Abstract
Oilseed crops are global commodities for their oil and protein seed content. We have engineered the oilseed Camelina sativa to exhibit increased protein content with a slight decrease in oil content. The introduction of a phytoene synthase gene with an RNAi cassette directed to suppress the storage protein 2S albumin resulted in seeds with an 11–24 % elevation in overall protein. The phytoene synthase cassette alone produced enhanced β-carotene content of an average 275 ± 6.10 μg/g dry seed and an overall altered seed composition of 11 % less protein and comparable nontransgenic amounts of both oil and carbohydrates. Stacking an RNAi to suppress the major 2S storage protein resulted in seeds that contain elevated protein and slight decrease in oil and carbohydrate amounts showing that Camelina rebalances its proteome within an enlarged protein content genotype. In both β-carotene enhanced seeds with/without RNAi2S suppression, the seed size was noticeably enlarged compared to nontransgenic counterpart seeds. Metabolic analysis of maturing seeds indicate that the enhanced β-carotene trait had the larger effect than the RNAi2S suppression on the seed metabolome. The use of a GRAS (generally regarded as safe) β-carotene as a visual marker in a floral dip transformation system, such as Camelina, might eliminate the need for costly regulatory and controversial antibiotic resistance markers. β-carotene enhanced RNAi2S suppressed Camelina seeds could be further developed as a rapid heterologous protein production platform in a nonfood crop leveraging its enlarged protein content and visual marker.





Similar content being viewed by others
References
Budin ST, Breene WM, Putnam DH (1995) Some compositional properties of camelina (Camelina sativa L. Crantz) seeds and oils. J Am Oil Chem Soc 72:309–315
Cober ER, Voldeng HD (2000) Developing high-protein, high-yield soybean populations and lines. Crop Sci 40:39–42
Costa-Font M, Gil JM, Traill WB (2008) Consumer acceptance, valuation of and attitudes towards genetically modified food: review and implications for food policy. Food Policy 33:99–111
DeHaven CD, Evans AM, Dai H, Lawton KA (2010) Organization of GC/MS and LC/MS metabolomics data into chemical libraries. J Cheminform 2:9
DeHaven CD, Evans AM, Dai H, Lawton KA (2012) Software techniques for enabling high-throughput analysis of metabolomics datasets. http://cdnintechweb.org/pdfs/28007.pdf
Doyle JJ (1990) Isolation of plant DNA from fresh tissue. Focus 12:13–15
Eskandari M, Cober ER, Rajcan I (2013) Genetic control of soybean seed oil: QTL and genes that increase oil concentration without protein or with increased seed yield. Theor Appl Genet 6:1677–1687
Evans AM, DeHaven CD, Barrett T, Mitchell M, Milgram E (2009) Integrated, nontargeted ultrahigh performance liquid chromatography/electrospray ionization tandem mass spectrometry platform for the identification and relative quantification of the small-molecule complement of biological systems. Anal Chem 81:6656–6667
Evans AM, Mitchell MW, Dai H, DeHaven CD (2012) Categorizing ion—features in liquid chromatography/mass spectrometry metabolomics data. Metabolomics 2:3
Farrow SC, Emery RJ (2012) Concurrent profiling of indole-3-acetic acid, abscisic acid and cytokinins and structurally related purines by high-performance liquid chromatography tandem electrospray mass spectrometry. Plant Methods 8:42
Fayle TM, Turner EC, Snaddon JL, Chey VK, Chung AYC, Eggleton P, Foster WA (2010) Oil palm expansion into rain forest greatly reduces ant biodiversity in canopy, epiphytes and leaf-litter. Basic Appl Ecol 11:337–345
Finkelstein RR, Gampala SSL, Rock CD (2002) Abscisic acid signaling in seeds. Plant Cell 14:S15–S45
Fleenor, R (2011) Plant guide for Camelina (Camelina sativa). USDA-Natural Resources Conservation Service, Spokane, WA 99201. http://plants.usda.gov/plantguide/pdf/pg_casa2.pdf
Galili G, Feldman M (1984) Intergenomic suppression of endosperm protein genes in common wheat. Can J Genet Cytol 26:651–656
Galili G, Hofgen R (2002) Metabolic engineering of amino acids and storage proteins in plants. Met Eng 4:3–11
Hajduch M, Ganapathy A, Stein JW, Thelen JJ (2005) A systematic proteomic study of seed filling in soybean. Establishment of high-resolution two-dimensional reference maps, expression profiles, and an interactive proteome database. Plant Physiol 137:1397–1419
Hegedus DD, Baron M, Labbe N, Coutu C, Lydiate D, Lui H, Rozwadowski K (2014) Strategy for targeting recombinant proteins to protein storage vacuoles by fusion to Brassica napus napin in napin-depleted seeds. Protein Expr Purif 95:162–168
Herman EM, Schmidt MA (2010) Industrial protein production crops: new needs and new opportunities. GM Crops 1:1–7
Herman EM, Schmidt MA (2015) Towards using biotechnology to modify soybean seeds as protein bioreactors. In: Azhakanandam K et al (eds) Recent advancements in gene expression and enabling technologies in crop plants. Springer, New York, pp 193–212
Hwang E-Y, Song Q, Jia G, Specht JE, Hyten DL, Costa J, Cregan PB (2014) A genome-wide association study of seed protein and oil content in soybean. BMC Genom 15:1
Jofuku KD, Omidyar PK, Gee Z, Okamuro JK (2005) Control of seed mass and seed yield by the floral homeotic gene APETALA2. Proc Natl Acad Sci 102:3117–3122
Julie-Galau S, Bellec Y, Faure J-D, Tepfer M (2014) Evaluation of the potential for interspecific hybridization between Camelina sativa and related wild Brassicaceae in anticipation of field trials of GM Camelina. Transgenic Res 23:67–74
Kagale S, Koh C, Nixon J, Bollina V, Clarke WE, Tuteja R, Spillane C, Robinson SJ, Links MG, Clarke C, Higgins EE, Huebert T, Sharpe AG, Parkin IAP (2014) The emerging biofuel crop Camelina sativa retains a highly undifferentiated hexaploid genome structure. Nat Commun 5:3706. doi:10.1038/ncomms4706
Kagaya Y, Okuda R, Ban A, Toyoshima R, Tsutsumida K, Usui H, Yamamoto A, Hattori T (2005) Indirect ABA-dependent regulation of seed storage protein genes by FUSCA3 transcription factor in Arabidopsis. Plant Cell Physiol 46:300–311
Kawakatsu T, Hirose S, Yasuda H, Takaiwa F (2010) Reducing rice seed storage protein accumulation leads to changes in nutrient quality and storage organelle formation. Plant Physiol 154:1842–1854
Kim WS, Gillman JD, Krishnan HB (2013) Identification of a plant introduction soybean line with genetic lesions affecting two distinct glycinin subunits and evaluation of impacts on protein content and composition. Mol Breed 32:291–298
Kinney AJ, Jung R, Herman EM (2001) Cosuppression of the α subunits of β-conglycinin in transgenic soybean seeds induces the formation of endoplasmic reticulum derived protein bodies. Plant Cell 13:1165–1178
Kumar T, Dweikat I, Sato S, Ge Z, Nersesian N, Chen H, Elthon T, Bean S, Ioerger BP, Tilley M, Clemente T (2012) Modulation of kernel storage proteins in grain sorghum (Sorghum bicolor (L.) Moench). Plant Biotechnol J 10:533–544
Lange R, Schumann M, Petrzika M, Busch H, Marquard R (1995) Glucosinolates in linseed dodder. Fat Sci Technol 97:146–152
Lau OS, Sun SSM (2009) Plant seeds as bioreactors for recombinant protein production. Biotechnol Adv 27:1015–1022
Lin Y, Pajak A, Marsolais F, McCourt P, Riggs CD (2013) Characterization of a cruciferin deficient mutant of Arabidopsis and its utility for overexpression of foreign proteins in plants. PLoS ONE 8:e64980
Livanos P, Galatis B, Apostolakos P (2014) The interplay between ROS and tubulin cytoskeleton in plants. Plant Signal Behav 9:e28069
Lu C, Kang J (2008) Generation of transgenic plants of a potential oilseed crop Camelina sativa by Agrobacterium mediated transformation. Plant Cell Rep 27:273–278
Marsolais F, Pajak A, Yin F, Taylor M, Gabriel M, Merino DM, Ma V, Kameka A, Vijayan P, Pham H, Huang S, Rivoal J, Bett K, Hernandez-Sebastia C, Liu Q, Bertrand A, Chapman R (2010) Proteomic analysis of common bean seed with storage protein deficiency reveals up-regulation of sulfur-rich proteins and starch and raffinose metabolic enzymes, and down-regulation of the secretory pathway. J Proteom 73:1587–1600
Masuko T, Minami A, Iwasaki N, Majima T, Nishimura S, Lee YC (2005) Carbohydrate analysis by a phenol-sulfuric acid method in microplate format. Anal Biochem 339:69–72
Moser BR (2012) Biodiesel from alternative oilseed feedstocks: Camelina and field pennycress. Biofuels 3:193–209
Mudalkar S, Golla R, Ghatty S, Reddy AR (2014) De novo transcriptome analysis of an imminent biofuel crop, Camelina sativa L. using Illumina GAIIX sequencing platform and identification of SSR markers. Plant Mol Biol 84:159–171
Nakashima K, Yamaguchi-Shinozaki K (2013) ABA signaling in stress-response and seed development. Plant Cell Rep 32:959–970
Nambara E, Marion-Poll A (2003) ABA action and interactions in seeds. Trends Plant Sci 8:213–217
Nambara E, Naito S, McCourt P (1992) A mutant Arabidopsis which is defective in seed development and storage protein accumulation in a new abi3 allele. Plant J 2:435–441
Nguyen HT, Silva JE, Podicheti R, Macrander J, Yang W, Nazarenus TJ, Nam J-W, Jaworski JG, Lu C, Scheffler BE, Mockaitis K, Cahoon EB (2013) Camelina seed transcriptome: a tool for meal and oil improvement and translational research. Plant Biotechnol J 11:759–769
Ohta T, Masutomi N, Tsutsui N, Sakairi T, Mitchell M, Milburn MV, Ryals JA, Beebe KD, Guo L (2009) Untargeted metabolomics profiling as an evaluative tool of fenobibrate-induced toxicology in Fischer 344 male rats. Toxicol Pathol 37:521–535
Paine JA, Shipton CA, Chaggar S, Howells RM, Kennedy MJ, Vernon G, Wright SY, Hinchliffe E, Adams JL, Silverstone AL, Drake R (2005) Improving the nutritional value of Golden Rice through increased pro-vitamin A content. Nat Biotechnol 23:482–487
Panthee DR, Pantalone VR, West DR, Saxton AM, Sams CE (2005) Quantiative trait loci for seed protein and oil concentration and seed size in soybean. Crop Sci 45:2015–2022
Ravanello MP, Ke D, Alvarez J, Huang B, Shewmaker CK (2003) Coordinated expression of multiple bacterial carotenoid genes in canola leading to altered carotenoid production. Metab Eng 5:255–263
Ruiz-Lopez N, Hasslam RP, Napier JA, Sayanova O (2014) Successful high level accumulation of the fish oil omega 3 long chain polyunsaturated fatty acids in a transgenic oilseed crop. Plant J. 77:198–208
Rukmini C (1994) Red palm oil to combat vitamin A deficiency in developing countries. Food Nutr Bull 15:126–129
Santander J, Martin T, Loh A, Pohlenz C, Gatlin DM, Curtiss R (2013) Mechanisms of intrinsic resistance to antimicrobial peptides of Edwardsiella ictaluri and its influence on fish gut inflammation and virulence. Microbiology 159:1471–1486
Schmidt MA, Herman EM (2008a) Proteome rebalancing in soybean seeds can be exploited to enhance foreign protein accumulation. Plant Biotechnol J 6:832–842
Schmidt MA, Herman EM (2008b) Suppression of soybean oleosin produces micro-oil bodies that aggregate into oil body/ER complexes. Mol Plant 1:910–924
Schmidt MA, Barbazuk WB, Sandford M, May G, Song Z, Zhou W, Nikolau BJ, Herman EM (2011) Silencing of soybean seed storage proteins results in a rebalanced protein composition preserving seed protein content without major collateral changes in the metabolome and transcriptome. Plant Physiol 156:330–345
Schmidt MA, Parrott WA, Hildebrand DF, Berg RH, Cooksey A, Pendarvis K, He Y, McCarthy F, Herman EM (2015) Transgenic soya bean seeds accumulating β-carotene exhibit the collateral enhancements of oleate and protein content traits. Plant Biotechnol J 13:590–600
Shigemitsu T, Ozaki S, Saito Y, Kuroda M, Morita S, Satoh S, Masumura T (2012) Production of human growth hormone in transgenic seeds: co-introduction of RNA interference cassette for suppressing the gene expression of endogenous storage proteins. Plant Cell Rep 31:539–549
Stoger E, Ma JK, Fisher R, Christou P (2005) Sowing the seeds of success: pharmaceutical proteins from plants. Curr Opin Biotechnol 16:167–173
Storey JD, Tibshirani R (2003) Statistical significance for genomewide studies. Proc Natl Acad Sci 100:9440–9445
Sundram K, Sambranthamurthi R, Tan Y-A (2003) Palm fruit chemistry and nutrition. Asia Pacific J Clin Nutr 12:355–362
Tada Y, Utsumi S, Takaiwa F (2003) Foreign gene products can be enhanced by introduced into low storage protein mutants. Plant Biotechnol J 1:411–422
Takahashi M, Uematsu Y, Kashiwaba K, Yagasaki K, Hajika M, Matsunagam R, Komatsu K, Ishimoto M (2003) Accumulation of high levels of free amino acids in soybean seeds through integration of mutations conferring seed protein deficiency. Planta 217:577–586
Takaiwa F (2013) Increasing the production yield of recombinant protein in transgenic seeds by expanding the deposition space within the intracellular compartment. Bioengineered 4:136–139
Teraishi M, Takahashi M, Hajika M, Matsunaga R, Uematsu Y, Ishimoto M (2001) Suppression of soybean β-conglycinin genes by a dominant gene, Scg-1. Theor Appl Genet 103:1266–1272
Utsumi S (1992) Plant food protein engineering. Advan Food Nutr Res 36:89–208
Van den Eede G, Aarts H, Corthier G, Flint HJ, Hammes W, Jacobsen B, Midtvedt T, van der Vossen J, von Wright A, Wachemagel WW, Wilcks A (2004) The relevance of gene transfer to the safety of food and feed derived from genetically modified (GM) plants. Food Chem Toxicol 42:1127–1156
Vera ML (1997) Effects of altitude and seed size on germination and seedling survival of heartland plants in north Spain. Plant Ecol 133:101–106
Vizcaino JA, Cote RG, Csordas A, Dianes JA, Fabregat A, Foster JM, Griss J, Alpi E, Birim M, Contell J, O’Kelly G, Schoenegger A, Ovelleiro D, Perez-Riverol Y, Reisinger F, Rios D, Wang R, Hermjakob H (2013) The proteomics identifications (PRIDE) database and associated tools: status in 2013. Nucleic Acids Res 41(D1):D1063–D1069
Walsh KD, Puttick DM, Hills MJ, Yang R-C, Topinka KC, Hall LM (2012) First report of outcrossing rates in camelina [Camelain sativa (L.) Crantz] a potential platform for bioindustrial oils. Can J Plant Sci 92:681–685
Wanasundara JPD (2011) Proteins of Brassicaceae oilseeds and their potential as a plant protein source. Crit Rev Food Sci Nutr 51:635–677
Wesley SV, Helliwell CA, Smith NA, Wang N, Rouse DT, Liu Q, Gooding PS, Singh SP, Abbott D, Stoutjesdijik Robinson SP, Gleave AP, Green AG, Waterhouse PM (2001) Construct design for efficient, effective and high-throughput gene silencing in plants. Plant J 27:581–590
Wich SA, Meijaard E, Marshall AJ, Husson S, Ancrenaz M, Lacy RC, van Schaik CP, Sugardjito J, Simorangkir T, Traylor-Holzer K, Doughty M, Supratna J, Dennis R, Gumal M, Knott CD, Singleton I (2008) Distribution and conservation status of the orang-utan (Pongo spp) Borneo and Sumatra: how many remain? Oryx 42:329–339
Wilcox JR, Shibles RM (1999) Interrelationships among seed quality attributes in soybean. Crop Sci 41:11–14
Withana-Gamage TS, Hegedus DD, Qiu X, Yu P, May T, Lydiate D, Wanasundara JPD (2013) Characterization of Arabidopsis thaliana lines with altered seed storage protein profiles using synchrotron-powered FT-IR spectromicroscopy. J Agric Food Chem 61:901–912
Wright ML, Pendarvis K, Nanduri B, Edelmann MJ, Jenkins HN, Reddy JS, Wilson JG, Ding X, Broadway PR, Ammari MG, Paul O, Roberts B, Donaldson JR (2016) The Effect of Oxygen on Bile Resistance in Listeria monocytogenes. J Proteomics Bioinform 4:107–119
Yamada T, Mori Y, Yasue K, Naruyama N, Kitamura K, Abe J (2014) Knockdown of the 7S globulin subunits shifts distribution of nitrogen sources to the residual protein fraction in transgenic soybean seeds. Plant Cell Rep 33:1963–1976
Yang L, Hirose S, Takahashi H, Kawakatsu T, Takaiwa F (2012) Recombinant protein yield in rice seed is enhanced by specific suppression of endogenous seed proteins at the same deposit site. Plant Biotechnol J 10:1035–1045
Acknowledgments
The authors are grateful to UC Davis Proteomics Core Facility for conducting the amino acid composition analysis and to Metabolon for metabolite analysis. We thank Howard Berg for photography assistance. Carbohydrate analysis was performed by the Complex Carbohydrate Research Center (University of Georgia) supported by the Chemical Sciences, Geosciences and Biosciences Division, Office of Basic Energy Sciences, US Department of Energy Grant (DE-FG02-93ER20097) to Parastoo Azadi at the Complex Carbohydrate Research Center.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
11248_2016_9992_MOESM1_ESM.tif
Fig. 1: Total amino acid composition analysis of crtB and RNAi2S/crtB transgenic seeds. Homozygous seeds from crtB and RNAi2S/crtB transgenic and nontransgenic Camelina seeds were analyzed by Proteomic Core Facility (UC Davis). The results are shown as an average ± standard error of 3 replicates. ANOVA Tukey with 95% confidence was performed. Means that do not share a letter are significantly different (p < 0.05)
11248_2016_9992_MOESM2_ESM.docx
Table 1: Mass spectroscopy data of selected proteins identified in the β-carotene (crtB) enhanced Camelina seeds with/without the SSP 2S suppression (RNAi2S/crtB). Data is presented by relative amounts of crtB proteins compared to nontransgenic, RNAi2S/crtB compared to nontransgenic and RNAi2S/crtB compared to crtB proteome. For full dataset see Suppl Table 2 and dataset identifier PXD002330 and 10.6019/PXD002330. The targeted 2S albumin SSP is severely reduced in the RNAi2S/crtB seeds while the other SSP, cruciferin, is notably increased during the seed protein rebalancing
11248_2016_9992_MOESM3_ESM.xlsx
Table 2: Proteins identified by mass spectrometry in crtB,RNAi2S/crtB and nontransgenic seed samples. Data is shown as normalized protein reads and pairwise comparisons of relative protein abundance within the samples
11248_2016_9992_MOESM4_ESM.docx
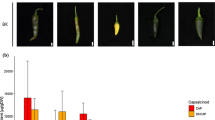
Table 3: Quantification of the phytohormone abscisic acid in developing Camelina seeds with enhanced β-carotene with/without endogenous SSP suppressed. Mid-mature seeds, approximately 10 mg, from plants grown side-by-side were used to quantitate the amount of abscisic acid via HPLC. Four technical replicates for nontransgenic seeds, two biological replicates of enhanced β-carotene (crtB-1 and crtB-2) and three biological replicates for enhanced β-carotene with the 2S storage protein (SSP) suppression (RNAi/crtB/-1, RNAi/crtB-2, RNAi/crtB-3) are shown. Values are mean ± standard deviation. ANOVA Tukey method with 95% confidence was performed. Means that do not share a letter are significantly different (p< 0.05). Most of the transgenic lines with enhanced β-carotene displayed elevated levels of this phytohormone in developing seeds. One line with both β-carotene and 2S suppression showed almost a 1400% increase in ABA in developing seeds
11248_2016_9992_MOESM5_ESM.xlsx
Table 4: Nontargeted metabolite analysis of dry and mid-maturation (immature) cotyledons of nontransgenic, crtB and RNAi2S/crtB Camelina seeds. Each biological sample was performed in triplicate and data presented as metabolite detected and quantity measured in each sample
11248_2016_9992_MOESM6_ESM.xlsx
Table 5: Oil composition analysis in crtB, RNAi2S/crtB and nontransgenic seed samples. Dry seeds samples from independent homozygous transgenics lines and nontransgenic control seeds were analyzed by gas chromatography analysis by the Experiment Station Chemical Laboratories at University of Missouri. No differences were detected in oil composition between the transgenics and nontrangenics
11248_2016_9992_MOESM7_ESM.doc
Table 6: Glycosyl composition analysis of crtB, RNAi2S/crtB and nontransgenic seed. Dry seeds from the transgenic lines of interest along with nontransgenic seeds were analyzed by gas chromatography/mass spectroscopy analysis by the Complex Carbohydrate Research Center (University of Georgia). Data for each of the triplicate samples are shown for the carbohydrates detected. No obvious differences were seen in the percentage of carbohydrate or composition of the transgenic seeds compared to the nontransgenic seeds
11248_2016_9992_MOESM8_ESM.docx
Table 7: Technical replicate data for measurements for protein, oil and carbohydrates in dry transgenic Camelina seeds compared to nontransgenic. Measurements shown were then used to calculate percentage of seed composition (protein, oil or carbohydrate) present in transgenic lines compared to nontransgenic seeds. Comparative composition analysis shown in Figure 4
Rights and permissions
About this article
Cite this article
Schmidt, M.A., Pendarvis, K. Proteome rebalancing in transgenic Camelina occurs within the enlarged proteome induced by β-carotene accumulation and storage protein suppression. Transgenic Res 26, 171–186 (2017). https://doi.org/10.1007/s11248-016-9992-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11248-016-9992-y