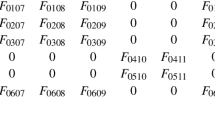Abstract
We present a theoretical investigation of a polymerization process catalyzed by an enzyme. A structural model of enzyme, sliding along the polymer chain as a Brownian particle, is proposed, and a stochastic approach is employed to describe the kinetics of the whole process. The key point of this work is the coupling mechanics/chemistry obtained by assuming that (1) some rates of chemical reaction depend on the position of the enzyme with respect to the polymer chain and (2) the potential energy and the friction coefficient in the Langevin equation depend on the chemical state of the polymerizing complex. We describe an algorithm for computing our stochastic model and a methodology to solve the Langevin equation numerically. We predict in particular: (1) the sudden arrest of the polymerization, (2) the decrease in the relative polydispersity with the increase in the length of the polymer chain, (3) the occurrence of four regimes, (4) the manifestation of the coupling mechanics/chemistry for one regime and (5) the possibility to evaluate the mechanical variables through classical chemical analysis. Although essentially devoted to the elongation phase, this work also briefly addresses the problem of phase termination and we propose a new device aimed at reducing the polydispersity of technical origin in actual polymerization processes.


















Similar content being viewed by others
Notes
The release of the monomer from \( \textsf{E}_{10}^\nu \) and \( \textsf{E}_{11}^\nu \) is already described by the backward reactions described by Eqs. (7a) and (7c).
For \( \textsf{A} = \textsf{B} \), the expression for the reaction rate transforms into \( K(t) = \kappa \left[\kern-0.15em\left[ \textsf{A} \right]\kern-0.15em\right]\left( {\left[\kern-0.15em\left[ \textsf{A} \right]\kern-0.15em\right] - 1} \right) \), in agreement with the fact that the reaction is impossible when \( \left[\kern-0.15em\left[ \textsf{A} \right]\kern-0.15em\right] = 1 \) (Gillespie 1992).
This remark also applies to the reactions described by Eqs. (7a)–(7g).
\( {\mathcal{H}}(q)\) (q) = 0 for q < 0 and \( {\mathcal{H}}(q) \) = 1 for q ≥ 0.
Here, the classical rates are assumed to be independent of the length of the polymer chain; for this reason, it is unnecessary to attach an index n to the symbol \( \chi_{i}^{ \pm } \).
We have \( p_{i}^{ \pm } = 1 \) since \( {\mathbb D}_{in}^\pm = {\mathbb R} \).
Note that if one would assume that the polymer and the fluid are fixed, while the enzyme is mobile with respect to the laboratory, then the friction coefficient would be associated with the enzyme and consequently independent of n.
The superscript sim in \( \tau_{\text{I}}^{\text{sim}} \) and \( \tau_{\text{II}}^{\text{sim}} \) indicates that these values result from the simulations of our model, while the subscripts I and II refer to the time periods \( {{\mathbb T}_{\text{I}}} \) and \( {{\mathbb T}_{\text{II}}} \), respectively.
That is confirmed by Fig. 13 that evidences a rate of polymerization of about 100 monomers per ms and per enzyme, that is, in average one monomer per enzyme during \( {{\mathbb T}_{\text{I}}} \cup {{\mathbb T}_{\text{II}}} \).
Note that this feature arises because the enzyme is assumed to be immobile with respect to the laboratory frame (see Sect. 3.3).
Note that \( {{k_{B} T} \mathord{\left/ {\vphantom {{k_{B} T} \alpha }} \right. \kern-0pt} \alpha } \) is the diffusion coefficient of the Brownian particle (Kubo et al. 1991).
A more rigourous approach should take into account the deterministic force, but that would make the analysis much more difficult.
References
Ait-Haddou R, Herzog W (2003) Brownian ratchet models of molecular motors. Cell Biochem Biophys 38:191–213
Anderson DF, Ermentrout B, Thomas PJ (2015) Stochastic representation of ion channel kinetics and exact stochastic simulation of neuronal dynamics. J Comput Neurosci 38:67–82
Arnold L (1992) Stochastic differential equations: theory and applications. Krieger Publishing Company, Malabar
Astumian RD, Bier M (1994) Fluctuation driven ratchets: molecular motors. Phys Rev Lett 72:1766–1769
Atzberger PJ, Peskin CS (2006) A Brownian dynamics model of kinesin in three dimensions incorporating the force-extension profile of the coiled-coil cargo tether. Bull Math Biol 68:131–160
Boscolo B, Trotta F, Ghibaudi E (2010) High catalytic performances of Pseudomonas fluorescens lipase adsorbed on a new type of cyclodextrin-based nanosponges. J Mol Catal B Enzym 62:155–161
Bustamante C, Keller D, Oster G (2001) The physics of molecular motors. Acc Chem Res 34:412–420
De Gennes P-G (1979) Scaling concepts in polymer physics. Cornell University Press, Ithaca, London
Dietrich-Buchecker C, Jimenez-Molero MC, Sartor V, Sauvage J-P (2003) Rotaxanes and catenanes as prototypes of molecular machines and motors. Pure Appl Chem 75:1383–1393
Fersht A (1999) Structure and mechanism in protein science. A guide to enzyme catalysis and protein folding. Freeman and Company, New York
Gao S, Wang Y, Wang W, Luo G, Dai Y (2010) Enhancing performance of lipase immobilized on methyl-modified silica aerogels at the adsorption and catalysis processes: effect of cosolvents. J Mol Catal B Enzym 62:218–224
Gardiner CW (1985) Handbook of stochastic method for physics, chemistry and natural science, 2edn. Springer, Berlin
Gillespie DT (1977) Exact stochastic simulation of coupled chemical reactions. J Phys Chem 81:2340–2361
Gillespie DT (1992) A rigorous derivation of the chemical master equation. Phys A 188:404–425
Gillespie DT (2000) The chemical Langevin equation. J Phys Chem 113:297–306
Haynie DT (2001) Biological thermodynamics. Cambridge University Press, Cambridge
Huang XJ, Chen PC, Huang F, Ou Y, Chen MR, Xu ZK (2011) Immobilization of Candida rugosa lipase on electrospun cellulose nanofiber membrane. J Mol Catal B Enzym 70:95–100
Huxley AF (1957) Muscle structure and theories of contraction. Prog Biophys Biophys Chem 7:257–318
Jülicher F, Ajdari A, Prost J (1997) Modeling molecular motors. Rev Mod Phys 69:1269–1281
Kubo R, Toda M, Hashitsume N (1991) Statistical physics II, nonequilibrium statistical mechanics, 2edn. Springer, Berlin
Laidler KJ (1987) Chemical kinetics. Harper Collins Publishers, New York
Landau LD, Lifshitz EM (2006) Fluid mechanics. Elsevier Butterworth-Heinemann, Oxford
Reimann P (2002) Brownian motors: noisy transport far from equilibrium. Phys Rep 361:57–265
Sakai S, Antoku K, Yamaguchi T, Watanabe R, Kawabe M, Kawakami K (2010) Electrospun PVA fibrous mats immobilizing lipase entrapped in alkylsilicate cages: application to continous production of fatty acid butyl ester. J Mol Catal B Enzym 63:57–61
Stubbe J, Tian J, He A, Sinskey AJ, Lawrence AG, Liu P (2005) Nontemplate-dependent polymerization processes: polyhydroxyalkanoate synthases as a paradigm. Annu Rev Biochem 74:433–480
Vale RD, Milligan RA (2000) The way things move: looking under the hood of molecular motor proteins. Science 288:88–95
Webber MJ (2016) Engineering responsive biomaterials: toward smart therapeutics. Bioeng Transl Med 1:252–266
Wilson MR, Solà J, Carlone A, Goldup SM, Lebrasseur N, Leigh DA (2016) An autonomous chemically fuelled small-molecule motor. Nature 534:235–240
Zhang XJ, Qian H, Qian M (2012) Stochastic theory of nonequilibrium steady states and its application Part I. Phys Rep 510:1–86
Acknowledgements
This work was partly supported by ‘Fundacão para a Ciência e a Tecnologia’ (Portugal) through Research Contract No. C2007-443-CENIMAT-1 to A. Véron.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Véron, A.R., Martins, A.F. Stochastic Mechanochemical Description of a Bioinspired Polymerization Process. Bull Math Biol 81, 155–192 (2019). https://doi.org/10.1007/s11538-018-0522-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11538-018-0522-3