Abstract
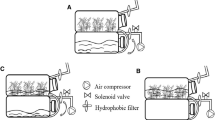
The cultivation of Vanilla planifolia is of great economic importance because vanillin, a chemical valued in the food and cosmetics industry, is extracted from its pods. The conventional propagation of this plant is limited by the low viability of its seeds and the very low germination rate. For this reason, in vitro micropropagation techniques using temporary immersion systems (TIS) represent an alternative propagation mechanism. This work assessed three different bioreactor systems in two different micropropagation phases (multiplication and rooting) of V. planifolia: Temporary Immersion Bioreactors (BIT®), Gravity Immersion Bioreactors (BIG), and Recipient for Automated Temporary Immersion (RITA®). A higher number of shoots/explant were observed in the multiplication phase in BIT® systems (18.06 shoots/explant), followed by RITA® (12.77) and BIG (6.83). In the rooting phase, a higher number of longer roots were obtained in BIT® compared with BIG and RITA®. However, higher chlorophyll content was observed in BIG, followed by RITA® and BIT®. A 100% survival was obtained in vitro micropropagated plantlets in BIT®, exceeding the survival rate observed in RITA® and BIG. In general, our findings confirm the utility of BIT® systems in the optimization of the commercial micropropagation of this species. Furthermore, this system reduces the costs associated with the use of RITA® systems.





Similar content being viewed by others
References
Adame-García J, Rodríguez-Guerra R, Iglesias-Andreu LG, Ramos-Prado JM, Luna-Rodríguez M (2014) Molecular identification and pathogenic variation of Fusarium species isolated from Vanilla planifolia in Papantla Mexico. Bot Sci 93:669–78
Akdemir H, Süzerer V, Onay A, Tilkat E, Ersali Y, Ozden Y (2014) Micropropagation of the pistachio and its rootstocks by temporary immersion system. Plant Cell Tiss Organ Cult 117:65–76
Aragón CE, Sánchez C, Gonzalez-Olmedo J, Escalona M, Carvalho L, Amâncio S (2014) Comparison of plantain plantlets propagated in temporary immersion bioreactors and gelled medium during in vitro growth and acclimatization. Biol Plant 58:29–38
Ascough GD, Fennel CW (2004) The regeneration of plant growth and development in liquid culture. S Afr J Bot 70:181–90
Bernal A, Machado P, Cortegaza L, Carmona ER, Rivero O, Zayas CM, Nodarse O, Perez A, Santana I, Arencibia AD (2008) Priming and biopriming integrated into the sugarcane micropropagation technology by Temporary Immersion Bioreactors (TIBS). Sugar Tech 10:42–7
Berthouly M, Etienne H (2005) Temporary immersion system: a new concept. In: Hvolsef-Eide A, Preil W (eds) Liquid culture systems for in vitro plant propagation. Springer, Dordrecht, pp 165–96
Castro-Bobadilla GA, Martínez J, Martínez ML, García-Franco JG (2011) Aplicación de riego localizado para aumentar la retención de frutos de Vanilla planifolia en el Totonacapan, Veracruz, México. Agrociencia 45:281–91
Ducos JP, Lambot C, Pétiard V (2007) Bioreactors for coffee mass propagation by somatic embryogenesis. Int J Dev Biol 1:1–12
Escalona M, Lorenzo JC, González B, Daquinta M, González JL, Desjardins Y, Borroto CG (1999) Pineapple (Ananas comosus L. Merr) micropropagation in temporary immersion systems. Plant Cell Rep 18:743–8
Etienne H, Berthouly M (2002) Temporary immersion systems in plant micropropagation. Plant Cell Tissue Organ Cult 69:215–31
Hazarika BN (2003) Acclimatization of tissue-cultured plants. Curr Sci 85:1704–12
Janarthanam B, Seshadri S (2008) Plantlet regeneration from leaf derived callus of Vanilla planifolia Andr. In Vitro Cell Dev Biol-Plant 44:84–9
Lee-Espinosa HE, Murguía-González J, García-Rosas B, Córdova-Contreras AL, Laguna-Cerda A, Mijangos-Cortés JO, Barahona-Pérez LF, Iglesias-Andréu LG, Santana-Buzzy N (2008) In vitro clonal propagation of vanilla (Vanilla planifolia ‘Andrews’). Hortic Sci 43:454–8
Martre P, Lacan D, Just D, Teisson C (2001) Physiological effects of temporary immersion on Hevea brasiliensis (Müll. Arg.) callus. Plant Cell Tissue Organ Cult 67:25–35
Mathur A, Mathur AK, Verma P, Yadav S, Gupta ML, Darokar MP (2008) Biological hardening and genetic fidelity testing of micro-cloned progeny of Chlorophytum borivilianum. Afr J Biotechnol 7:1046–53
Mengesha A, Ayenew B, Gebremariam E, Tadesse T (2012) Micro-propagation of Vanilla planifolia using Enset (Ensete ventricosum (Welw, cheesman)) starch as a gelling agent. Curr Res J Biol Sci 4:519–25
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue culture. Physiol Plantarum 15:473–97
Niemenak N, Noah AM, Omokolo ND (2013) Micropropagation of cocoyam (Xanthosoma sagittifolium L. Schott) in temporary immersion bioreactor. Plant Biotechnol Rep 7:383–90
Niemenak N, Saare-Surminski K, Rohsius C, Omokolo ND, Lieberei R (2008) Regeneration of somatic embryos in Theobroma cacao L. in temporary immersion bioreactor and analysis of free amino acids in different tissues. Plant Cell Rep 27:667–76
Oliveira SOD, Meneses R, Aparecida T, Scherwinski-Pereirad JE (2013) A new procedure for in vitro propagation of vanilla (Vanilla planifolia) using a double-phase culture system. Sci Hortic 161:204–9
Pérez M, Bueno MA, Escalona M, Toorop P, Rodríguez R, Cañal MJ (2013) Temporary immersion systems (RITA) for the improvement of cork oak somatic embryogenic culture proliferation and somatic embryo production. Trees 27:1277–84
Porra RJ, Thompson WA, Kriedelman PE (1989) Determination of accurate extraction and simultaneously equation for assaying chlorophyll a and b extracted with different solvents: verification of the concentration of chlorophyll standards by atomic absorption spectroscopy. Biochem Biophys Acta 975:384–94
Quiala E, Canal MJ, Meijon M, Rodriguez R, Chavez M, Valledor L, Feria M, Barbon R (2012) Morphological and physiological responses of proliferating shoots of teak to temporary immersion and BA treatments. Plant Cell Tiss Org Cult 109:223–34
Ramírez-Mosqueda MA, Iglesias-Andreu LG (2015) Indirect organogenesis and assessment of somaclonal variation in plantlets of Vanilla planifolia Jacks. Plant Cell Tiss Organ Cult 123:657–664
Ramírez-Mosqueda MA, Iglesias-Andreu LG, Luna-Rodríguez M, Castro-Luna AA (2015) In vitro phytotoxicity of culture filtrates of Fusarium oxysporum f. sp.vanillae in Vanilla planifolia Jacks. Sci Hortic 197:573–578
Ramos-Castellá A, Iglesias-Andreu LG, Bello-Bello J, Lee-Espinosa H (2014) Improved propagation of vanilla (Vanilla planifolia Jacks. ex Andrews) using a temporary immersion system. In Vitro Cell Dev Biol—Plant 50:576–81
Roels S, Escalona M, Cejas I, Noceda C, Rodriguez R, Canal MJ, Sandoval J, Debergh P (2005) Optimization of plantain (Musa AAB) micropropagation by temporary immersion system. Plant Cell Tiss Org Cult 82:57–66
Ruffoni B, Savona M (2013) Physiological and biochemical analysis of growth abnormalities associated with plant tissue culture. Hort Environ Biotechnol 54:191–205
Shin KS, Park SY, Paek KY (2014) Physiological and biochemical changes during acclimatization in a Doritaenopsis hybrid cultivated in different microenvironments in vitro. Environ Exp Bot 100:26–33
Soto-Arenas MA (2003) Vanilla. In: Pridgeon AM, Cribb PJ, Chase MW, Rasmussen FN (eds) Genera Orchidacearum, vol 3, Orchidoideae (Part 2) Vanilloideae. Oxford University Press, Oxford, pp 321–34
Sreedhar RV (2009) Novel approaches for molecular analyses, micropropagation and curing of vanilla (Vanilla planifolia), PhD thesis. University of Mysore, New Delhi
Sreedhar RV, Venkatachalam L, Neelwarne B (2009) Hyperhydricity-related morphologic and biochemical changes in vanilla (Vanilla planifolia). J Plant Growth Regul 28:46–57
Tan BC, Chin CF, Alderson P (2011) Optimization of plantlet regeneration from leaf and nodal derived callus of Vanilla planifolia Andrews. Plant Cell Tissue Organ Cult 105:457–63
Tan BC, Chin CF, Alderson P (2013) Effects of sodium nitroprusside on shoot multiplication and regeneration of Vanilla planifolia Andrews. Plant Cell Tissue Organ Cult 105:457–63
Teisson C, Alvard D (1995) A new concept of plant in vitro cultivation liquid medium: temporary immersion. In: Terzi M et al (eds) Current issues in plant molecular and cellular biology. Kluwer Academic Publishers, Dordrecht, pp 105–10
Teisson C, Alvard D, Berthouly B, Cote F, Escalant V, Etienne H, Lartaud M (1996) Simple apparatus to perform plant tissue culture by temporary immersion. Acta Hortic 440:521–6
Torres-González MJ, Aguirre-Medina JF, Iracheta-Donjuan L (2011) Germinación de semillas y obtención de plántulas de Vanilla planifolia Andrews en condiciones in vitro. Agroproductividad 4:3–8 (in Spanish)
Ziv M (2005) Simple bioreactors for mass propagation of plants. In: Hvoslef-Eide AK, Preil W (eds) Liquid culture systems for in vitro plant propagation. Springer, Dordrecht, pp 328–56
Zuraida AR, Liyana KHF, Nazreena OA, Wan WS, Che CMZ, Zamri Z, Sreeramanan S (2013) A simple and efficient protocol for the mass propagation of Vanilla planifolia. A J P S 4:1685–92
Acknowledgments
The authors would like to thank the “Programa para el Desarrollo Profesional Docente (PRODEP)” for financial support provided for the project “Biotechnological Basis for the Genetic Improvement of Vanilla planifolia” within the “Conservation, Management and Plant Breeding network. MARM thanks the Consejo Nacional de Ciencia y Tecnología (CONACyT) for the grant scholarship No. 275736, which allows the realization of this work.
Author information
Authors and Affiliations
Corresponding author
Additional information
Editor: Ewen Mullins
Rights and permissions
About this article
Cite this article
Ramírez-Mosqueda, M.A., Iglesias-Andreu, L.G. Evaluation of different temporary immersion systems (BIT®, BIG, and RITA®) in the micropropagation of Vanilla planifolia Jacks. In Vitro Cell.Dev.Biol.-Plant 52, 154–160 (2016). https://doi.org/10.1007/s11627-015-9735-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11627-015-9735-4