Abstract
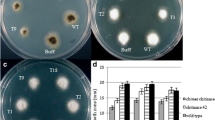
The oriental melon (Cucumis melo L. var. makuwa cv. ‘Silver Light’) is an important fruit crop in the tropical and subtropical regions. However, oriental melon production is severely decreased by fungal diseases. In this study, antifungal protein (AFP) and chitinase (CHI) fusion genes were introduced into oriental melons to control fungal diseases caused by Rhizoctonia solani and Fusarium oxysporum. Transformation of oriental melon (Cucumis melo L. var. makuwa cv. ‘Silver Light’) with Agrobacterium tumefaciens strain LBA4404 containing antifungal protein (AFP) and chitinase (CHI) fusion genes under the control of the cauliflower mosaic virus (CaMV) 35S promoter and neomycin phosphotransferase (nptII) gene as a selectable marker was performed. Cotyledon explants of oriental melon were inoculated by Agrobacterium suspensions with pBI121–AFP–CHI and cultured in a regeneration medium. After regeneration, genomic DNA polymerase chain reaction (PCR) was conducted to confirm the presence of putative transgenic shoots. Southern blot analysis confirmed that the AFP–CHI fusion gene was incorporated into the genomic DNA of the PCR-positive lines. RT-PCR analysis showed that the AFP–CHI fusion gene was expressed in the individual transgenic lines. Western blot analysis revealed the accumulation of CHI protein in leaves. A segregation analysis of the T1 generation confirmed the inheritance of the transgene. Our results demonstrated that the AFP–CHI fusion gene was effective in protecting the transgenic melon plants against fungal disease caused by Rhizoctonia solani and Fusarium oxysporum.






Similar content being viewed by others
References
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Carvalho AO, Gomes VM (2009) Plant defensins-Prospects for the biological functions and biotechnological properties. Peptides 30:1007–1020
Chen SC, Liu AR, Wang FH, Ahammed GJ (2009) Combined overexpression of chitinase and defensin genesin transgenic tomato enhances resistance to Botrytis cinerea. Afr J Biotechnol 8:5182–5188
Chhikara S, Chaudhury D, Dhankher OP, Jaiwal PK (2012) Combined expression of a barley class II chitinase and type I ribosome inactivating protein in transgenic Brassica juncea provides protection against Alternaria brassicae. Plant Cell Tissue Organ Cult 108:83–89
Choi JY, Shin JS, Chung YS, Hyung NI (2012) An efficient selection and regeneration protocol for Agrobacterium-mediated transformation of oriental melon (Cucumis melo L. var. makuwa). Plant Cell Tissue Organ Cult 110:133–140
Collinge DB, Kragh KM, Mikkelsen JD, Nielsen KK, Rasmussen U, Vad K (1993) Plant chitinases. Plant J 3:31–40
Cornelisen BJC, Melchers LS (1993) Strategies for control of fungal diseases with transgenic plants. Plant Physiol 101:709–712
Corrado G, Arciello S, Fanti P, Fiandra L, Garonna A, Digilio MC, Lorito M, Giordana B, Pennacchio F, Rao R (2007) The Chitinase A from the baculovirus AcMNPV enhances resistance to both fungi and herbivorous pests in tobacco. Transgenic Res. doi:10.1007/s11248-007-9129-4
Datta K, Tu J, Oliva N, Ona I, Velazhahan R, Mew TW, Muthukrishnan S, Datta SK (2001) Enhanced resistance to sheath blight by constitutive expression of infection-related rice chitinase in transgenic elite indica rice cultivars. Plant Sci 160:405–414
Dieter P, He PL, Rainer F, Fritz K, Yu CL (2004) Fusion proteins comprising a Fusarium—specific antibody linked to antifungal peptides protect plants against a fungal pathogen. Nat Biotechnol 22:732–738
Elliott PE, Lewis RS, Shew HD, Gutierrez WA, Nicholson JS (2008) Evaluation of tobacco germplasm for seedling resistance to stem rot and target spot caused by Thanatephorus cucumeris. Plant Dis 92:425–430
Fang G, Grumet R (1990) Agrobacterium tumefaciens mediated transformation and regeneration of muskmelon plants. Plant Cell Rep 9:160–164
Fiocchetti F, D Amore R, De Palma M, Bertini L, Caruso C, Caporale C, Testa A, Cristinzio G, Saccardo F, Tucci M (2008) Constitutive over-expression of two wheat pathogenesis-related genes enhances resistance of tobacco plants to Phytophthora nicotianae. Plant Cell Tissue Organ Cult 92:73–84
Ganesan M, Bhanumathi P, Ganesh Kumari K, Lakshmi Prabha A, Song P-S, Jayabalan N (2009) Transgenic Indian cotton (Gossypium hirsutum) harboring rice chitinase gene (Chi II) confers resistance to two fungal pathogens. Am J Plant Biochem Biotechnol 5(2):63–74
Gilbert MO, Zhang YY, Punja ZK (1996) Introduction and expression of chitinase encoding genes in carrot following Agrobacterium-mediated transformation. In vitro Cell Dev Biol Plant 32:171–178
Girhepuje PV, Shinde GB (2011) Transgenic tomato plants expressing a wheat endochitinase gene demonstrate enhanced resistance to Fusarium oxysporum f. sp. lycopersici. Plant Cell Tissue Organ Cult 105:243–251
Gutierrez WA, Shew HD, Melton TA (1997) Source of inoculum and management for Rhizoctonia solani damping-off on tobacco transplants under greenhouse conditions. Plant Dis 81:604–606
Hsiao YL, Chiling SC, Liang SL, Chien AL, Ming TC, Yee YC (2003) Over-expression of Arabidopsis thaliana heat shock factor gene (AtHsfA1b) enhances chilling tolerance in transgenic tomato. Bot Bull Acad Sin 44:129–140
Institute SAS (1999) SAS system version 8 for windows. SAS Institute, Cary
Jean-Yves P, Douglas KB, Martin BD, Robert MH, Harjeet KK, James LD (2011) Apoptosis-related genes confer resistance to Fusarium wilt in transgenic ‘Lady Finger’ bananas. Plant Biotechnol J 9:1141–1148
Jongedijk E, Tigelaar H, Van Roekel JSC (1995) Synergistic activity of chitinase and β- 1,3 glucanases enhances fungal resistance in transgenic tomato plants. Euphytica 85:173–180
Kwon KM, Hong RJ, Kim HY, Kim CK (2001) Soil-environmental factors involved in the development of root rot/vine on cucurbits caused by Monosporascus cannonballus. Plant Pathol J 17:45–51
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
Lakshman DK, Natarajan SS, Lakshman S, Garrett WM, Dhar AK (2008) Optimized protein extraction methods for proteomic analysis of Rhizoctonia solani. Mycologia 100:867–875
Li P, Pei Y, Sang X, Ling Y, Yang Z, He G (2009) Transgenic indica rice expressing a bitter melon (Momordica charantia) class I chitinase gene (McCHIT1) confers enhanced resistance to Magnaporthe grisea and Rhizoctonia solani. Eur J Plant Pathol 125:533–543
Liu L, Kakihara F, Kato M (2004) Characterization of six varieties of Cucumis melo L. based on morphological and physiological characters, including shelf-life of fruit. Euphytica 135:305–313
Luria SE, Burrous JW (1957) Hybridization between Escherichia coli and Shigella. J Bacteriol 74:461–476
Monforte AJ, Oliver M, Gonzalo MJ, Alvarez JM, Dolcet–Sanjuan R, Arús P (2004) Identification of quantitative trait loci involved in fruit quality traits in melon (Cucumis melo L.). Theor Appl Genet 108:750–758
Moreno AB, Peñas G, Rufat M, Bravo JM, Estopà M, Messeguer J, San Segundo B (2005) Pathogen-induced production of the antifungal AFP protein from Aspergillus giganteus confers resistance to the blast fungus Magnaporthe grisea in transgenic rice. Mol Plant Microbe Interact 9:960–972
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plantarum 15:473–497
Nirala NK, Das DK, Srivatsava PS, Sopory SK, Upadhyaya KC (2010) Expression of a rice chitinase gene enhances antifungal potential in transgenic grapevine (Vitis vinifera L.). Vitis 49(4):181–187
Nookaraju A, Agrawal DC (2012) Enhanced tolerance of transgenic grapevines expressing chitinase and β-1,3-glucanase genes to downy mildew. Plant Cell Tissue Organ Cult. doi:10.1007/s11240-012-0166-1
Ntui VO, Thirukkumaran G, Azadi P, Khan RS, Nakamura I, Mii M (2010) Stable integration and expression of wasabi defensin gene in “Egusi” melon (Colocynthis citrullus L.) confers resistance to Fusarium wilt and Alternia leaf spot. Plant Cell Rep 29:943–954
Nuñez–Palenius HG, Gomez–Lim M, Ochoa–Alejo N, Grumet R, Lester G, Cantliffe DJ (2008) Melon fruits: genetic diversity, physiology, and biotechnology features. Crit Rev Biotechnol 28:13–55
Ohnuma T, Taira T, Yamagami T, Aso Y, Ishiguro M (2004) Molecular cloning, functional expression, and mutagenesis of cDNA encoding class I chitinase from rye (Secale cereale) seeds. Biosci Biotechnol Biochem 68:324–332
Oldach KH, Becker D, Lorz H (2001) Heterologous expression of the genes mediating enhanced fungal resistance in transgenic wheat. Mol Plant Microbe Interact 14:832–838
Punja ZK (2001) Transgenic carrots with enhanced tolerance to fungal pathogens. In: Khachatourians GG, McHughen A, Scorza R, Nip WK, Hui YH (eds) Transgenic plants and crops. Marcel Decker, New York, pp 503–521
Robert N, Roche K, Lebeau Y, Breda C, Boulay M, Esnault R, Buffard D (2002) Expression of grapevine chitinase gene in berries and leaves infected by fungal or bacterial pathogen. Plant Sci 162:389–400
Sambrook J, Fritsch EF, Maniatis T (1989) Molecular cloning: a laboratory manual, 2nd edn. Cold Spring Harbor Laboratory Press, Cold Spring Harbor Laboratory, New York
Scher FM, Baker R (1982) Effect of Pseudomonas putida and a synthetic iron chelator on induction of soil supressiviness to Fusarium wilt pathogens. Phytopathology 72:1567–1573
Shin S, Mackintosh CA, Lewis J, Heinen SJ, Radmer L, Dill-Macky R, Baldridge GD, Zeyen RJ, Muehlbauer GJ (2008) Transgenic wheat expressing a barley class II chitinase gene has enhanced resistance against Fusarium graminearum. J Exp Bot 59(9):2371–2378
Stepansky A, Kovalski I, Perl–Treves R (1999) Intraspecific classification of melons (Cucumis melo L.) in view of their phenotypic and molecular variation. Plant Syst Evol 217:313–333
Stintzi A, Heitz T, Prasad V, Wiedemann Merdinoglu S, Geoffroy P, Legrand M, Fritig B (1993) Plant pathogenesis-related proteins and their role in defense against pathogens. Biochimice 75:687–706
Terras FR, Eggermant K, Kovalega V, Raikhel NV, Osborn RW, Kester A, Rees SB, Torrekens S, Van Leuven F, Vanderleyden J (1995) Small cysteine-rich antifungal proteins from radish: their role in host defense. Plant Cell 7:573–588
Wang Y, Kausch AP, Chandlee JM, Luo H, Ruemmele BA, Browning M, Jackson N, Goldsmith MR (2003) Co-transfer and expression of chitinase, glucanase, and bar genes in creeping bentgrass for conferring fungal disease resistance. Plant Sci 165:497–506
Wu HW, Yu TA, Raja JAJ, Wang HC, Yeh SD (2009) Generation of transgenic oriental melon resistant to Zucchini yellow mosaic virus by an improved cotyledon-cutting method. Plant Cell Rep 28:1053–1064
Yevtushenko DP, Romero R, Forward BS, Hancock RE, Kay WW, Misra S (2005) Pathogen-induced expression of a cecropin A-melittin antimicrobial peptide gene confers antifungal resistance in transgenic tobacco. J Exp Bot 56:1685–1690
Acknowledgments
This work was supported by the National Science Council of Taiwan, Republic of China (98-2321-B- 005-009-MY3).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Bezirganoglu, I., Hwang, SY., Fang, T.J. et al. Transgenic lines of melon (Cucumis melo L. var. makuwa cv. ‘Silver Light’) expressing antifungal protein and chitinase genes exhibit enhanced resistance to fungal pathogens. Plant Cell Tiss Organ Cult 112, 227–237 (2013). https://doi.org/10.1007/s11240-012-0227-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-012-0227-5