP on the arterial minus end-tidal carbon dioxide gradient. Chest 92:451–454
Mascia L, Grasso S, Puntillo F, Majorano M, Cafarelli A, Bruno F, Fiore T, Ancona G, Brienza A, Ranieri VM (2000) The effects of PEEP on cerebral hemodynamics in severe brain injured patients with acute lung injury. Int Care Med S123
Teasdale G, Jennett B (1974) Assessment of coma and impaired consciousness. A practical scale. Lancet 13:81–84
Bernard GR, Artigas A, Brigham KL, Carlet J, Falke K, Hudson L, Lamy M, Legall JR, Morris A, Spragg R (1994) The American-European Consensus Conference on ARDS. Definitions, mechanisms, relevant outcomes, and clinical trial coordination. Am J Respir Crit Care Med 149:818–824
Brain Trauma Foundation, American Association of Neurological Surgeons, Joint Section on Neurotrauma and Critical Care (1996) Guidelines for the management of severe head injury. J Neurotrauma 13:641–734
Chan KH, Miller JD, Dearden NM, Andrews PJ, Midgley S (1992) The effect of changes in cerebral perfusion pressure upon middle cerebral artery blood flow velocity and jugular bulb venous oxygen saturation after severe brain injury. J Neurosurg 77:55–61
Gattinoni L, Vagginelli F, Carlesso E, Taccone P, Conte V, Chiumello D, Valenza F, Caironi P, Pesenti A, for the Prone-Supine Study Group (2004) Decrease in PaCO2 with prone position is predictive of improved outcome in acute respiratory distress syndrome. Crit Care Med 31:2727–2733
Pinsky M, Desmet JM, Vincent JL (1991) Effects of PEEP on right ventricular function in humans. Am Rev Respir Dis 143:25–31
Markwalder TM, Grolimund P, Seiler RW, Roth F, Aaslid R (1984) Dependency of blood flow velocity in the middle cerebral artery on end tidal carbon dioxide partial pressure—a transcranial ultrasound Doppler study. J Cereb Blood Flow Metab 4:368–372
Toung TJ, Aizawa H, Traystman RJ (2000) Effects of positive end-expiratory pressure ventilation on cerebral venous pressure with head elevation in dogs. J Appl Physiol 88:655–661
Rosner MJ, Rosner SD, Johnson AH (1995) Cerebral perfusion pressure: management protocol and clinical results. J Neurosurg 83:949–962
Robertson CS, Gopinath S, Goodman C, Contant CF, Valadka A, Narayan RK (1995) SjvO2 monitoring in head injured patients. J Neurotrauma 12:891–896
Buunk G, van der Hoeven JG, Meinders AE (1997) Cerebrovascular reactivity in comatose patients resuscitated from a cardiac arrest. Stroke 28:1569–1573
Permutt S, Riley RL (1963) Hemodynamics of collapsible vessels with tone: the vascular waterfall. J Appl Physiol Sep 18:924–932
Lodrini S, Montolivo M, Pluchino F, Borroni V (1989) Positive end-expiratory pressure in supine and sitting positions: its effects on intrathoracic and intracranial pressures. Neurosurgery 24:873–877
Moraine JJ, Berre J, Melot C (2000) Is cerebral perfusion pressure a major determinant of cerebral blood flow during head elevation in comatose patients with severe intracranial lesions? J Neurosurg 92:606–614
Feldman Z, Kanter MJ, Robertson CS, Contant CF, Hayes C, Sheinberg MA, Villareal C, Narayan R, Grossman R (1992) Effect of head elevation on intracranial pressure, cerebral perfusion pressure, and cerebral blood flow in head-injured patients. J Neurosurg 76:207–211
Epstein HM, Linde HW, Crampton AR, Ciric IS, Eckenhoff JE (1970) The vertebral venous plexus as a major cerebral venous outflow tract. Anesthesiology 32:332–338
Author information
Authors and Affiliations
Corresponding author
Additional information
Supported by Ministero dell’Università e della Ricerca Scientifica e Tecnologica
Rights and permissions
About this article
Cite this article
Mascia, L., Grasso, S., Fiore, T. et al. Cerebro-pulmonary interactions during the application of low levels of positive end-expiratory pressure. Intensive Care Med 31, 373–379 (2005). https://doi.org/10.1007/s00134-004-2491-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-004-2491-2