Abstract
The phase equilibria of the ternary system Bi-Mn-Sb were experimentally investigated with focus on the possible existence of a ferromagnetic continuous solid solution between α-BiMn and MnSb at 320 °C (both B81, NiAs structure type). Isothermal sections at 320 and 600 °C were studied applying powder x-ray diffraction and scanning electron microscopy including energy dispersive x-ray spectroscopy. The corresponding isothermal sections at 320 and 600 °C were established based on these results. A complete ternary reaction scheme (Scheil diagram) and a liquidus surface projection based on primary crystallization analyses and DTA (differential thermal analysis) were constructed. Furthermore, the vertical section at 10 at.% Sb was studied by DTA. A continuous solid solution between the isostructural phases α-BiMn and MnSb, as reported in the literature, could not be confirmed. A new hexagonal P63/mmc setting of the known orthorhombic P2221 ternary phase BixMnSb1−x, based on single-crystal x-ray diffraction data, is described.
Similar content being viewed by others
1 Introduction
An increase in the demand of rare earth elements (REEs), particularly for the production of efficient permanent magnets, will be a major factor for both cost and availability of these elements in the near future. Thus, extensive research has been devoted to design ferromagnetic materials free of REEs for more than two decades. An extensive review on potential compounds was given by Kramer et al.[1] Possible candidates for such REE-free ferromagnetic materials are among others, several Mn-based compounds, one of them being the compound BiMn, in particular the low temperature modification α-BiMn.
According to Massalski et al.,[2] α-BiMn crystallizes in the hexagonal NiAs (B81) type structure (space group P63/mmc) and is stable up to 353 °C where it decomposes in a peritectic reaction into a Bi-rich liquid and the high-temperature modification β-BiMn (which itself decomposes peritectically at 446 °C). Unfortunately, it has not been possible to synthesize α-BiMn as a bulk material without impurities, despite several decades of intensive research (see e.g. Ref 3,4,5,6,7,8). A possible approach to circumvent these problems was considered the addition of a third component such as Ni, Pt or Rh, which forms an intermetallic phase with Bi that is iso-typic with α-BiMn.[9,10]
Another promising possibility was the partial substitution of Bi by Sb, which forms two ferromagnetic phases with Mn, i.e. Mn2Sb and MnSb. Of these, hexagonal MnSb is isotypic with α-BiMn; in addition, Bi and Sb are completely miscible with each other.
In the present study it was attempted to clarify the phase equilibria in the ternary Bi-Mn-Sb system, in particular to check for a possible stabilization of the ferromagnetic α-BiMn phase by the addition of Sb. This possibility had been indicated by an early report by Dannöhl and Gmöhling[11] who found a continuous solid solution between α-BiMn and MnSb at 250 °C.
2 Literature Overview
2.1 The Binary Systems
Experimental phase diagram information on the binary boundary systems was taken from the literature. For the Mn-Sb system, the results by Kainzbauer et al.[12] were used who established a thoroughly revised version of the Mn-Sb phase diagram.
Information on the Bi-Sb system, which exhibits a continuous solid solution, was taken from the phase diagram compilation of Okamoto.[13] The miscibility gap below 170 °C, concluded by Ohtani and Ishida[14] based on thermodynamic calculations, was not considered in the present investigation.
The exact phase equilibria in the Bi-Mn system are still disputed, especially at temperatures above the peritectic reaction L + δ-Mn = γ-Mn at 1138 °C. For the present study the Bi-Mn phase diagram, up to the temperature of the peritectic reaction L + β-Mn = α-Mn at 727 °C, was accepted as shown by Massalski et al.[2] based on experimental data. Due to a lack of available experimental data above 727 °C, the phase diagram assessment by Okamoto[15] is accepted. This assessment is based on a thermodynamic analysis by Oikawa et al.[16] and does not include a liquid miscibility gap originally shown by Massalski et al.[2] and Wang et al.[17] The existence of this liquid miscibility gap originates from an early study by Siebe[18] who observed two separate liquid layers between 23 and 99.5 wt.% Mn. However, it has to be mentioned that Siebe used for the synthesis of his big 20 g samples Mn metal which was contaminated with 3 wt.% Al, Si and Fe. The Bi-Mn phase diagram as used in the present study is shown in Fig. 1.
2.2 The Ternary System
Only little information has been available on the ternary Bi-Mn-Sb system. In an early study, Dannöhl and Gmöhling[11] reported a continuous ferromagnetic solid solution between the two isotypic B8 phases MnSb and α-BiMn at 250 °C and showed a liquidus projection of the system. This continuous solid solution was in stark contrast to a later study by Ahlborn et al.[19] who investigated magnetic properties in the section MnSb-BiMn but could not confirm any continuous solid solubility. A study of the MnSb liquidus close to the monovariant line in the MnSb-Sb-Bi system, together with the determination of the corresponding tie lines at different compositions along this monovariant line, was published by Durand-Charre et al.[20]
Several authors, among them Göble et al.[21] as well as Andersen et al.,[22] reported the existence of a ternary compound BixMnSb1−x with a composition close to the Bi-Mn binary side (see below at section 2.3.). In a thermodynamic analysis of the entire ternary system by Uemitsu et al.,[23] this phase was not included although its existence was mentioned in the text.
Beside this limited phase diagram information, only studies of Bi-Mn-Sb thin films were reported in the literature by Kang et al.[24] as well as Wang et al.[25]
2.3 The Ternary Phase BixMnSb1−x
Andresen et al.[22] reported that the binary high-temperature phase β-BiMn can be produced as a stable phase at low temperature by the addition of small amounts of Sb. They described the crystal structure of this phase with the composition Bi0.9MnSb0.1as orthorhombic with the space-group symmetry P2221 which was confirmed later on by Göble et al.[21]
In contrast, Ahlborn et al.[19] used the hexagonal setting derived from the NiAs-type of α-BiMn in their study of this ternary compound. Generally, the orthorhombic P2221 structure can be derived from the hexagonal NiAs-type structure by aorth = ahex; borth = √3ahex; corth = chex.
In all studies regarding this phase, independently of sample preparation, impurities of unreacted Bi, Sb, Mn, as well as of MnO and Mn2Sb are mentioned. Although these impurities, especially Mn2Sb influence the magnetic measurements the investigations showed that the ternary phase BixMnSb1−x is not ferromagnetic or has a smaller permanent magnetic moment compared to BiMn and MnSb.
A list of crystallographic data of binary and ternary compounds relevant for the current work is given in Table 1 together with relevant literature.
3 Experimental
3.1 Sample Preparation
Starting materials for all samples were pieces of Mn (99.95%, Alfa Aesar, Johnson Matthey Chemicals, Karlsruhe, Germany), Bi (99.999%, ASARCO, New Jersy, USA) and Sb (99.999% Alfa Aesar, Johnson Matthey Chemicals, Karlsruhe, Germany).
All sample handling was done, in a glove box (Labmaster SP MBraun, moisture and oxygen level below 0.1 ppm) under an Ar atmosphere to prevent oxidation of Mn.
For the 600 °C isothermal section, the metals were manually pulverized and sieved (grain size < 0.09 mm). Calculated amounts of the powders were mixed and pressed into pellets in a 5 mm pressing cylinder under a load of 20-25 kN. The pellets were placed into alumina crucibles which were sealed into evacuated silica glass tubes (~ 10−3 mbar) and heated up to 1100 °C for 5 min, then cooled down within 30 min to 600 °C and annealed at this temperature for at least 2 weeks.
For the 320 °C isothermal section, the element pieces were sealed into evacuated silica glass tubes (~ 10−3 mbar) and melted over an oxyhydrogen flame under shaking with optical control of the melting process. The annealing time was at least 3 weeks. All heating and annealing processes were carried out in muffle furnaces (Nabertherm, Germany) with a temperature accuracy of approximately ± 10 °C depending on the sample location.
The samples containing 80 at.% Mn were produced as powder pellets and placed in closed tantalum crucibles. To prevent the formation of the intermetallic phase Mn2Ta a tungsten inlay was used. The materials were rapidly heated up to 1200 °C and held for 5 min at this temperature in an induction furnace using an IR—laser pyrometer for the temperature control.
All the samples, except those for determination of the crystallisation, were annealed at 600 or 320 °C, quenched in cold water and investigated by standard analytical methods as described below. Samples used to determine the primary crystallisation were quenched from the liquid and analyzed in their as-cast state. Details of sample preparation and annealing parameters are listed in Table 2.
3.2 Characterization
Metallographic investigations were performed on polished samples utilizing a scanning electron microscope (SEM) (Zeiss Supra 55 VP) with energy-dispersive x-ray spectroscopy (EDX). Backscattered electrons were used to visualize the surface at 20 kV acceleration voltage. The EDX detector was calibrated by the pure elements Bi, Mn and Sb, for the energy calibration Co was employed. To minimize statistical errors, the average phase composition was obtained from at least eight spot/area scans.
Differential thermal analyses (DTA) were performed on a DSC 404F1 Pegasus (Netzsch, Selb, Germany), applying evacuated silica glass crucibles (~ 10−3 mbar) as sample containers. In order to prevent a reaction between sample and silica glass, the crucibles were protected by a carbon layer on the inside. The temperature program included usually two heating/cooling cycles with a heating/cooling rate of 5 K/min starting from room temperature up to about 30° above the estimated liquidus temperature. Temperature measurements were performed with type S (Pt/PtRh) thermocouples calibrated at the melting points of Ag, Au, Sb and Sn. Zr was used as reference material.
Invariant effects were evaluated from the peak onset, both on heating and cooling. Liquidus effects were evaluated from the peak maximum on heating and from the peak onset on cooling. Generally, the effects evaluated in the first heating run were deemed most reliable.
The phase identification was performed at ambient conditions by powder-XRD on a Bruker D8 Advance Diffractometer in Bragg–Brentano pseudo-focusing geometry (reflection setting), using Cu-Kα radiation and a LynxEye® one-dimensional silicon strip detector (exposure time: 1 h). For evaluation and Rietveld refinement of all diffraction patterns the TOPAS® 4.2 software[26] was used.
For the determination of the crystal structure of the phase BixMnSb1−x a single crystal was mechanically separated from a sample containing the new phase with a composition of Bi41.3Mn50.7Sb8 together with remaining Bi. The composition was determined by SEM and the structure confirmed by powder-XRD. A NoniusKappa CCD four-circle diffractometer was used for the single crystal measurement employing graphite monochromatized MoKα radiation; it was equipped with a 300 µm capillary-optics collimator, using a rotation angle of 2° per image, a detector-to-sample distance of 30 mm and a CCD detector (frame size 621×576 pixels). For data collection, the Collect software[27,28] was used. The obtained unit cell (least-squares refinements of 2θ values) and correctional parameters (Lorentz, polarization and absorption effects determined by multi-scan method) were used in the subsequent structure determination by the SHELXL-2014 software.[29,30]
4 Results and Discussion
A total of 48 ternary Bi-Mn-Sb samples were prepared and investigated for the construction of the isothermal sections and 17 samples for the liquidus surface projection.
The combined results of XRD and SEM measurements are listed together with experimental information in Table 2 and were used to construct the isothermal sections at 320 and 600 °C; they are illustrated in Fig. 2 and 3. EDX area scans were used to determine the composition of the matrix solidified from the liquid during quenching, especially in the 600 °C section, which has been denoted by “solidified liquid = L” in Table 2. DTA results are collected in Table 3. Boundaries of phase fields confirmed by at least one sample are drawn using thin solid lines.
4.1 Isothermal Section at 320 °C
The 320 °C isothermal section, illustrated in Fig. 2, shows the three binary phases α-BiMn, Mn2Sb and MnSb together with the ternary phase ~ MnBi0.9Sb0.1, designated here by τ, but there is no indication whatsoever of a continuous solid solution between α-BiMn and MnSb as it had been found at 250 °C by Dannöhl and Gmöhling.[11] Despite the difference of 70°, it must be concluded that such a continuous solid solution does not exist at any temperature. This is the more probable as the experimental conditions, i.e. annealing at 250 °C for 750 h, would not be sufficient to achieve equilibrium conditions. Consequently, the optimized phase diagram by Uemitsu et al.,[23] which is mostly based on the experimental work by Dannöhl and Gmöhling,[11] cannot be correct.
The ternary τ phase, first described by Andresen et al.,[22] is located close to the α-BiMn phase field. Powder XRD as well as SEM/EDX measurements confirmed that it is a true ternary compound and not an extended solubility of Sb in α-BiMn. According to the phase equilibria shown in Fig. 2 the solubility of Sb in α-BiMn is less than 1 at.% at 320 °C. Single crystal measurements showed that the ternary phase has a hexagonal structure (see chapter 4.4.). The narrow single-phase field of τ ranges from about 4 to 10.3 at.% Sb and from about 49.2 to 53.4 at.% Mn.
The isothermal section shows six three-phase fields of which five were confirmed by at least one sample. The three-phase field (L + α-BiMn + τ) was also confirmed by one sample but EDX results showed that its composition is very close to the phase boundary; therefore, it is shown by dashed lines. Both binary compounds, Mn2Sb and MnSb, show a rather low solid solubility of less than 4 at.% Bi. In samples containing MnSb in equilibrium with solid Bi1−xSbx, no ternary solid solubility was detected at all.
4.2 Isothermal Section at 600 °C
The isothermal section at 600 °C contains no additional solid phase besides the binary phases Mn2Sb and MnSb and the solid solution Bi1−xSbx. Two three-phase fields could be experimentally confirmed.
Concluding from the two binary systems, a continuous liquid phase field is suggested at this temperature, starting between ~ 72 and 100 at.% Bi on the Bi-Mn side, and ending between 13 and 21 at.% Mn on the Mn-Sb side. However, all SEM/EDX measurements of samples containing MnSb and L show a Mn content of less than 2 at.% for the quenched liquid phase as determined from area scans (Table 2). This is, especially on the Sb rich side, significantly lower than expected from the binary systems. It must be assumed that, already during the quenching process, Mn and Sb crystallize as MnSb in addition to the MnSb crystallites growing during the annealing process. The crystallization during quenching leads to a depletion of Mn in the liquid. The same applies to MnSb present in samples located in the single phase field L. An example is shown in Fig. 4(a) (sample composition Bi9.5Mn5Sb85.5) where the MnSb crystallites are directly surrounded by brighter areas containing less Sb. A further indication of MnSb phase formation during quenching is the small size of those crystallites.
SEM-BSE images of selected samples (black structures are either holes or cracks caused by sample preparation); (a) Bi9.5Mn5Sb85.5 (annealed at 600 °C), showing L and MnSb which crystallized during quenching, light gray/white areas around MnSb crystallites are depleted in Sb compared to the average concentration in L; (b) detail image of sample Bi86Mn8Sb6 (600 °C), showing Bi, MnSb and Mn2Sb; (c) sample Bi89Mn3Sb8 (600 °C); (d) sample Bi2Mn25Sb73 (600 °C), showing a globular eutectic structure composed of Sb and MnSb; (e) as-cast sample Bi83.7Mn10Sb6.3, showing Bi, MnSb and Mn2Sb; (f) sample Bi41Mn50Sb9 (annealed at 450 °C), showing Bi and Bi0.8MnSb0.2
The shape of the liquid phase boundary in the Bi-rich corner is deduced from the two samples Bi86Mn8Sb6 and Bi89Mn3Sb8. Corresponding SEM backscattered images are shown in Fig. 4(b) and (c). A detailed micrograph of sample Bi86Mn8Sb6 shows that primary crystals of MnSb are surrounded by Mn2Sb. This indicates an equilibrium of the three phases MnSb, Mn2Sb and L, whereby the sample must be located outside of the liquid boundary. On the other hand, sample Bi89Mn3Sb8 shows long thin needles of MnSb in an L(Bi) matrix (with L(Bi) designating Bi that had crystallized out of the liquid on quenching), which precipitated fast during the quenching process. This indicates that the sample is located in the single phase field L close to the phase boundary. The composition of the liquid was determined through EDX area scans of the solidified matrix in quenched samples. However, the concentration values of Bi and Sb show some scattering. All compositions listed in Table 2 are average values. Due to the discrepancies in the Mn content of the quenched liquid, the phase boundary itself is shown as a dashed line.
In the Sb-rich corner of the ternary phase diagram, a small section consists of L and solid (Sb). The boundaries of this two-phase field are defined on the one hand by the binary diagrams and on the other hand by a sample with the composition Bi4Mn7Sb89. This latter sample shows the three phases MnSb (formed during the quenching process), (Sb) and L; on the other hand, the sample exhibits a melting point of 589 °C, which is 11° lower than the annealing temperature. Thus, it can be assumed that it is situated in the L single-phase field close to the L + (Sb) two-phase field. The sample Bi4Mn7Sb89 shows, similar to sample Bi2Mn25Sb73 (Fig. 4d), a globular eutectic structure composed of L(Sb) and MnSb.
In contrast to the isothermal section at 320 °C the binary MnSb phase field is shifted to higher Mn contents and dissolves more, i.e. up to 6 at.% Bi. Figure 3 represents the best compromise of all available experimental results.
4.3 Liquidus Projection, Fields of Primary Crystallization and Ternary Reactions
All observed liquidus values from DTA measurements and results of a study of primary crystallization, based on 17 as-cast samples, were combined with literature data for the binary subsystems to construct a liquidus surface projection of the entire Bi-Mn-Sb system. It is shown in Fig. 5, and the corresponding experimental values are listed in Table 4. Bold solid lines correspond to monovariant lines which are based on experimental results, whereas dashed lines indicate estimated monovariant lines; in both cases arrows point to lower temperatures. The isothermal lines are drawn as thin dotted lines.
Liquidus surface projection of the ternary Bi-Mn-Sb system including primary crystallization fields. Gray open circles are values by Durand-Charre et al.[20] The rhombus represents the sample Bi83.7Mn10Sb6.3 in which the primary crystallization cannot be identified by the micrographic texture (see Fig. 4e). Solid lines: monovariant lines based on experimental results; dashed lines estimated monovariant lines; dotted lines: isotherms; filled circles: estimated compositions of liquid at invariant reactions. The enlarged schematic segment shows the Bi rich corner with the very narrow primary crystallization fields of τ, α-, β-BiMn, MnSb, and Mn2Sb
Because of using carbonized crucibles, the temperatures of thermal effects evaluated from DTA measurements are subject to an uncertainty, estimated to be up to 10 °C.
Compared with the data by Dannöhl and Gmöhling[11] and Uemitsu et al.[23] the liquidus projection in Fig. 5, including the fields of primary crystallization, looks quite different. On the other hand, the monovariant line originating from the binary eutectic MnSb + Sb is in perfect agreement with the results by Durand-Charre et al.[20] (shown in Fig. 5 by small open circles) and by Dannöhl and Gmöhling.[11]
The liquidus surface is dominated by the two large primary crystallization fields of MnSb and Mn2Sb and the four fields of the different Mn modifications. Because it is not possible to quench the γ- and δ-Mn modifications, their primary crystallization fields are estimated from the binary phase diagrams and drawn with dashed lines. Both the fields of primary crystallization of MnSb and Mn2Sb reach far to the Bi corner, i.e. to around 95 at.% Bi or slightly more.
From the micrographic texture of sample Bi83.7Mn10Sb6.3 (Fig. 4e) it is ambiguous if MnSb or Mn2Sb is the primary crystallization phase. Therefore, the sample is shown, as the only one, directly on the border between the two crystallization fields as a rhombus.
As can be seen from the liquidus projection in Fig. 5, a series of invariant reactions occurs in the a very narrow composition range (estimated between about 94 and 97 at.% Bi), as well as in a very narrow temperature range. Although it is virtually impossible to deduce the correct sequence of reactions from experimental results, it is attempted to provide the most logical Scheil diagram in Fig. 6. A corresponding schematic magnification of the liquidus projection in the Bi-rich corner is presented as an inset in Fig. 5. It shows very narrow fields of primary crystallization for the ternary phase τ as well as for α-BiMn and β-BiMn where the ternary phase τ itself is formed in a ternary peritectic reaction.
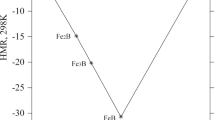
Figure 7 provides an isopleth for a constant Sb concentration of 10 at.%. Since the phase fields in the Bi-rich corner cannot be derived unequivocally from the experimental results (DTA and XRD) this part has been left out in the diagram.
Vertical sections at 10 at.% Sb; triangle up: liquidus on heating; triangle down: liquidus on cooling; open circles: invariant thermal effect; filled circles: other thermal effect; open dotted circles: thermal effects at cooling; gray crosses: thermal effect of Bi. The phase fields in the Bi-rich corner (up to 20 at.% Mn) cannot be derived unequivocally from the experimental results (DTA and XRD); this part has been left out
4.4 The Ternary Phase BixMnSb1−x
The DTA measurements of several bulk phases, containing the τ phase, indicated that it is stable up to the ternary peritectic reaction at about 500 °C (see Tables 3 and 5).
In contrast to Andresen et al.[22] or Göbel et al.[21] who found an orthorhombic structure, space group P2221, a hexagonal structure (space group P63/mmc) was found in the current study for this ternary phase.
Bi0.8MnSb0.2 crystallizes with its own structure type that can be derived from the NiAs structure. The asymmetric unit of the structure contains 5 atomic positions which are listed together with their Wyckoff sites and site symmetries in Table 6. However, one of these positions (Sb, 6h) is essentially vacant as the refined occupation factor is only 1.5%. This position which is only poorly occupied, seems to be a partial delocalization of Sb from the Bi position. Compared with the basic NiAs-type—realized e.g. in MnSb and BiMn—the cell parameters a, b = 8.5714(4) Å are approximately doubled whereas the parameter c = 5.7113(3) Å is slightly shorter.
In Bi0.8MnSb0.2 the ratio of Mn-atoms to main group element atoms is 1:1, similar to the NiAs-type. However, the distribution of atoms in the unit cell differs significantly from the NiAs- and the related filled Ni2In-type structure, as discussed below.
The NiAs and Ni2In structures can be described based on the different stacking sequences of the 36 triangular nets (NiAs: Ni A0 As B1/4 Ni A1/2 As C3/4 ; Ni2In: Ni A0 Ni C1/4 In B1/4 Ni A1/2 Ni B3/4 In C3/4 ) where the subscript representing the z value and superscript the different point positions relative to the cell origin. In the case of Bi0.8MnSb0.2, the 36 net for the main group elements at z = 1/4 and z = 3/4 is formed by the mixed position M2 (2c) together with Bi1 (6h). The respective 36 net for the transition metal at z = 0 and z = 1/2 is incomplete, as it contains only the fully occupied Mn1 position, but the corresponding 2a position is not occupied resulting in a vacancy on every 4th position of the 36 net. The Mn2 (2d) position, on the other hand, is part of an incomplete 36 net at z = 1/4 and z = 3/4 with only one quarter of the net positions being occupied.
Thus, the crystal structure of Bi0.8MnSb0.2 is related to NiAs as well as to Ni2In. The main group elements Bi and Sb occupy exactly the same net positions at z = 1/4 and z = 3/4 as in NiAs (As B1/4 and As C3/4 ) and in Ni2In (In B1/4 and In C3/4 ). The Mn1 atoms occupy three quarter of the Ni A0 and Ni A1/2 net (present in NiAs as well as in Ni2In) and the Mn2 corresponds to one quarter of the additional Ni-net in Ni2In (Ni C1/4 and Ni B3/4 ).
As mentioned previously the crystal structure of the phase was described in the space group P2221 by Andresen et al.[22] and Göbel et al.[21] (Fig. 8a and b). A transformation of the orthorhombic cell according to [2 0 0/-1 1 0/0 0 1] from a = 4.304(5) Å, b = 7.449(4) Å, c = 5.742(4) Å, V = 181.4 Å3[22] results in a cell metric of a = 8.608(10) Å, b = 8.603(4) Å, c = 5.742(4) Å, α = β = 90°, γ = 120.02(9)°, V = 368.2 Å3. This is close to the hexagonal cell a = 8.5714(4), c = 5.7113(3) Å, V = 363.39(3) Å3 of the present investigation determined by single crystal XRD. Not considering any partial occupation of distinct atom positions, the crystal structure of Bi0.8MnSb0.2 reported by Göbel et al.,[21] is structurally related to.[22] A vacant position in the crystal structure described by Andresen et al.[22] is occupied by Mn atoms. A transformation of the orthorhombic cell parameters a = 4.27, b = 7.40, c = 5.70 Å by [2 0 0/− 1 1 0/0 0 1] results in a pseudo-hexagonal cell metric of a = 8.54, b = 8.54, c = 5.70 Å, α = β = 90°, γ = 119.88°.
Clinographic view of the BixMnSb1−x crystal structures. (a) Bi0.8MnSb0.2 after Göbel et al.,[21] colour coding: green Bi1/Sb1, dark green Bi2/Sb2, violet Mn1, light blue Mn2, red Mn3; (b) Bi0.9MnSb0.1 after Andresen et al.,[22] colour coding see (a); (c) Bi0.8MnSb0.2 (τ) this work, view down the c axes, colour coding: green Bi1, orange M2; violet Mn1, light blue Mn2; grey Sb (~ 2% interstitial) (Color figure online)
Topological relations between Bi0.9MnSb0.1[22] respectively Bi0.8MnSb0.2[21] and the compound presented here are not evident. A comparison of the three different crystal structures is shown in Figs. 8(a) to (c).
According to the current study, a violation of the hexagonal cell symmetry of the ternary phase BixMnSb1−x is not likely as the anisotropies of the displacement parameters of all atoms are not conspicuous. An attempt to release the restriction of the cell parameters a and b results in insignificant differences only. Merging of symmetry equivalent reflections gives no hint for the requirements of a lowering of the Laue symmetry. One could argue that the ratio of the occupation factors for the mixed occupied position M2 (Bi2:Sb2 ~ 1:2) might indicate a reduction of the P63/mmc symmetry. However, these atoms occupy the position 2c with site symmetry −6m2; order might be achieved only by an enlarged unit cell. A careful investigation of the reciprocal lattice obtained from the single-crystal experiments gave no evidence for any super structure reflections.
The composition measurements by SEM (BSE image in Fig. 4f) resulting in Bi41.3Mn50.7Sb8, are in good agreement with the calculated value Bi41.7Mn49.7Sb8.6 (Bi0.8MnSb0.2) from single crystal model. Sb and Bi occupy the Wyckoff position 2c jointly.
Structural parameters, coordinates and displacement parameters as well as interatomic distances are given in Tables 6, 7, and 8. The refined atomic coordinates and structural parameters obtained by powder XRD are given in Table 9; Fig. 9 displays a 2θ segment of the powder XRD pattern between 19° and 69°. The crystal structure itself is shown in Fig. 8(c).
Powder XRD patterns of the sample Bi41Mn50Sb9, (annealed at 450 °C), measured at ambient conditions. The pattern contains Bi0.8MnSb0.2 (τ) and Bi. The experimental pattern (above), the calculated pattern of Bi0.8MnSb0.2 (middle) and the difference curve (bottom) are shown. Miller indices are given for the first 11 Bi0.8MnSb0.2 reflexes. The refinement is based on the results of the single crystal structure analyses
Further structural information was deposited with Fachinformationszentrum KarlsruheFootnote 1 and can be obtained on quoting the depository number CCDC 1881822.
5 Conclusion
In an attempt to stabilize the ferromagnetic phase α-BiMn by the addition of a third element, the ternary Bi-Mn-Sb phase diagram was investigated experimentally. Earlier literature reports that the two NiAs-type phases BiMn and MnSb form a continuous solid solution could not be confirmed in the present study.
Based on the results of thermal analyses (DTA), powder XRD, and SEM–EDX a large part of the ternary phase diagram could be elucidated. This is shown in form of two isothermal sections at 320 °C, i.e. at a temperature below the peritectic decomposition of α-BiMn (Fig. 2), and 600 °C (Fig. 3) as well as one isopleth at a constant Sb content of 10 at.% (Fig. 7). A liquidus surface projection was constructed (Fig. 5) based on DTA results supported by the study of quenched samples to define the fields of primary crystallization. The liquidus projection indicates that several invariant reactions occur in the Bi-rich corner in a very narrow temperature and composition range between about 94 and 97 at.% Bi.
Instead of a continuous solid solution between α-BiMn and MnSb, a ternary intermetallic compound τ with the approximate composition Bi0.8MnSb0.2 was established. A ternary compound with this composition had already been known from literature, but its exact crystal structure was disputed. This ternary phase is formed in a peritectic reaction at about 500 °C; single crystal XRD allowed to provide detailed information on its crystal structure (Fig. 8).
Notes
FIZ Karlsruhe, 76344 Eggenstein-Leopoldshafen, Germany, (Fax: (49) 7247-808-666; e-mail: crysdata@fiz.karlsruhe.de).
References
M.J. Kramer, R.W. McCallum, I.A. Anderson, and S. Constantinides, Prospects for Non Rare Earth Permanent Magnets for Traction Motors and Generators, JOM, 2012, 64(7), p 752-763
T.B. Massalski, H. Okamoto, P.R. Subramanian, and L. Kacprzak, Ed., Binary Alloy Phase Diagrams, 2nd ed., ASM International, Materials Park, OH, 1996, p 759-761
M.C.J. Marker, P. Terzieff, P. Kainzbauer, M. Bobnard, K.W. Richter, and H. Ipser, BiMn: Synthesis Separation by Centrifugation, and Characterization, J. Alloys Compd., 2018, 741, p 682-688
Y. Liu, J. Zhang, G. Jia, Y. Zhang, Zh. Ren, X. Li, Ch. Jing, Sh. Cao, and K. Deng, Magnetic Anisotropy Properties and Spin Reorientation for Textured Bi-Mn Alloys Fabricated by a Field-Inducing Technique, Phys. Rev. B, 2004, 70, p 184424
N.V. Rama Rao, A.M. Gabay, and G.C. Hadjipanayis, Anisotropic Fully Dense MnBi Permanent Magnet with High Energy Product and Coercivity at Elevated Temperatures, J. Phys. D Appl. Phys., 2013, 46, p 062001
C. Cui, J.P. Choi, G. Li, E. Polikarpov, J. Darsell, N. Overman, M. Olszta, D. Schreiber, M. Bowden, T. Droubay, M.J. Kramer, N.A. Zarkevich, L.L. Wang, D.D. Johnson, M. Marinescu, I. Takeuchi, Q.Z. Huang, H. Wu, H. Reeve, N.V. Vuong, and J.P. Liu, Thermal Stability of MnBi Magnetic Materials, J. Phys. Condens. Matter, 2014, 26, p 064212
J. Cui, J.P. Choi, E. Polikarpov, M.E. Bowden, W. Xie, G. Li, Z. Nie, N. Zarkevich, M.J. Kramer, and D. Johnson, Effect of Composition and Heat Treatment on MnBi Magnetic Materials, Acta Mater., 2014, 79, p 374-381
Y-Ch. Chen, G. Gregori, A. Leineweber, F. Qu, Ch-Ch. Chen, T. Tietze, H. Kronmüller, G. Schütz, and E. Goering, Unique High-Temperature Performance of Highly Condensed MnBi Permanent Magnets, Scr. Mater., 2015, 107, p 131-135
M. Ellner, Über die kristallchemischen Parameter der Ni-, Co- und Fe-haltigen Phasen vom NiAs-Typ, J. Less-Common Met., 1976, 48, p 21 (in German)
V. Taufour, S. Thimmaiah, S. March, S. Saunders, K. Sun, T.N. Lamichhane, M.J. Kramer, S.L. Budko, and P.C. Canfield, Structural and Ferromagnetic Properties of an Orthorhombic Phase of MnBi Stabilized with Rh Addition, Phys. Rev. Appl., 2015, 4, p 014021
H.D. Dannöhl and W. Gmöhling, Untersuchungen über den Zusatz von Kobalt und Antimon zu Mangan Wismuth-Legierungen, Z. Metallkde, 1963, 54, p 564-569 (in German)
P. Kainzbauer, K.W. Richter, and H. Ipser, Experimental Investigation of the Binary Mn-Sb Phase, J. Phase Equilib. Diffus., 2016, 37(4), p 459-468
H. Okamoto, Bi-Sb (Bismuth-Antimony), J. Phase Equilib. Diffus., 2012, 33(6), p 493-494
H. Ohtani and K. Ishida, A Thermodynamic Study of the Phase Equilibria in the Bi-Sb-Sn System, J. Electron. Mater., 1994, 23(8), p 747-755
H. Okamoto, Supplemental Literature Review of Binary Phase Diagrams: Ag-Co, Ag-er, Ag-Pd, B-C, Bi-La, Bi-Mn, Cu-G, Cu-Tm, Er-Y, Gd-Tl, H-La and Hg-Te, J. Phase Equilib. Diffus., 2015, 36, p 10-21
K. Oikawa, Y. Mitsui, K. Koyama, and K. Anzai, Thermodynamic Assessment of the Bi-Mn System, Mater. Trans., 2011, 52(11), p 2032-2039
C.P. Wang, W.J. Yu, Z.S. Li, X.J. Liu, A.T. Tang, and F.S. Pan, Thermodynamic Assessments of the Bi-U and Bi-Mn Systems, J. Nucl. Mater., 2011, 412, p 66-71
P. Siebe, Die Legierungen: Mangan-Wismut, Mangan-Zink und Mangan Silber, Z. Allg. Anorg. Chem., 1919, 108(1), p 161-183 (in German)
K. Ahlborn, K. Bärber, and W. Schröter, Magnetic Properties of Alloys in the System MnSb-MnBi, Phys. Status Solidi A Appl. Res., 1975, 30(1), p 251-261
M. Durand-Charre, G. Marcon, G. Massart, and F. Durand, Determination du liquidus et des conodes au voisinage de la Ligne eutectique MnSb-(Sb, Bi), J. Less Common Metals, 1973, 32, p 103-109 (in French)
H. Göbel, E. Wolfgang, and H. Harms, Properties of MnBi Compounds Partially Substituted with Cu, Zn, Ti, Sb, Te, Phys. Status Solidi A Appl. Res., 1976, 34, p 553-563
A.F. Andresen, J.E. Engebretsen, and J. Refsnes, Neutron Diffraction Investigations on Quenched MnBi and MnBi0.9Sb0.1, Acta Chem. Scand., 1972, 26, p 175-190
M. Uemitsu, M. Enoki, S. Likubo, and H. Ohtani, Thermodynamic Analysis of Phase Equilibria in the Mn-Bi-Sb Ternary System, Nippon Kinzoku Gakkaishi, 2014, 78(9), p 327-336 (in Japanese)
K. Kang, G. Kravets, and A.K. Petford-Long, Crystalline Structure and Magnetic and Magneto-Optical Properties of MnSbBi Thin Films, Thin Solid Films, 2002, 410, p 28-37
Y.J. Wang, J.X. Shen, and Q. Tang, The Structure and the Magnetic Properties of Mn0.5(Bi1−xSbx)0.5 Thin Films, J. Mag. Mag. Mater., 1988, 74, p 365-369
TOPAS 4.2, Bruker AXS Inc., Karlsruhe, Germany, 2011.
R.W.W. Hooft, Software COLLECT, Nonius BV, Delft, the Nederlands, 1999
Z. Otwinowski and W. Minor, Processing of x-ray Diffraction Data Collected in Oscillation Mode, Method Enzymol, 1997, 276, p 307-326
G.M. Sheldrick, A Short History of SHELX, Acta Cryst. A, 2008, 64, p 112-122
G.M. Sheldrick, Crystal Structure Refinement with SHELXL, Acta Cryst. C, 2015, 71, p 3-8
R.A. Robinson, A. Purwanto, M. Koghi, P.C. Canfield, T. Kamiyama, T. Ishigaki, J.W. Lynn, R. Erwin, E. Peterson, and R. Movshovich, Crystallography and Magnetism of the heavy-Fermion Compound Yb Bi Pt, Phys. Rev. B, 1994, 50(13), p 9595-9598
T. Yamada, N. Kunitomi, Y. Nakai, D.E. Cox, and G. Shirane, Magnetic Structure of alpha-Mn, J. Phys. Soc. Jpn., 1970, 28(3), p 615-627
O.B. Karlsen, A. Kjekshus, H. Fjellvag, P. Ravindran, R. Vidya, and B.C. Hauback, Structure and Magnetism of the beta-Mn-Co Solid Solution Phase, J. Alloys Compd., 2009, 476, p 9-13
C.S. Barrett, P. Cucka, and K. Haefner, The Crystal Structure of Antimony at 4.2, 78 and 298 K, Acta Cryst., 1963, 16, p 451-453
T. Chen, Contribution to the Equilibrium Phase Diagram of the Mn-Bi System Near MnBi, J. Appl. Phys., 1974, 45(5), p 2358-2360
V.G. Vanyarkho, N.A. Moshchalkova, V.M. Gunchenko, and N.V. Fadeeva, On the Existence of the Compound MnSb, Izv. Akad. Nauk SSSR, Neorg. Mater., 1988, 24(6), p 900-903 (in Russian)
W.B. Pearson, The Cu2Sb and Related Structures, Z. Kristallorg., 1985, 171, p 23-39
Acknowledgments
Open access funding provided by University of Vienna. Financial support of this work by the Austria Science Fund (FWF) through Project No. P 26023 is gratefully acknowledged. Open access funding was provided by the University of Vienna. The authors also want to thank Dr. Stephan Puchegger from the Center for Nano Structure Research, University of Vienna, for support with the SEM/EDX measurements.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This invited article is part of a special issue of the Journal of Phase Equilibria and Diffusion in honor of the 2018 J. Willard Gibbs Phase Equilibria Award winner Dr. John Morral. The award was presented to Dr. Morral during MS&T’18, October 14-18, 2018, in Columbus, Ohio, “for fundamental and applied research on topology of phase diagrams and theory of phase equilibria resulting in major advances in the calculation and interpretation of phase equilibria and diffusion.”
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Kainzbauer, P., Richter, K.W., Effenberger, H.S. et al. The Ternary Bi-Mn-Sb Phase Diagram and the Crystal Structure of the Ternary Τ Phase Bi0.8MnSb0.2. J. Phase Equilib. Diffus. 40, 462–481 (2019). https://doi.org/10.1007/s11669-019-00719-x
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11669-019-00719-x