Abstract
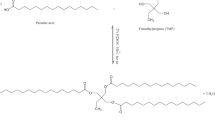
Palm fatty acid distillate (PFAD) is the by-product obtained during physical refining of crude palm oil, which mainly consists of free fatty acids along with minor amounts of glycerides, bioactive compounds such as tocopherols, tocotrienols, phytosterols, squalene, other hydrocarbons. In the present work, an eco-friendly alkyd resin was prepared using sustainable feedstock such as PFAD along with rosin. The various physico-chemical properties of PFAD-based alkyd resin (PFAD-AR) such as acid value, saponification value, viscosity and volatile matter were determined and compared to palm oil based alkyd resin (PO-AR). The structural properties of the alkyd resins were investigated using Fourier transform infrared (FTIR) and proton nuclear magnetic resonance (1H NMR) spectroscopic techniques. The study presents the utilization of PFAD-based alkyd resin with sodium lauryl sulfate (SLS) and sodium lauryl ether sulfate (SLES) in the liquid detergent formulation. The performance properties of the PFAD-based alkyd resin liquid detergent formulations such as surface tension, wetting power and detergency were comparable with palm oil based alkyd resin liquid detergent formulations and with commercial liquid detergent (CLD). Surface tensions of liquid detergent formulations varied from 20 to 30 mN/m with decrease in concentration. The foaming properties of alkyd resin based liquid detergents are reduced with the increase in the amount of alkyd resin polymer in the formulations. Therefore, it has potential application as a foam reducer in detergent for washing machines.










Similar content being viewed by others
Abbreviations
- AOS:
-
Alpha olefin sulfonate
- CLD:
-
Commercial liquid detergent
- FTIR:
-
Fourier transform infrared
- 1H NMR:
-
Proton nuclear magnetic resonance
- LABS:
-
Linear alkyl benzene sulfonate
- MTO:
-
Mineral turpentine oil
- PFAD:
-
Palm fatty acid distillate
- PFAD-AR:
-
PFAD-based alkyd resin
- PO-AR:
-
Palm oil based alkyd resin
- PVA:
-
Polyvinyl alcohol
- SFT:
-
Surface tension
- SLES:
-
Sodium lauryl ether sulfate
- SLS:
-
Sodium lauryl sulfate
References
Hou CT, Shaw J, Luxem FJBK. Biodiesel from acidulated soapstock (acid oil). Biocatalysis and bioenergy. New York: Wiley; 2008. p. 11.
Haslenda H, Jamaludin MZ. Industry to industry by-products exchange network towards zero waste in palm oil refining processes. Resour Conserv Recycl. 2011;55:713–8.
Piloto-Rodríguez R, Melo EA, Goyos-Pérezl L, Verhelst S. Conversion of by-products from the vegetable oil industry into biodiesel and its use in internal combustion engines: a review. Braz J Chem Eng. 2014;31:287–301.
Jadidi N, Adib B, Malihi FB. Synergism and performance optimization in liquid detergents containing binary mixtures of anionic–nonionic and anionic–cationic surfactants. J Surfact Deterg. 2013;16:115–21.
Rosen MJ. Surfactants and interfacial phenomena. New York: Wiley; 1989.
Bertleff W, Neumann P, Baur R, Kiessling D. Aspects of polymer use in detergents. J Surfact Deterg. 1998;1:419–24.
Turner GPA. Introduction to paint chemistry and principles of paint technology. London: Chapman & Hall; 1988. p. 168.
De Silva SHUI, Amarasinghe ADUS, Premachandra BAJK, Prashantha MAB. Effect of karawila (Momordica charantia) seed oil on synthesizing the alkyd resins based on soya bean (Glycine max) oil. Prog Org Coat. 2012;74:228–32.
Gogate BB, Agrawal RS. Starch-sorbitol based co-polymer (a substitute for LABS). J Soaps Deterg Toilet Rev. 2003;34:25–8.
Gogate BB, Agrawal RS. Sorbitol based polymeric surfactant for detergent powder. J Soaps Deter Toilet Rev. 2003;34:19–22.
Parasuram KS. Soaps and detergent. New Delhi: Tata McGraw-Hill; 1995.
Kharkate SK, Karadbhajne VK, Gogate BB. Some resin based ecofriendly liquid detergents. J Sci Ind Res. 2005;64:752–5.
Toliwal SD, Patel D, Patel JV, Jadhav K. Utilization of byproducts of oil processing industry in liquid detergent. Indian J Chem Technol. 2009;16:373–6.
Toliwal SD, Patel D, Patel JV, Jadhav K. Liquid detergent from acid oil based polymer. J Appl Chem Res. 2010;14:14–22.
Gapoor A, Hassan WHW, Sulong M. Phytochemical for nutraceutical from the by product of palm oil refining. Palm Oil Develop. 2002;36:13–9.
Nang HLL, Wafti NSA, May CY. Palm fatty acid distillate. MPOB Information Series. 2009; MPOB No. 471.
Cheah KY, Toh TS, Koh PM. Palm fatty acid distillate biodiesel: next generation palm biodiesel. Inform. Champaign: AOCS; 2010.
Deshmane VG, Gogate PR, Pandit AB. Ultrasound-assisted synthesis of biodiesel from palm fatty acid distillate. Ind Eng Chem Res. 2009;48:7923–7.
Chabukswar DD, Kaur HP, Gaikar VG. Esterification of palm fatty acid distillate using heterogeneous sulfonated microcrystalline cellulose catalyst and its comparison with H2SO4 catalyzed reaction. Ind Eng Chem Res. 2013;52:7316–26.
Paul S. Surface coating. New York: Wiley; 1985. p. 70–134.
Harris JC. Detergency evaluation and testing. New York: Wiley; 1954.
Rosen MJ. Surfactants and interfacial phenomena. New York: Wiley; 2004. p. 277–302.
Ross J, Miles GD. An apparatus for comparison of foaming properties of soaps and detergents. J Am Oil Chem Soc. 1941;18:99–102.
Seyferth H, Morgan OM. The canvas disc wetting test. Am Dyestuff Rep. 1938;27:525–9.
ASTM. Annual book of ASTM standard. Philadelphia: American Society for Testing Materials; 1973.
Aigbodion AI, Okieimen FE. Kinetics of the preparation of rubber seed oil alkyds. Eur Polym J. 1996;32:1105–8.
Pavia DL, Lampman GM, Kriz GS, Vyvyan JR. Introduction to spectroscopy. Belmont: Brooks/Cole, Cengage Learning; 2008.
Cervantes-Martinez A, Maldonado A. Foaming behaviour of polymer–surfactant solutions. J Phys Condens Matter. 2007;19:246101.
Moulay S, Zenimi A, Dib MJ. Rosin/acid oil-based liquid soap. J Surfact Deterg. 2005;8:169–74.
Kissa E. Wetting and detergency. Pure Appl Chem. 2009;53:2255–68.
Acknowledgements
The authors gratefully acknowledge the Institute of Chemical Technology and financial support under the University Grants Commission (UGC), New Delhi, Government of India.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Chiplunkar, P.P., Shinde, V.V. & Pratap, A.P. Synthesis and Application of Palm Fatty Acid Distillate Based Alkyd Resin in Liquid Detergent. J Surfact Deterg 20, 137–149 (2017). https://doi.org/10.1007/s11743-016-1905-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11743-016-1905-9